Abstract
The present study investigated the anticonvulsant activity of different root extracts of Calotropis procera R. Br. (Asclepiadaceae) in rats in order to evaluate the traditional use of this plant. The anticonvulsant activity of different extracts of Calotropis procera roots was studied against seizures induced by maximal electroshock seizures (MES), pentylenetetrazol (PTZ), lithium-pilocarpine and electrical kindling seizures. In the MES test, the chloroform extract of Calotropis procera roots showed the most significant (p < 0.01) anticonvulsant effect by decreasing the duration of hind limb extension (extensor phase), clonus and also the duration of stupor phase, as compared to control. In the PTZ test, the chloroform extract showed a highly significant (p < 0.001) effect, whereas the aqueous extract showed the most significant (p < 0.01) effect as compared to control by delaying the onset of convulsions. The extracts also inhibited convulsions induced by lithium-pilocarpine and electrical kindling. The results of this study indicate that the chloroform extract and aqueous extract of Calotropis procera roots may be beneficial in the absence (petitmal) and tonic-clonic (grand mal) type of seizures.
Introduction
Epilepsy is a common neurological disorder affecting an estimated 40–50 million people worldwide (Rudiger, 2002). Prevalence of chronic epilepsy is in the range of 4–10 per 1,000 people. The incidence of epilepsy is highest among children below 7 years-of-age and in individuals of above 55 years. The reported prevalence of epilepsy in India is about 5.5 to 7.9 per 1,000 people, which is about 1/18th of the world population (CitationNag, 2000).
Currently available synthetic anti-epileptic drugs are unable to control epileptic seizures in as many as 25% of the patients. Further, the combination of anti-epileptic drugs (AEDs) has high risk of toxicity and drug interactions, which may complicate clinical management. These limitations with conventional AEDs demand the need for developing newer anti-epileptic agents (CitationGupta et al., 1999; CitationGupta & Malhotra, 2000). Presently, scientists are keen to obtain drugs from plant origin due to their specific curative properties and relatively low adverse effects. The Ayurvedic system of medicine has a quite sophistical classification of medicinal plants as per the dominant pharmacological/therapeutic activity of mental functions (CitationVaidhya, 1997).
A literature survey revealed that various parts (latex, stem, bark, roots, seeds, flower tops and leaves) of Calotropis procera (Ait.) R. Br. (Asclepiadaceae) are used in the Ayurvedic system of medicine for its different pharmacological activities. Roots are used to treat cancer, dysentery, hysteria, cramps, leprosy, worms, fever, gout, and snake bites. In traditional medicine, roots and bark are used for all kinds of fits, epilepsy, convulsions, and paralysis complaints (CitationChatterjee & Prakash, 1994; CitationNadakarni, 2002). An ethanol extract of Calotropis procera roots and flowers has been reported for antifertility and hormonal, anti-inflammatory, antipyretic, analgesic and antimicrobial activities (CitationMascoloa et al., 1988; CitationCircosta et al., 2001; CitationKamath & Rana, 2002).
The present study was carried out to evaluate anticonvulsant effect of Calotropis procera roots by maximal electroshock seizure (MES), pentylenetetrazole (PTZ), lithium-pilocarpine and electrical kindling induced seizures.
Materials and methods
Plant material
The matured roots of Calotropis procera were collected from Dandeli forest, Belgaum, Karnataka, India, in November 2006 and were authenticated by R. S. Goudar, Department of Botany, R. L. Science Institute, Belgaum Karnataka, India. A voucher specimen (Md-01) of the plant material is kept at the pharmacognosy museum of K. L. E. Society’s College of Pharmacy Belgaum, Karnataka, India for future reference.
Calotropis procera roots were shade-dried at room temperature and powdered to 40 mesh particle size. The powdered material (300 g) was extracted with petroleum ether (40°–60°C), chloroform and alcohol as solvents by using a Soxhlet apparatus. The aqueous extraction was prepared by cold maceration process using water as solvent. The extracts obtained were filtered and the solvents were removed in vacuo to yield dry extracts. The yield was pet-ether (40°–60°C) 4.03 g, chloroform 5.20 g, alcohol 8.75 g and aqueous extract 14.30 g. All the extractive preparations were suspended in Tween-80 for administration to animals.
Drugs and chemicals
Pentylenetetrazole (Sigma, USA), phenytoin sodium (Epsolin injection, Zydus Neurosciences, India), diazepam (Calmpose injection, Ranbaxy, India), lithium-pilocarpine (Merk-Aldrich, India) were dissolved/diluted in water in a volume of 9, 5, 1, and 4 mg/ml, respectively, and administered.
Animals
Female Albino Wistar rats (180-200 g) were obtained from the animal house, Department of Livestock Production, Government Veterinary College, Hebbal, Bangalore, India. All experiments with each group of rats were then carried out within the next week to minimize the effect of increasing age on seizure susceptibility. Each rat was used for only one experiment. Female rats were employed because they are known to eliminate several anti-epileptic drugs less rapidly than males (CitationSchmutz, 1985) and this was thought to be an advantage for drug potency studies.
The rats were kept in groups of 6 in plastic cages at controlled temperature (25°C) and humidity (about 40%) with a 12 h light cycle beginning at 7 am. The rats were provided with standard diet and tap water ad libitum. All experiments were carried out between 8 am and 12 noon at an ambient temperature of 23°–25°C (CitationLoscher et al., 1991a). Food but not water was withdrawn 8 h before and during experiments. The Institutional Animal Ethical Committee approved the protocol of this study (Resolution No. 08).
Acute toxicity study
The median lethal dose of the pet-ether (40°-60°C), chloroform, alcohol and aqueous was determined by orally administering the extracts in increasing dose levels of 1, 2, 3, 4, and 5 g/kg body weight to healthy adult Wistar albino rats of either sex. The animals will be observed continuously for 2 h under the following profiles:
Behavioral profile: alertness, restlessness, irritability and fearfulness
Neurological profile: spontaneous activity, reactivity, touches response, pain response and gait
Autonomic profile: defecation and urination
After a period of 24 h, the animals were observed for any lethality or death (% of mortality).
Assessment of anticonvulsant activity
Maximal electroshock seizure (MES) test
A total of 36 rats were divided into six groups. Group 1 received 1 ml/rat of saline, group 2 received 25 mg/kg of phenytoin, groups 3, 5, and 6 received 500 mg/kg of petroleum ether (40–60°C), ethanol, and aqueous extracts, respectively, while group 4 received 200 mg/kg of chloroform extract of Calotropis procera roots. The saline and standard reference drug were administered 45 min before induction of MES, whereas the test extracts of roots of Calotropis procera were administered 1 h before induction of MES.
To induce convulsions in the control and drug treated animals, the maximal (tonic hind limb extension) electroshock seizure (MES) test with supramaximal stimulation was carried out via transauricular copper electrodes (introduced bilaterally into the ears) with the apparatus (Inco Electroconvulsometer model# 100-3), using a fixed current 150 mA in rats for 0.2 sec. The duration of each phase for each animal (in sec) was measured by using stopwatch. The tonic extension of the hind limbs (extensor phase) and mortality were recorded.
Pentylenetetrazole-induced seizure test
A total of 36 female rats were used. The rats were divided into 6 groups of 6 each. Group I received 1 ml/rat saline; group 2 received 4 mg/kg of diazepam; groups 3, 5, and 6 received 500 mg/kg petroleum ether (40–60°C), alcohol and aqueous extracts, respectively, while group 4 received 200 mg/kg chloroform extract of Calotropis procera roots. Pentylenetetrazole (PTZ), 90 mg/kg, was administered intraperitoneally to induce convulsions in the animals of all six groups 45 min, to 1 h prior to administration of saline, standard drug and different extracts of Calotropis procera roots. The anticonvulsant property of the drug in this model was assessed by its ability to delay the onset of action (animals were observed for 30 min after injection of PTZ), protection against PTZ-induced convulsions, and percentage of mortality was recorded (CitationLoscher et al., 1991b; CitationPaschoa et al., 1997; CitationHosseinzadeh & Khosravan, 2002; CitationVogel, 2002).
Lithium–pilocarpine-induced seizures
The effect of fractions on the seizures induced by lithium–pilocarpine was studied in rats. The animals received lithium sulphate (3 meq/kg i.p.) 24 h before pilocarpine (30 mg/kg i.p.). The animals received the extracts of Calotropis procera roots, diazepam (2 mg/kg i.p.) or vehicle 30 min before pilocarpine. The severity of convulsions was assessed, until the maximum effect was produced, using the following stages. No response, stage 0; fictive scratching, stage 1; tremors, stage 2; head nodding, stage 3; forelimb clonus, stage 4; rearing, falling and clonus, stage 5 (CitationPatel et al., 1988).
Electrically kindled seizures
Six rats were used in each group. The animals received two sub-convulsive electric shocks per day (21 mA, 0.1 s duration, 3 h apart) until all animals exhibited full blown clonic convulsions (CitationGoddard et al., 1969). Ten shocks were required to induce convulsions in each animal. Each animal, after one shock-free day, received the different extracts of Calotropis procera roots 30 min before electrical stimulation. The severity of seizures was graded according to the following criteria: Grade I, facial movements; Grade II, prominent head nodding; Grade III, rising of forelimb and mild forelimb clonus; Grade IV, marked rearing with oral movements and forelimb clonus; Grade V, repeated falling on back and clonic seizures (CitationMcNamara et al., 1993).
Statistical analysis
The values are expressed as mean ± SEM and the data were analyzed using one way ANOVA followed by Dunnett’s test. The level of significance was set at P < 0.05.
Results
Acute toxicity test
From the data, it is evident that the plant extracts were well tolerated orally in rats up to the dose of 5 g/kg except chloroform extract. Because of this, the LD50 for pet-ether, alcohol and aqueous extracts was considered as 5 g/kg body weight, and the ED50 (1/10th dose LD50) for these extracts was taken as 500 mg/kg. The LD50 for the chloroform extract was 2 g/kg body weight and the ED50 was taken as 200 mg/kg ().
Table 1. Acute toxicity of Calotropis procera R. Br root extracts.
Maximal electroshock seizure (MES) test
From it is revealed that the chloroform extract of Calotropis procera roots decreased the duration of hind limb extension by (7.00 ± 0.97 sec) which is most significant (p < 0.01) when compared to the effect produced by the control (4.83 ± 1.54 sec), pet-ether extract (11.17 ± 1.40 sec), alcohol extract (18.17 ± 0.95 sec) and aqueous extract (13.17 ± 0.94 sec) of Calotropis procera roots. The chloroform extract also decreased the duration of clonus and stupor phase of MES induced convulsions as compared to control.
Table 2. Effect of Calotropis procera R. Br root extracts against MES induced convulsions.
Pentylenetetrazole (PTZ)-induced seizures test
The effects of Calotropis procera root extracts in PTZ-induced convulsions are shown in . Statistical data obtained from the anticonvulsant effect of Calotropis procera roots against PTZ induced seizure has revealed that chloroform extract of Calotropis procera root delayed the onset of convulsions by 543.20 ± 1.96 sec as compared to the effect produced by the control (113.3 ± 4.97 sec) and other extracts. The chloroform extract not only delayed the onset action of seizures but 50% of animals in the group treated were protected against seizures induction. Moreover, all the animals were recovered and there was no incidence of mortality in the group of animals treated with chloroform extract of Calotropis procera.
Table 3. Effect of Calotropis procera R. Br root extracts against PTZ induced seizures.
The aqueous extract has delayed the onset of action (138.00 ± 11.20 sec) as compared to the control group (113.3 ± 4.97 sec) and no incidence of mortality was observed. While in the animals treated with normal saline (control group), pet-ether extract and alcohol extract, 100% mortality was observed.
Lithium–pilocarpine-induced seizures
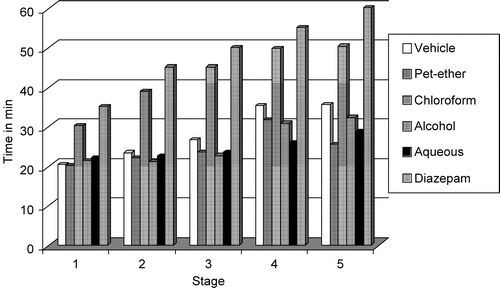
The chloroform extract prevented the occurrence of Lithium–pilocarpine-induced seizures as it increased the latency to various stages of seizures (fictive scratching; tremors; head nodding; forelimb clonus and rearing, falling and clonus) significantly (p < 0.05). The chloroform extract was most effective during stage 4, i.e., forelimb clonus. The effect is comparable to control and diazepam ().
Figure 1. Inhibitory effect Calotropis procera R. Br root extracts and diazepam on the latency to various stages (stage 1, fictive scratching; stage 2, tremors; stage 3, head nodding; stage 4, forelimb clonus; and stage 5, rearing, falling and clonus) of lithium–pilocarpine-induced status epilepticus in rats.
Electrically kindled seizures
The severity of seizures was recorded as different grades: Grade I, facial movements; Grade II, prominent head nodding; Grade III, raising of forelimb and mild forelimb clonus; Grade IV, marked rearing with oral movements and forelimb clonus; Grade V, repeated falling on back and clonic seizures. Chloroform and aqueous extract showed statistically significant (p < 0.05) effect in electrically kindled seizures. However, only the chloroform extract was able to decrease the duration of forelimb clonus, marked rearing with oral movement and also the duration of repeated falling back and clonic seizures, which indicates the chloroform extract does possess potent anticonvulsant activity against electrically kindled seizures, as compared to control.
Discussion
The results of the current study indicate that the chloroform extract of Calotropis procera roots delayed the onset of convulsions induction, and was able to decrease the duration of hind limb extension, clonus and stupor phases of MES induced convulsion, as compared to control and the effects produced by petroleum-ether, alcohol and aqueous extract of the Calotropis procera roots.
Petroleum-ether and aqueous extracts showed statistically significant (p < 0.05) effects in phases of stupor. The standard drug, phenytoin, at a dose of 25 mg/kg body weight, provided 100% protection and also significantly reduced the duration of stupor when compared to control. In other words, the chloroform extract is able to decrease the duration of hind limb extension (extensor phase), clonus and also the duration of stupor phase, which indicates the chloroform extract does possess potent anticonvulsant activity against generalized tonic clonic seizure (grand mal), while other extracts, viz., petroleum ether extract, alcohol and aqueous extracts of roots of Calotropis procera did not show statistically any significant effect in the extensor phase as compared to control.
The chloroform extract of Calotropis procera roots delayed the onset of convulsion in PTZ-induced convulsions and protected 50% of the animals from induction of convulsions, i.e., 50% of the animals treated with chloroform extract did not show any signs of convulsions and no incidence of mortality was observed in the group treated with chloroform extract. This suggests that the anticonvulsant action of the chloroform extract is mediated by the chloride channel of GABA/benzodiazepine receptor complex and not by the chloride channel of glycine receptors. Since PTZ is a GABAA receptor antagonist, the extract may be acting by increasing GABA concentration in the brain. The extract also inhibited seizures due to electrical kindling. Kindling is widely accepted as a model of chronic epilepsy (CitationLothman & Williamson, 1994). Baclofen, a GABAB agonist, exerts an anticonvulsant activity in amygdala kindling (CitationWurpel, 1994).
Anticonvulsant drugs may be needed to reduce the risk of having another seizure and are not usually prescribed for only one generalized seizure for which no cause can be found. But anticonvulsants drugs are necessary for those who have had more than one seizure. Anticonvulsants drugs can completely prevent convulsive seizures in more than 50% of the affected and greatly reduce the frequency of seizures. No one drug can control all types of seizures (CitationMark, 2003).
The chloroform and aqueous extracts of Calotropis procera roots also reduced the severity of status epilepticus induced by lithium–pilocarpine. Lithium alone does not have a proconvulsant effect in rats (CitationClifford et al., 1985; CitationOrmandy et al., 1991; CitationKofman et al., 1991, Citation1992). However, rats pretreated with lithium have limbic seizures, following subconvulsant doses of pilocarpine. The combined treatment with lithium–pilocarpine results in an accumulation of inositol monophosphate and re-reduction in cortical inositol that are about ten times greater than the effects obtained with either drugs alone (CitationSherman et al., 1985). There is a massive increase in the concentration of acetylcholine and choline in the cerebral cortex and hippocampus, which may play a role in seizure maintenance and lethality associated with status epilepticus (CitationJope & Gu, 1991). The chloroform and aqueous extracts of Calotropis procera roots and diazepam prevented the occurrence of lithium–pilocarpine-induced seizures (CitationSherman, 1991). Since the status epilepticus appears to be linked to low GABA levels in brain, we therefore investigated the effect of the fractions on the brain contents of GABA. The chloroform and aqueous extracts of Calotropis procera roots increased GABA contents of the brain and this could be the mechanism of anticonvulsant action of the extracts used in this study.
Acknowledgments
The authors express their thanks to the K.L.E. Society, Belgaum, Karnataka, India for providing the facilities necessary to carry out the research work.
Declaration of interest: The authors report no conflicts of interest.
References
- Chatterjee A, Prakash SC (1994): In: The Treatise of Indian Medicinal Plants. New Delhi, Publications and Information Directorate, pp. 130–131.
- Circosta C, Sanogo R, Occhiuto F (2001): Effects of Calotropis procera on oestrous cycle and on oestrogenic functionality in rats. Il Farmaco 56: 373–378.
- Clifford DB, Podolski A, Zorumski CP (1985): Acute effects of lithium on hippocampal kindled seizures. Epilepsia 26: 689–692.
- Goddard GV, McIntyre DC, Leech CK (1969): A permanent change in brain function resulting from daily electrical stimulation. Exp Neul 25: 295–330.
- Gupta YK, Malhotra J, George B, Kulkarni SK (1999): Methods and consideration for experimental evaluation of antiepileptic drugs. Indian J Physiol Pharmacol 43: 25–43.
- Gupta YK, Malhotra J (2000): Antiepileptic drug therapy in the twenty first century. Indian J Physiol Pharmacol 44: 8–23.
- Hosseinzadeh H, Khosravan V (2002): Anticonvulsant effects of aqueous and enthanolic extracts of Crocus sativus L. stigmas in mice. Arch Iran Med 5: 44–47.
- Jope RS, Gu X (1991): Seizures increase acetylcholine and choline concentration in rat brain regions. Neurochem Res 16: 1219–1226.
- Kamath JV, Rana AC (2002): Preliminary study on antifertility activity of Calotropis procera roots in female rats. Fitoterapia 73: 111–115.
- Kofman O, Belmaker RH, Grisaru N (1991): Myoinositol attenuates two specific behavioral effects of acute lithium in rats. Psychopharmacol Bull 27: 185–190.
- Kofman O, Sherman WR, Katz V, Belmaker RH (1992): Restoration of brain myo-inositol level in rat increases latency to lithium–pilocarpine seizures. Psychopharmacology 110: 229–234.
- Kohling R (2002): Voltage-gated sodium channel in epilepsy. Epilepsia 43: 1278–1296.
- Lothman EW, Williamson JM (1994): Closely spaced recurrent hippocampal seizures elicit two types of heightened epileptogenesis: A rapidly developing, transient kindling and a slowly developing, enduring kindling. Brain Res 649: 71–84.
- Loscher W, Fassbender CP, Nolting B (1991a): The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. II. Maximal electroshock seizure models. Epilepsy Res 8: 79–94.
- Loscher W, Fassbender CP, Nolting B (1991b): The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. III. Pentylenetetrazole seizure models. Epilepsy Res 8: 171–189.
- Mark HB (2003): In: The Merck Manual of Medical Information. New Jersey, Merck Publishing Group, pp. 495–500.
- Mascoloa N, Sharmab R, Jainb SC, Capasso F (1988): Ethnopharmacology of Calotropis procera flowers. J Ethnopharmacol 22: 211–221.
- McNamara JO, Bonhaus DW, Shin C (1993): The kindling model of epilepsy. In: eds., Schwartz KP, Concepts and Models in Epilepsy Research. New York, Cambridge University Press, pp. 27–47.
- Nadakarni KM (2002): In: Indian Materia Medica. Popular Prakashan, Mumbai, pp. 242–247.
- Nag D (2000): Gender and epilepsy – A clinician’s experience. Neurol Ind 48: 99–104.
- Ormandy GC, Song L, Jope RS (1991): Analysis of the convulsant potentiating effects of lithium in rats. Exp Neurl 111: 356–361.
- Paschoa OED, Kruk MR, Hamstra R, Voskuyl RA, Danhof M (1997): Seizure patterns in kindling and cortical stimulation models of experimental epilepsy. Brain Res 770: 221–227.
- Patel S, Meldrum BS, Fine A (1988): Susceptibility of pilocarpine-induced seizures in rats increases with age. Behav Brain Res 31: 165–167.
- Rudiger K (2002): Voltage-gated sodiusm channel in epilepsy. Epilepsia 43: 1278–1296.
- Schmutz M (1985): Carbamazepine. In: Hand book of Experimental Pharmacology. Berlin, Springer, pp. 479–506.
- Sherman WR, Honchar MP, Munsell LY (1985): Detection of receptor-linked phosphoinositide metabolism in brain of lithium treated rats. In: eds., Bleasdale JE, Eichborg J, Hauser C, Inositol and Phosphoinositides: Metabolism and Regulation. London, Academic Press, pp. 251–382.
- Sherman WR (1991): Lithium and the phosphoinositide signaling system. In: ed., Birch NJ, Lithium and the Cell. London, Academic Press, pp. 121–157.
- Vaidhya ADB (1997): Status and scope of Indian medicinal plants acting on central nervous system. Indian J Pharmacol 29: 5340–5353.
- Vogel HG (2002): In: Drug Discovery and Evaluation: Pharmacological Assays. New York, Springer, pp. 487–488.
- Wurpel JND (1994): Baclofen prevents rapid amygdala kindling in adult rats. Experientia 50: 475–478.