Abstract
This study was designed to determine the polyphenolic contents of the extracts and to evaluate the antioxidant activity of Hypericum origanifolium Willd. and H. montbretii Spach. (Guttiferae (Hypericaceae)), The possible composition activity relationship was investigated and the results were compared with that of H. perforatum L. Methanol, ethyl acetate, and water were used as solvents to produce extracts from flowers and leaves of the plants. The determination of phenolic acids in the Hypericum species was achieved by using a modified Reverse phase-High pressure liquid chromatography (RP-HPLC) method adopting an internal standard. It was observed that chlorogenic and caffeic acids were higher in all extracts. The highest values were found in ethyl acetate extracts for total phenolic content as gallic acid and for the flavonoids and flavonols as rutin equivalents (all measurements are mg/g), respectively. Hypericum extracts were evaluated for their radical scavenging activity by the 2,2-diphenyl-1-picryhydrazyl radical (DPPH) and their oxidative stability by the Rancimat method. Results were compared with Butyllated hidroxy toluene (BHT), a synthetic antioxidant, and with a reference plant, H. perforatum. A good correlation between antioxidant activity and total phenol content in the extracts was observed. In an antioxidant activity assay, the leaf extracts of H. origanifolium were found to be two or three times more active than those of BHT, H. perforatum, and H. montbretii leaves and flowers. In an antiradical activity assay, leaves and flowers of H. montbretii and leaves of H. origanifolium were the most active at the tested concentrations, exhibiting an activity comparable to that of the positive control BHT, but all of the extracts, with the exception of the leaves of H. montbretii, showed activity weaker than the leaves and flowers of H. perforatum, the reference plant.
Introduction
The genus Hypericum L., a member of the Guttiferae (Hypericaceae) family, contains about 400 species in the world, about 80 species in Turkey, all small herbaceous perennials (CitationRobson, 1967, Citation1988; CitationDönmez, 2000). From a medicinal viewpoint, H. perforatum is the most important species of this genus, commonly known as St. John’s wort. It has traditionally been used on a widespread basis almost all over the world. There are many studies that have been conducted with H. perforatum, but very limited studies for the other species, like H. montbretii and H. origanifolium. Although there is only one anatomical study on these (CitationPotoğlu-Erkara & Tokur, 2004), several phytochemical studies have been previously performed to determine their constituents. According to the results of phytochemical studies, the aerial parts of H. origanifolium contain naphthodiantrones (hypericin, pseudohypericin, proto-pseudohypericin, emodin and frangulin), flavonoids (rutin, quercetin, myrcetin, hyperoside), and xanthones (mangiferin and iso-magniferin) (CitationMathis & Ourisson, 1964; CitationMakovestśka, 1999; CitationSirvent et al., 2002; CitationKitanov & Nedialkov, 1998); H. montbretii has naphthoadiantrones (hypericin, pseudohypericin, proto-pseudohypericin, emodin and frangulin), flavonoids (rutin, quercetin, myrcetin, myrcitrin, quercitrin, iso-quercitrin, hyperoside, (+)-catechin), xanthones (mangiferin and iso-magniferin), biflavonoids (13,118-biapigenin), phenolic acid (chlorogenic acid), and volatile compounds (α-, δ-, γ-cadinene, α-cadinol, d-germacrene, tr-nerolidol and valecene) (CitationMathis & Ourisson, 1964; CitationKitanov & Nedialkov, 1998; CitationSakar et al., 1991; CitationErken et al., 2001; CitationMakovestśka, 2000).
Data on the biological activity of H. montbretii and H. origanifolium are also limited to a few previous reports, which have demonstrated their antimicrobial, antibacterial and antiyeast activities (CitationSakar et al., 1988; CitationSakar & Tamer, 1990). To the best of our knowledge, there is no previous study indicating their antioxidant activities.
Hence, the primary aim of this study was to investigate the contents of phenolic acids and antioxidant potential of the extracts of H. origanifolium and H. montbretii by comparing with H. perforatum as a reference plant and to determine their relationships with the total phenolics, flavonoid and flavonol compositions by definite analytical procedures
Materials and methods
Chemicals
The chemicals gallic acid (GA), protocathechuic acid (protoCA), p-hydroxy benzoic acid (p-hydBA), caffeic acid (CA), chlorogenic acid (ChA), syringic acid (SA), p-coumaric acid (p-COU), ferulic acid (FA), o-coumaric acid (o-COU), trans-cinnamic acid (tr-CIN), propyl paraben, rutin, and Folin-Ciocalteu phenol reagent were provided by Sigma Co. (St. Louis, MO), 2,2-diphenyl-1-picryhydrazyl radical (DPPH·) was from Aldrich Chemical Co. (Milwaukee, WI), and methanol, ethyl acetate, and formic acid were purchased Merck GmbH (Darmstadt, Germany), respectively. They were all analytical grade and high purity more than 99.9% purity. Crude olive oil was kindly provided by üstün Co. (Balıkesir, Turkey).
Apparatus
An HPLC system consisting of a model of 600 E HPLC pump, 717 plus Autosampler, 996 photodiode array dedector (PAD), data processor of a Millennium 32 was used for the analysis (Waters Co., MA). Rancimat apparatus (743 Metrohm AG, SW) was employed. A 160 A model spectrophotometer (Shimadzu, Kyoto, Japan) with quartz cells was used. Ultra pure water was supplied from Human UP 9000 System (18 mΩ) water purification system. Reverse phase, C18 Ultrasphere column (Teknokroma, Barcelona, Spain) (100 × 4.6 mm i.d., 3 μ) was employed for the analysis of phenolic acids.
Plant materials
H. origanifolium (HO), H. montbretii (HM), and H. perforatum (HP) were collected around Eskişehir, Turkey. H. origanifolium Sivrihisar, Tekören village, 1100 m, June 2003 (OUFE 10334), H. montbretii Kalabak village, 1300 m, June 2003 (OUFE 10332), and H. perforatum Türkmen Mountains, upper parts of Kalabak, 1300 m, June 2003 (OUFE 10337). The plants were identified according to Flora of Turkey and the East Aegean Islands (CitationRobson 1967, Citation1988; CitationDönmez, 2000).
Preparation of extracts
Extraction procedures were applied as described elsewhere (Citationöztürk et al., 2006; CitationTsao & Den, 2004). Dried flowers and leaves of Hypericum species were ground and extracted with petroleum ether in a Soxhlet apparatus. Fat-free air-dried material was extracted with methanol:water (70:30, v/v) at 40°C, 30 min four times. The extract was concentrated to dryness in vacuum and aqueous solution was lyophilized (ME). Second extracts were prepared as follows: fat-free air-dried material was extracted with methanol:water (70:30 v/v) at 40°C, 30 min four times, and it was concentrated in vacuum, and the aqueous phase was extracted with ethyl acetate at room temperature. Then it was concentrated to dryness in vacuum (EA) and the aqueous solution was lyophilized (W). Thus, recovery of the phenolic acids with some solvents of different polarity was attempted. All extracts obtained were weighed to determine the yields of soluble constituents.
Determination of total phenolic content (Folin-Ciocalteu method)
The amounts of total phenols were determined spectrophotometrically at 750 nm, based on a colorimetric measurement for extracts as described in a previous method (CitationFolin & Ciocalteu, 1928). This method gives a general measurement of phenolic content, as it is not completely specific for phenolic compounds and not all phenolic compounds exhibit the same level of activity in the assay.
Determination of total flavonoid content
The flavonoids were determined as a species of rutin as it was proposed in a study (CitationMiliauskas et al., 2004). One ml of plant extract in methanol (10 g/l) was mixed with 1 ml of aluminum chloride in ethanol (20 g/l) and diluted with ethanol to 25 ml. To 0.5 ml of each sample or standard rutin solution, 0.5 ml aluminum chloride solution was added and the absorption at 415 nm was read after 60 min at ambient temperature. Blank samples were prepared from 1 ml plant extract and 1 drop acetic acid, and diluted to 25 ml. The flavonoid contents were found by comparing the absorbance values of the extracts with those of the standard rutin solutions which were prepared as a stock solution of 0.05 g rutin. All determinations were carried out in duplicate. The amount of flavonoids in plant extracts as rutin equivalents (RE) was calculated by the following formula:
X = (A • mo • 10 )/(Ao • m)
where: X = flavonoid content, mg/g plant extract in RE; A = the absorption of plant extract solution; Ao = the absorption of standard rutin solution; m = the weight of plant extract, g; mo = the weight of rutin in the solution, g.
Determination of total flavonol content
Flavonols were determined as a species of rutin as proposed previously (CitationMiliauskas et al., 2004). The calibration curve of rutin was constructed by mixing 2 ml of rutin solution having concentrations in the range of 0.5-0.017 mg/ml with 2 ml (20 g/l) aluminum chloride and 6 ml (50 g/l) sodium acetate. The absorption at 440 nm was read after 2.5 h at ambient temperature. The same procedure was applied to plant extracts similar to rutin solution. The experiments were always duplicated. The content of flavonols, in rutin equivalents (RE), was calculated by the following formula:
X = C • V/m
where: X = flavonol content, mg/g plant extract in RE; C = the concentration of rutin solution, established from the calibration curve, mg/ml; V, m = the volume and the weight of plant extract, ml, g.
HPLC analysis
Chromatographic separation was carried out using two solvents system: A) methanol:water:formic acid (10:88:2, v/v/v); B) methanol:water:formic acid (90:8:2, v/v/v), as reported elsewhere (Citationöztürk et al., 2007). The analyses were performed by using a linear gradient program. Initial condition was 100% A; 0-15 min, changed to 100% A; 15-20 min, to 85% A; 20-30 min, to 50%; 30-35 min to 0% A; 36-42 min, went back to 100% A. The flow-rate was 1 ml•min−1 and the injection volume was 10 μl. Signals were detected at 280 nm. Besides, IS technique was applied to the analysis to increase the repeatability.
The relevant extracts were dissolved in a mixture of methanol and water (1:1 v/v) and injected into the HPLC.
Radical scavenging activity using the DPPH method
DPPH assay was used as a rapid spectroscopic method to provide an evaluation of antioxidant activity due to scavenging free radicals. Being a stable free radical with purple color, DPPH· is reduced into the yellow colored diphenylpicryl hydrazine.
Free radical scavenging effects of the fractions on DPPH· were estimated according to the method of CitationSanchez-Moreno et al. (1998) with some modifications. The reaction mixture was left at ambient temperature for 30 min in darkness; absorbance of the resulting solution was then measured spectrophotometrically at 517 nm. The scavenging activity was plotted against concentration and the concentration that showed 50% DPPH scavenging activity (IC50) was calculated following the logarithmic procedures.
Antioxidant activity by the Rancimat method
Antioxidant activities of extracts obtained from Hypericum species were also measured by the Rancimat method (A 743 Rancimat apparatus, Metrohm AG, SW) at the concentration of 1% (CitationExarchou et al., 2002). A flow of air (20 L/h) was bubbled through the oil heated at 110°C, and the volatile compounds were collected in cold water, increasing the water conductivity. The conductivity was monitored continuously until a sudden rise signified the end of the induction period. Each sample was dispersed in 3 g of olive oil rich in linoleic acid (65% of fatty acids) at the concentration of 1%. Olive oil without antioxidant as the control was run similarly. The test was applied in triplicate. Induction index was calculated by the following equation:
Induction Index (II) = Induction time of sample/ Induction time of control
As it was observed from the formula, a higher induction index indicates higher antioxidant activity (CitationEsquivel et al., 1999).
Results and discussion
shows a summary of results of Hypericum species employed in this study comprising the extraction yields, total phenols, flavonoid and flavonol contents for the extracts recovered with solvents of different polarities.
Table 1. The extraction yields, contents of the total phenols, flavonoids and flavonols in various extracts of H. montbretii (HM), H. origanifolium (HO) and reference plant H. perforatum (HP).
The extraction yield as a percentage of plant material ranges from 7.27 for HOF-EA to 39.63 for HPF-ME. Methanol extracts of all species showed a higher yield was obtained compared to the others. This might be due to the presence of more polar compounds in the methanol extracts of plants, as methanol is a solvent with higher polarity.
The contents of total phenolic compounds, flavonoids, and flavonols in the extracts were determined as stated in the experimental section. The content of total phenols is expressed as gallic acid equivalent (mg gallic acid/g extract) and flavonoids and flavonols as rutin equivalents (mg rutin/g extract). The values are given as means of triplicate analyses ().
The amounts of total phenols in the extracts were investigated in different solvent polarities. Total phenolic content among all samples varied between 104 and 451 mg/g in gallic acid equivalents. Significantly, highest results were found in ethyl acetate extracts in the order of increase HOF-EA < HPF-EA < HML-EA < HMF-EA < HPL-EA < HOL-EA. It is easily observed in that water extracts have the lowest content of total phenols.
The content of flavonoids, in rutin equivalents in mg/g of plant extract, varied from 1.16 to 55.33 mg/g. The highest results of flavonoids were found in the ethyl acetate extracts, in turn, increasingly HML-EA < HPF-EA < HPL-EA < HOF-EA < HOL-EA < HMF-EA. Water extracts also had the lowest content of total flavonoids, similar to the results of those of total phenolic contents of extracts.
The concentrations of flavonols are expressed in rutin equivalent in mg/g of plant extract and they varied from 0.14 to 7.49 mg/g. The highest results of flavonols were also found in the ethyl acetate extracts as mentioned above with the order of HML-EA < HPF-EA < HOF-EA < HOL-EA < HMF-EA < HPL-EA. As expected theoretically from solvent polarity, these results are in general agreement.
In the Hypericum species studied, ten phenolic acids (gallic, protocatechuic, p-hydroxybenzoic, caffeic, chlorogenic, syringic, p-coumaric, ferulic, tr-cinnamic and o-coumaric acids) were determined by an HPLC gradient system using a modified method, which was described elsewhere (Citationöztürk et al., 2007). All of the phenolic acids were resolved entirely from each other. The HPLC chromatograms of ethyl acetate extracts obtained from leaves of H. montbretii are demonstrated in . It may be seen that no additional clean-up step to purify the extracts is necessary and all phenolic acids could be quantified.
Figure 1. The chromatogram of ethyl acetate extract obtained from H. montbretti leaves in the conditions as mentioned in experimental. GA (1), pro-CA (2), CA (4), ChA (5), SA (6), p-COU (7), FA (8), tr-CIN (10), IS (11).
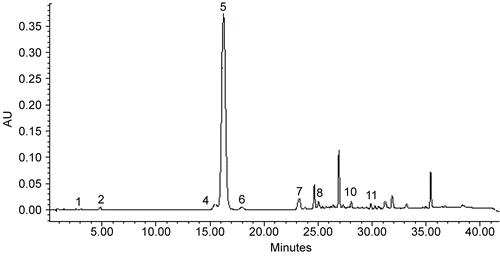
The integrated peak areas and their retention times were computed to obtain the rate of PN of the relevant phenolic acids, and their amounts were calculated in the related extracts via their calibration curves. They are given in .
Table 2. The contents of phenolic acids (as a percentage) in various extracts of studied Hypericum species.
The results show that chlorogenic acid (ChA) dominated in all species, and the concentration varied from one to another in flower and leaves. Especially, H. montbretti leaves and flowers contained mostly ChA. In addition, protoCA, FA, tr-CIN, o- and p-COU acids were detected in nearly all species.
Some researchers have determined only ChA and CA in Hypericum species (CitationHolzl & Petersen, 2003). A more detailed study has listed neochlorogenic acid, 3-O-[E]-4-coumarylquinic acid, 3-O-[Z]-4-coumarylquinic acid, cryptochlorogenic acid and protoCA (CitationJurgenliemk & Nahrstedt, 2002).
There are many radical ions in nature and they are scavenged by some compounds with different chemical reactions. Relatively stable organic radical DPPH is one of them, and it has been widely used for the determination of the antioxidant activity of pure compounds as well as the different plant extracts (CitationBrand-Williams et al., 1995). One parameter that has been introduced recently for the interpretation of the results from the DPPH method is the efficient concentration or EC50 value, otherwise called inhibitory concentration, IC50 value. This is defined as the concentration of substrate that causes 50% loss of the DPPH activity (CitationMolyneux, 2004). The higher antioxidant activity corresponds to the lower value of IC50.
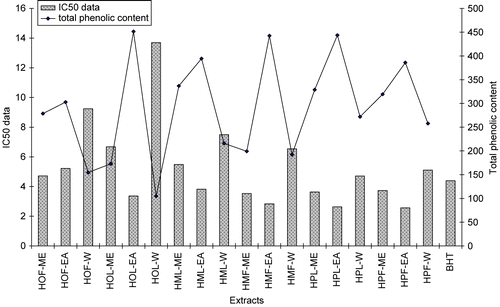
Radical scavenging activities of each extract measured employing different concentrations (9.6 × 10−4, 1.8 × 10−3 and 3.6 × 10−3 μg·mL−1), and the results were presented as IC50 values (). The highest radical scavenging activity was exhibited by the extracts of ethyl acetate. The lowest activity was observed in water extracts. Leaves and flowers of H. montbretii and leaves of H. origanifolium were the most active at the tested concentrations, exhibiting an activity comparable to that of the positive control BHT, but with the exception of the leaves of H. montbretii, all of the extracts showed weaker activity than those of leaves and flowers H. perforatum, the reference plant ().
Table 3. Radical scavenging and antioxidant activities of extracts obtained from studied Hypericum species.
Since there is no previous study reporting the chemical contents and antioxidant activity of H. montbretii and H. origanifolium, the results obtained here can be only compared to those of H. perforatum, with known antioxidant activity (CitationHunt et al., 2001; CitationSilva et al., 2005; CitationBenedi et al., 2004; CitationConforti et al., 2005), and with the synthetic antioxidant BHT. Generally, leaves of plants carry higher antioxidant activity as phenolic compounds when compared to flowers.
Significantly, the lowest IC50 data were found in ethyl acetate fractions, in turn, HOF-EA < BHT < HMF-EA < HOL-EA < HML-EA < HPF-EA < HPL-EA. Water extracts also had the lowest antiradical activities. The total phenolic compounds show mainly correlation with those results of radical scavenging activity. These results and their comparisons are demonstrated in .
Figure 2. Comparisons of radical-scavenging activities (IC50) and total phenolic contents of studied Hypericum extracts.
The Rancimat method is commonly used to evaluate the antioxidative properties of various natural and synthetic antioxidants and is based on the increase of electrical conductivity due to the formation of volatile compounds as a result of lipid oxidation.
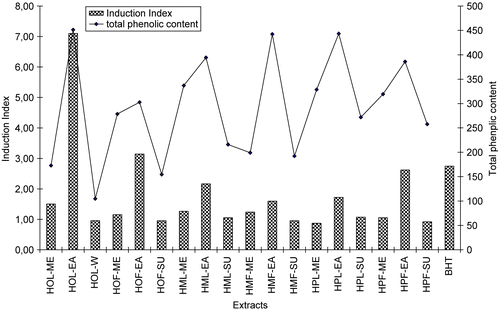
As shown in , extracts obtained from Hypericum species exhibited different effects on retarding olive oil oxidation. The highest induction index (II) data were found in ethyl acetate extracts, in turn, HOL-EA > HOF-EA > BHT > HPF-EA > HML-EA > HPL-EA > HMF-EA. On the contrary, methanol and water extracts of Hypericum species did not inhibit the oxidation of olive oil at the studied concentrations.
Among the Hypericum species, extracts of H. origanifolium were found to have extremely potent antioxidant activity in olive oil. Its induction index value at the studied concentration was about three times more active than that of BHT (synthetic antioxidant) and H. perforatum (reference plant). The induction index of H. montbretii extracts was also approximately equal to that of BHT and H. perforatum. The total phenolic compounds show mainly correlation with the results of antioxidant activity. These results and their comparisons are shown in .
Figure 3. Comparisons of Induction Index values in Rancimat method and total phenolic contents of studied Hypericum extracts.
The strong antioxidant activities of plant materials are believed to be a result of phenolic compounds (flavonoid, flavonol, phenolic acid, etc.) (CitationSalvador et al., 2001). In general, extracts or fractions with higher radical scavenging and antioxidant activities showed a higher phenolic content, and some good correlations were found with these parameters.
Conclusion
In this study, antioxidant and radical scavenging capacities of the extracts of different Hypericum species were evaluated to investigate a relationship between phenolic contents and antioxidant activity. These results showed for the first time that the extracts of H. montbretii and H. origanifolium leaves and flowers, possesses significant antioxidant activity. This activity is related to the presence of polyphenolic compounds (flavonoids, flavonols, and phenolic acids).
Of all Hypericum species examined in this study, leaf extracts showed higher antioxidant and radical scavenging activities than flower extracts, except for H. perforatum. In addition, ethyl acetate extracts exhibited the highest antioxidant activity as demonstrated by two antioxidant methods used in our study. Water extracts demonstrated lower antioxidant and antiradical activity than methanol extracts. There was a good linear correlation between phenolic concentration and antioxidant activity in extracts. The best results were obtained in the extracts of HPL-EA and HML-EA. It was found that chlorogenic acid (ChA) was higher in all species. H. montbretii leaves and flowers contained mostly ChA. ProtoCA, FA, tr-CIN, o- and p-COU acids were also detected as phenolic acids.
Although the radical scavenging activity of H. perforatum was significantly higher than that of H. montbretii and H. origanifolium, which was due to the difference in their phenolic contents, oxidative stability of H. origanifolium leaves was about three times higher than that of BHT, H. perforatum and H. montbretii leaves and flowers.
The results of this study indicate that H. montbretii and H. origanifolium, like H. perforatum, can serve as natural sources to develop the free radical scavengers and might prevent radical attack and increase oxidative stability in biological and food systems. Hence, these species could possess therapeutic effects in different areas and they could be considered as useful sources of materials for human health and as antioxidant food preservatives.
Acknowledgments
The authors acknowledge the Research Foundation of the University of Anadolu (Project No: 30353) and the Plant, Drug and Scientific Research Centre of Anadolu University (AUBIBAM) for their kind support of this study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Benedi J, Arroyo R, Romero C, Martin-Aragon S, Villar AM (2004): Antioxidant properties and protective effects of a standardized extract of Hypericum perforatum on hydrogen peroxide-induced oxidative damage in PC12 cells. Life Sci 75: 1263–1276.
- Brand-Williams W, Cuvelier ME, Berset C (1995): Use of free radical method to evaluate antioxidant activity. LWT- Food Sci Technol 28: 25–30.
- Conforti F, Statti GA, Tundis-Bianchi RA, Agrimontib C, Sacchetti G, Andreotti E, Menichini F, Poli F (2005): Comparative chemical composition and variability of biological activity of methanolic extracts from Hypericum perforatum L. Nat Prod Res 19: 295–303.
- Dönmez AA (2000): Hypericum L. In: eds. Güner A, özhatay N, Ekim T & Baser KHC. Flora of Turkey and the East Aegean Islands. Vol.11. Edinburgh, Edinburgh University Press, pp. 71–72.
- El-Sherbiny DA, Khalifa AE, Attia AS, Eldenshary ES (2003): Hypericum perforatum extract demonstrates antioxidant properties against elevated rat brain oxidative status induced by amnestic dose of scopolamine. Pharm Biochem Beh 76: 525–533.
- Erken S, Malyer H, Demirci F, Demirci B, Baser KHC (2001): Chemical investigations of some Hypericum species growing in Turkey I. Chem Nat Comp 37: 434–438.
- Esquivel MM, Ribeiro MA, Bernardo-Gil MG (1999): Supercritical extraction of savory oil: Study of antioxidant activity and extract characterization. J Supercrit Fluid 14: 129–135.
- Exarchou V, Nenadis N, Tsimidou M, Gerothanasis IP, Troganis A, Boskou D (2002): Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage, and summer savory. J Agr Food Chem 50: 5294–5299.
- Folin O, Ciocalteu V (1928): On tyrosine and tryptophane determinations in proteins. J Biol Chem 73: 627–50.
- Holzl J, Petersen M (2003): Chemical constituents of Hypericum ssp. In: ed. Ernst E, Hypericum, The Genus Hypericum. London, Taylor & Francis Group, pp. 77–94.
- Hunt EJ, Lester CE, Lester EA, Tackett RL (2001): Effect of St. John’s wort on free radical production. Life Sci 69: 181–190.
- Jurgenliemk G, Nahrstedt J (2002): Phenolic compounds from Hypericum perforatum. Planta Med 68: 88–91.
- Kitanov GM, Nedialkov PT (1998): Mangiferin and isomangiferin in some Hypericum species. Biochem Syst Ecol 26: 647–653.
- Makovestśka OY (1999): Research of biologically active substances of Hypericum L. species. Report VI. Sections olympia (Spach) Nyman, oampylopus Boiss. and origanifolia Stef. Farm Zh (Kiev) 6: 46–50.
- Makovestśka OY (2000): Research on biologically active substances of Hypericum L. species. Report VII. Sections drosocarpium Spach., oligostema (Boiss.) Stef., thasia Boiss. and crossophyllum Spach. Farm Zh (Kiev) 3: 53–59.
- Mathis C, Ourisson G (1964): Chemo-taxonomic study of the genus Hypericum. III. The distribution of saturated hydrocarbons and monoterpenes from the essential oil of Hypericum. Phytochemistry 3: 133–141.
- Miliauskas G, Venskutonis PR, van Beek TA (2004): Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem 85: 231–237.
- Molyneux P (2004): The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol 26: 211–219.
- öztürk N, Bozan B, Konuklugil B (2006): Antioxidant and free radical scavenging activities of Linum olympicum extracts. Anadolu University J Sci Technol 7: 31–39.
- öztürk N, Tunçel M, Tunçel NB (2007): Determination of phenolic acids by a modified HPLC: Its application to various plant materials. J Liq Chromatogr R T 30: 587–596.
- Potoglu-Erkara I, Tokur S (2004): Morphological and anatomical investigations on some Hypericum L., species growing naturally in and around Eskisehir. Trakya Univ J Sci 5: 97–105.
- Robson NKB (1967): Hypericum L. In: ed. Davis PH, Flora of Turkey and the East Aegean Islands, Vol. 2. Edinburgh, Edinburgh University Press, p. 355.
- Robson NKB (1988): Hypericum L. In: eds. Davis PH, Mill RR, & Tan K, Flora of Turkey and the East Aegean Islands, Vol. 10, Edinburgh, Edinburgh University Press, p. 96.
- Sakar MK, Tamer AU, Tokur S (1988): Antimicrobial activities of some Hypericum species growing in Turkey. Fitoterapia 59: 49–52.
- Sakar MK, Tamer AU (1990): Antimicrobial activity of different extracts from some Hypericum species. Fitoterapia 61: 464–466.
- Sakar MK, Ezer N, Engelshowe R (1991): Constituents of Hypericum montbretii. Int J Pharmacog 29: 228–230.
- Salvador MD, Aranda F, Gomez-Alonso S, Fregapane G (2001): Cornicabra virgin olive oil: A study of five crop seasons. Composition, quality, and oxidative stability. Food Chem 74: 267–274.
- Sanchez-Moreno C, Larrauri JA, Saura-Calixto F (1998): A procedure to measure the antiradical efficiency of phenols. J Sci Food Agric 76: 270–276.
- Silva BA, Ferreres F, Malva J, Dias APC (2005): Phytochemical and antioxidant characterization of Hypericum perforatum alcoholic extracts. Food Chem 90: 157–167.
- Sirvent TM, Walker L, Vance N, Gibson DM (2002): Variation in hypericins from wild populations of Hypericum perforatum L. in the Pacific Northwest of the USA. Econ Bot 56: 41–48.
- Tsao R, Den Z (2004): Separation procedures for naturally occurring antioxidant phytochemicals. J Chromatogr B 812: 85–99.