Abstract
Hepatitis C virus (HCV) causes chronic hepatitis, cirrhosis and hepatocellular carcinoma. Some patients are resistant to interferon-α (IFN-α) treatment, and thus there is urgent need to improve anti-HCV therapies and discover novel therapeutic approaches in the form of new antiviral agents. Using real-time PCR (RT-PCR) and the MTS assay, we examined the suppression of HCV replication and the cytotoxicity of 11 aqueous extracts and eight compounds using Chinese herbs traditionally used for liver protection. Curcuma aromatica Salisb. (Zingiberaceae), Canna indica L. (Cannaceae), and two commercial extracts from Ganoderma tsugae Murr. (Aphyllophoromycetideae), Triterpenoids Enterprise (Shuang Hor Lingzhi®) and Polysaccharides Enterprise (Shuang Hor Supreme Lingzhi®) substantially inhibited HCV replication at 1 mg/ml in Huh-7 human hepatoma cells containing an HCV subgenomic replicon. In addition, HCV-Huh-7 cells treated with a combination of a low dose (10 IU/ml) of IFN-α and 1 mg/ml of one of the four herbal extracts also exhibited significant inhibition of HCV replication. Thus, C. aromatica, C. indica, Triterpenoids Enterprise (Shuang Hor Lingzhi®), and Polysaccharides Enterprise (Shuang Hor Supreme Lingzhi®) are possible sources of potent anti-HCV agents.
Introduction
Chronic hepatitis C virus (HCV) infection is associated with a high risk of cirrhosis that often leads to hepatic failure and hepatocellular carcinoma (CitationDi Bisceglie, 1998; CitationFeinman et al., 1988; CitationSimmonds, 1995). According to World Health Organization reports, more than 170 million people are infected with HCV worldwide (CitationSzabo & Dolganiuc, 2006). About 80% of HCV infections result in cirrhosis and chronic hepatitis. The HCV genome is highly variable due to the poor fidelity of the viral RNA-dependent RNA polymerase and the lack of a genome repair mechanism. HCV genomic variability lacks uniformity throughout the genome (CitationYao & Tavis, 2005; CitationAlter et al., 1995; CitationButler et al., 2004; CitationCrofts et al., 1995; CitationSydiskis et al., 1991). HCV genomic variable nonstructural 5A (NS5A) protein relates to resistance to the drug IFN-α (CitationWohnsland et al., 2007). NS5B protein is an RNA-dependent RNA polymerase that is involved in HCV virus replication (CitationChoi et al., 2003, Citation2004; CitationVaraklioti et al., 2002; CitationWalewski et al., 2001; CitationXu et al., 2001).
The HCV infection rate in Taiwan is extremely high, with an HCV antibody-positive rate of the general adult population of about 2%, and some aboriginal communities have extraordinarily high HCV infection rates of about 32% (CitationHuang et al., 2003). The majority of these cases are HCV type 1b (86%). The second largest group is type 2a (8%), with the remaining cases types 1a (3%) and 2a (3%) (CitationHuang, 2003). It has been observed that type 1b is most resistant to IFN-α treatment (CitationXu et al., 2001; CitationTaylor et al., 2000; CitationTakada et al., 1992). Because IFN-α is poorly tolerated and expensive, identifying more effective and economical alternative therapies is critical (CitationDonadel et al., 1998; CitationGlue et al., 2000; CitationNeumann et al., 1998; CitationShields et al., 2000; CitationShindo et al., 1991).
Natural products may serve as a good source of new drugs. Ganoderma (Aphyllophoromycetideae) is a genus of mushrooms with traditional Chinese medicinal applications widely used in Asian countries. Ganoderma lucidum (Leyss, ex Fr.) Karst, a basidiomycetes mushroom, is one of the most popular chemopreventive mushrooms. Many bioactive components, often polysaccharides or triterpenes, have been identified from its fruiting bodies, mycelia, spores, and culture media. Antitumor-active polysaccharides from the fruiting bodies of G. tsugae Murr. have also been characterized (CitationWang et al., 2002). Some reports have shown that triterpenes have antioxidative (CitationZhu et al., 1999; CitationTsuboi et al., 2004), cholesterol stasis (CitationKomoda et al., 1989) and hepatoprotective (CitationKim et al., 1999) activities. In addition, parthenolide has anti-HCV activities in vitro (CitationHwang et al., 2006). A traditional Chinese herb, Curcuma aromatica Salisb. (Zingiberaceae), inhibited cell proliferative activity, and is useful in the treatment of some cancers in mice (CitationWu et al., 2000). Glycyrrhizin normalizes plasma alanine aminotransferase in hepatitis C-infected patients (CitationRino et al., 2006).
We report here the utilization of a cell-based assay using the hepatoma-derived Huh-7 cell line that supports replication of an engineered copy of subgenomic HCV RNA in place of the viral structural genes (CitationLohmann et al., 1999) to investigate the effects of herbal extracts used in conventional therapy or components purified from them on HCV replication.
Materials and methods
Chemicals and herbal extract preparation
Herbs were selected for testing based on their anti-inflammatory or liver protection properties. Sailosaponin A, sailosaponin C, glycyrrhizin, paeconiflorin and baicalein were purchased from Yoneyama Co. (Tokyo, Japan). Safranin O, bromelain and ribavirin were obtained from Sigma (St. Louis, MO). All were dissolved in DMSO. IFN-αB2 was obtained from PBL Biomedical Laboratories (NJ, USA).
Samples of G. tsugae were kindly provided by the Double Crane Group, Yung-Kien Industry Corp., Taiwan, Republic of China. Two commercial products, Triterpenoids Enterprise (Shuang Hor Lingzhi®) and Polysaccharides Enterprise (Shuang Hor Supreme Lingzhi®), prepared from G. tsugae have been shown to have anti-inflammatory effects on hepatitis (CitationLi & Wang, 2006; CitationPark et al., 1997). According to a commercial formula, dry powders from G. tsugae mycelia and fruiting bodies are mixed in a fixed ratio and preserved at room temperature to produce Triterpenoids Enterprise (Shuang Hor Lingzhi®) and Polysaccharides Enterprise (Shuang Hor Supreme Lingzhi®). The two products consist of different polysaccharides and triterpenes.
Anemarrhena asphodeloides Bunge (Anthericaceae), Echeveria peacockii Baker (Crassulaceae), Lonicera japonica Thunb. (Caprifoliaceae), Scutellaria baicalensis Georgi (Lamiaceae), Gynostemma simplicifolium Blume (Cucurbitaceae), Lavandula officinalis Chaix (Lamiaceae), Curcuma aromatica, and Canna indica L. (Cannaceae) were obtained from a plantation of the Green Health Biotechnology Corporation (Yunlin, Taiwan). Their authenticity was confirmed by Jung-Chou Chen. The leaves of these plants were harvested and extracted with hot water (110°C) twice. The hot-water extracts were spray-dried to obtain a dry powder. All powders were dissolved and suspended in distilled water to a concentration of 100 mg/ml. The mixtures were sterilized under high pressure for 10 min and then put in a Beckman JA-14 rotor for centrifugation at 3,000 rpm for 10 min (CitationHuang et al., 1997).
HCV-Huh-7 cell culture
A subgenomic HCV RNA replicon (kindly supplied by Professor J. Ou, Department of Molecular Microbiology and Immunology, University of Southern California) (CitationXu et al., 2001), and self-replicating HCV genomic RNA were used to evaluate candidate anti-HCV reagents with regard to HCV RNA replication. Human hepatoma-derived Huh-7 cells (5 × 105) containing the subgenomic HCV RNA replicon were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 800 μg/ml G418, 100 ng/ml penicillin, and 100 U/ml streptomycin. The MTS assay (Promega, Madison, WI) was used to determine the effects of the extracts and purified chemicals on HCV-Huh-7 cell viability. In metabolically active cells, MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy phenyl)-2-(4-sulfophenyl)-2H- tetrazolium (Promega), is reduced by dehydrogenase activity to an aqueous, soluble formazan product. The quantity of formazan is considered to be proportional to the number of viable cells in the culture. Briefly, 8 × 103 cells were incubated in 96-well plates containing 200 μl growth medium. After 24 h incubation, the medium was carefully removed, and 200 μl fresh medium containing various concentrations of extracts or chemicals was added to the wells for 48 h. At the end of this process, 20 μl/well of combined MTS/phenazine ethosulfate (PMS) solution was added and the wells were incubated for 1 h at 37°C in a humidified incubator. Absorbance was measured on a VERSAmax microplate reader (Molecular Devices, CA) at 490 nm. Absorbance values are presented as the mean ± SE of three replicates for each treatment. Absorbance of untreated cells was considered as 1.0.
RT-PCR and real-time RT-PCR
Total RNA was extracted from HCV-Huh-7 cells using a RareRNA kit (Genepure Technology, Taiwan) following the manufacturer’s protocol. HCV RNA was quantified by RT-PCR and real-time RT-PCR as described (CitationYao & Tavis, 2005; CitationPawlotsky et al., 2000).
cDNA was reverse-transcribed from 1 μg total cellular RNA using random hexamer primers and murine leukemia virus reverse transcriptase. cDNA (50 ng in 1 μl) was amplified for 35 cycles in a volume of 50 μl that contained 2 U of Taq polymerase (ProTaq, Taiwan), 200 mM dNTPs, 10 mM Tris-HCl (pH 8.0), 1.5 mM MgCl2, 75 mM KCl and 20 pmol of the nonstructural 5B (NS5B) sense and antisense primers (nt 2-31, 59-TgggATCCCgTATgATACCCgCTgCTTTgA-39 and nt 402-373, 59-ggCggAATTCCTggTCATAgCCTCCgTgAA-39, respectively) (CitationVerbeeck et al., 2006). The PCR reaction included a 5-min denaturation (94°C) followed by 35 cycles, each consisting of denaturation (94°C, 1 min), annealing (60°C, 1 min) and extension (72°C, 2 min), with a final extension phase (10 min). PCR reactions were performed in a programmable thermal controller instrument-thermal cycler Model 2720 (Applied Biosystems, CA). A 401-bp fragment of the NS5B gene was amplified. The same amount of cDNA was amplified using β-actin-specific sense and antisense primers (59-CAGGGAGTGATGGTGGGCA-39, 59-CAAACATCATC TGGTCATCTTCTC-39, respectively) that were according to the manufacturer’s instructions (Life Technologies, Grand Island, NY). The samples were subjected to 27 cycles that included denaturation (94°C, 1 min), annealing (60°C, 1 min) and extension (72°C, 2 min) with a final extension phase (10 min). The products were visualized via electrophoresis on a 1.5% agarose gel and stained with ethidium bromide. The ratio of NS5B RNA was calculated by normalizing the NS5B band intensity to that of β-actin. We confirmed the quality of cellular mRNA based on the intensity of the β-actin PCR product band intensity was quantified with the ChemiImager System (Alpha Innotech Corp.).
HCV RNA was quantified in a real-time 59 exonuclease RT-PCR (TaqMan, Applied Biosystems assay using the ABI 7700 Sequence Detector (PE Biosystems, CA). The primers and probe were derived from the conserved region of the 59 non-coding region and were selected using the Primer Express software designed for this purpose (PE Biosystems). The forward primer covered nt 149-167 (59 TGCGGAACCGGTGAGTACA 39), the reverse primer covered nt 210-191 (59 CGGGTTTATCCAAGAAAGGA 39), and the probe was from nt 189-169 (59 CCGGTCGTCCTGGCAATTCCG 39). The fluorogenic probe was labeled with FAM, and BHQ-1 and was obtained from Biosearch Technologies, Inc. (Novato, CA). The primers and probe were used at 10 pmol in a 50 μl reaction. The reactions were performed using a TaqMan Gold RT-PCR kit (PE Biosystems) and a 30 min 48°C reverse transcription step, followed by 10 min at 95°C and 50 cycles of amplification under universal TaqMan standardized conditions: 15 s at 95°C for denaturation and 1 min at 60°C for annealing and extension. All measurements were performed in triplicate.
Results
Cytotoxic effects of herbal extracts on HCV-Huh-7 cells
The MTS assay was performed and cell morphology was analyzed to explore the cytotoxic properties of chemicals purified from herbal medicines and extracts. We found that sailosaponin A and safranin O had the highest cytotoxic activities and caused cell death (). Treatment of HCV-Huh-7 cells with 1 mg/ml Triterpenoids Enterprise (Shuang Hor Lingzhi®), Polysaccharides Enterprise (Shuang Hor Supreme Lingzhi®), and extracts of C. aromatica and C. indica did not affect cell viability. The effect of Triterpenoids Enterprise (Shuang Hor Lingzhi®), Polysaccharides Enterprise (Shuang Hor Supreme Lingzhi®), and extracts from C. aromatica and C. indica on growth inhibition of HCV-Huh-7 cells was further tested at concentrations of 4, 2, 1, and 0.5 mg/ml using the MTS assay (). Substantial effects were only observed at the highest concentration of 4 mg/ml for Triterpenoids Enterprise (Shuang Hor Lingzhi®) and C. indica. Concentrations in the range of 0.5–2.0 mg/ml did not show significant cytotoxicity ( and ). There was no inhibition of HCV replication with A. asphodeloides, E. peacockii, L. japonica, S. baicalensis, or G. simplicifolium; therefore, we did not test the cytotoxic effects of these herbal extracts ().
Table 1. Herbal extracts, fungal extracts, and purified components from herbal medicines and their cytotoxic effects on HCV-Huh-7 cells, inhibitory effects on subgenomic HCV RNA replication and synergistic effects with IFN-α.
Table 2. Effects of fungal and herbal extracts on cytotoxicity of Huh-7 cells containing the HCV subgenomic replicon.
HCV-Huh-7 cells treated with 10 or 100 U/ml of IFN-α showed no loss of cell viability (). Furthermore, no toxicity was observed in cells treated with 400 μM ribavirin in combination with IFN-α at 10 and 100 IU/ml (). At concentrations of 4 and 2 mg/ml, all four extracts had cell survival rates of less than 80%. HCV-Huh-7 cells exposed to 1 mg/ml Triterpenoids Enterprise (Shuang Hor Lingzhi®) and Polysaccharides Enterprise (Shuang Hor Supreme Lingzhi®) exhibited survival rates of 0.73 ± 014 and 0.61 ± 0.07, respectively, and almost no additional decrease in survival was observed at 0.5 mg/ml. At concentrations of 1 and 0.5 mg/ml, C. aromatica and C. indica showed no obvious cytotoxic effects ().
Table 3. Effects of herbal extracts in conjunction with IFN-α on cytotoxicity of Huh-7 cells containing the HCV subgenomic replicon.
Anti-HCV effects of herbal extracts
To test the ability of herbal extracts or molecules purified from them to inhibit HCV replication, they were tested in a cell-based HCV subgenomic replicon assay. HCV-Huh-7 cells were treated with herbal extracts or purified components known to have anti-inflammatory or hepatoprotective properties at 1 mg/ml for 48 h. Subgenomic HCV RNA levels were measured following agarose gel electrophoresis of RT reactions. Among the eight purified compounds and 10 plant extracts tested, four extracts (at 1 mg/ml) inhibited HCV RNA replication (). As shown in -, sailosaponin C, sailosaponin A, glycyrrhizin, paeoniflorin, bromelain, baicalein, and extracts from L. japonica, G. simplicifolium, E. peacockii, and L. officinalis neither inhibited replication of HCV NS5B RNA nor synergized the inhibition of replication with IFN-α. Treatment with the commercially available Triterpenoids Enterprise (Shuang Hor Lingzhi®) and Polysaccharides Enterprise (Shuang Hor Supreme Lingzhi®) or extracts of C. aromatica or C. indica led to a decrease in HCV RNA NS5B level (, lanes 9 and 12, and , lanes 9 and 12). The dose-dependent inhibition of HCV subgenomic RNA replication in the presence of 5, 10 and 100 IU/ml of IFN-α is shown in and , lanes 2-4. Only at a concentration of 10 IU/ml IFN-α was notable inhibition observed in the presence of 1 mg/ml of fungal or herbal extract (, lanes 10 and 13, and , lanes 10 and 13. These results suggest that IFN-α has a stronger anti-HCV effect in combination with these four herbal extracts than with ribavirin ( and , lanes 6 and 7) and that these four herbal extracts may potentially allow the use of a lower OR have the potential to reduce the concentration of IFN- α administered in clinical therapies. At 500 μg/ml of extract with IFN-α, the anti-HCV effects of these four herbal extracts were modest (data not shown).
Figure 1. Replication inhibition effect on Huh-7 cells containing an HCV subgenomic replicon by purified components of herbal medicines. (A) Huh-7 cells (7 × 106/ml) containing an HCV subgenomic replicon were treated with sailosaponin C, sailosaponin A, glycyrrhizin, or paeoniflorin at the concentrations indicated in the figure for 48 h. For analysis of NS5B, 1 μg RNA was extracted for RT reactions and PCR. PCR products (4 μl) were applied to a 1.5% agarose gel. As a control, 1 μg RNA was extracted from untreated Huh-7 cells containing the HCV subgenomic replicon (Lane 1). (B) HCV-Huh-7 cells were treated with purified components of herbal medicines in the presence of 10 IU/ml IFN-α as above. (C) HCV-Huh-7 cells treated with herbal extracts in the presence of 10 IU/ml IFN-μ. Ratio levels presented are the means of triplicates. Cell treatment at each concentration has been repeated in three separate experiments.
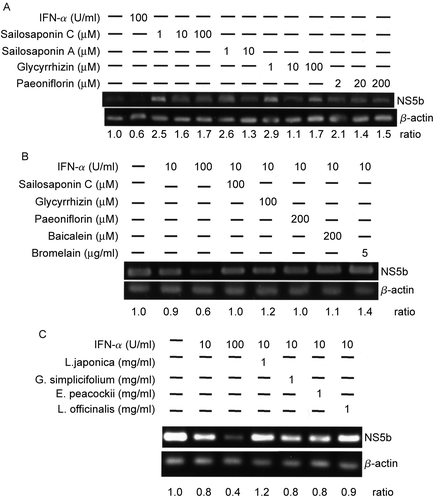
Figure 2. Replication inhibition effect on Huh-7 cells containing an HCV subgenomic replicon by herbal extracts in combination with IFN-α. (A) Huh-7 cells (7 × 106/ml) containing an HCV subgenomic replicon were treated with Triterpenoids Enterprise (Shuang Hor Lingzhi®), Polysaccharides Enterprise (Shuang Hor Supreme Lingzhi®) alone or in combination with IFN-α for 48 h at the concentrations given in the figure, and 1 μg RNA was extracted to perform RT reactions and PCR for NS5B. (B) Huh-7 cells (7 × 106/ml) containing an HCV subgenomic replicon were treated with extracts of C. aromatica or C. indica alone or in combination with IFN-α for 48 h at the concentrations given in the figure, and 1 μg RNA was extracted to perform RT and PCR. RCR products (4 μl) were applied to a 1.5% agarose gel. As a negative control, 1 μg RNA was extracted from untreated Huh-7 cells containing the HCV subgenomic replicon (lane 1). As a positive control, RNA was also extracted from HCV-Huh-7 cells treated with IFN-α alone or in combination with ribavirin. Cell treatment at each concentration has been repeated in three separate experiments.
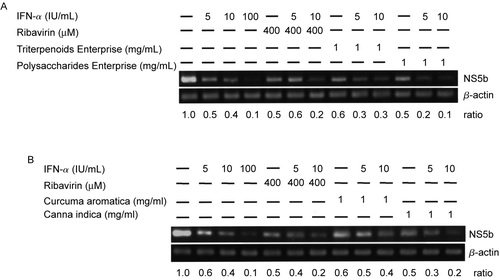
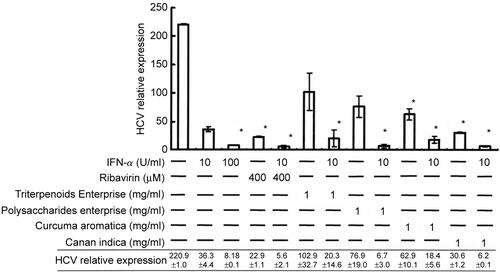
To verify the viral replication suppression effects of these four herbal extracts, real-time RT-PCR analysis was performed. HCV-Huh-7 cells were treated with the extracts at 1 mg/ml for 48 h. Then, the relative HCV subgenomic RNA titers were compared with untreated cells. The relative subgenomic HCV titers of cells treated with 1?mg/ml Triterpenoids Enterprise (Shuang Hor Lingzhi®), Polysaccharides Enterprise (Shuang Hor Supreme Lingzhi®), C. aromatica extract or C. indica extract were 102.9 ± 32.7, 76.9 ± 19.0, 62.9 ± 10.1 and 30.6 ± 1.2, respectively (). Substantial replication suppression effects were seen with C. aromatica and C. indica) extract treatment. Furthermore, when cells were treated with 10 IU/ml IFN- α in combination with 1 mg/ml Triterpenoids Enterprise (Shuang Hor Lingzhi®), Polysaccharides Enterprise (Shuang Hor Supreme Lingzhi®), C. aromatica extract or C. indica extract, the relative titers were 20.3 ± 14.6, 6.7 ± 3.0, 18.4 ± 5.6 and 6.2 ± 0.1, respectively ().
Figure 3. Replication inhibition effect on Huh-7 cells containing an HCV subgenomic replicon by herbal and fungal extracts with real-time quantitative PCR analysis. HCV-Huh-7 cells (7 × 106/ml) were treated with Triterpenoids Enterprise (Shuang Hor Lingzhi®), Polysaccharides Enterprise (Shuang Hor Supreme Lingzhi®), or extracts from C. aromatica or C. indica at 1 mg/ml for 48 h, and 1 μg RNA was extracted to perform real-time quantitative PCR. The relative HCV RNA titers were compared with those of untreated cells. Relative HCV RNA titers of cells treated with the four herbal extracts in combination with 10 IU/ml of IFN-α were also obtained as indicated. Results are expressed as the mean ± standard deviation for three replicate wells. Cell treatment at each concentration has been repeated in three separate experiments.
Discussion
Our results show that aqueous extracts of C. aromatica and C. indica can effectively inhibit the replication of HCV in Huh-7 cells containing an HCV subgenomic replicon and that the fungal products Triterpenoids Enterprise (Shuang Hor Lingzhi®) and Polysaccharides Enterprise (Shuang Hor Supreme Lingzhi®) and an extract of C. aromatica and C. indica can suppress HCV replication when combined with low doses of IFN-α, suggesting these substances may be useful as anti-viral agents in combination with low doses of IFN-α.
The mechanism of inhibition of HCV subgenomic replicon replication by Triterpenoids Enterprise (Shuang Hor Lingzhi®), Polysaccharides Enterprise (Shuang Hor Supreme Lingzhi®), and extracts of C. aromatica and C. indica remains unclear. These agents do not appear to bind the RNA-dependent RNA polymerase or to induce reactive oxygen species (unpublished observations). The pathogenicity of HCV is somehow related to abnormal secretion by CD8+ HCV-specific killer T lymphocytes (CitationShields et al., 2000; CitationLemon & Brown, 1994; CitationMondelli et al., 1988; CitationMartin et al., 1999) and may involve abnormal secretion of TNF-α, IFN-α, GM-CSF, IL-8 or IL-10 (CitationLemon & Brown, 1994; CitationMondelli et al., 1988). Ineffective or non-specific T cell activation possibly plays a central role in viral persistence and disease outcome. Thus, monitoring the cytokine profiles of peripheral blood mononuclear cells (PBMCs) from patients with chronic hepatitis C might have prognostic value. Our previous report demonstrated that cytokine production in PBMCs can be selectively regulated by Xiao Chai Hu Tang, a traditional Chinese medicinal herb (CitationHuang et al., 2003). However, Xiao Chai Hu Tang does not inhibit HCV subgenomic RNA replication. On the other hand, the cytokine profiles of PBMCs from HCV patients are altered after treatment with Triterpenoids Enterprise (Shuang Hor Lingzhi®), Polysaccharides Enterprise (Shuang Hor Supreme Lingzhi®) or an extract of C. aromatica or C. indica (unpublished observations). This implies that these herbal extracts may not suppress the replication of the HCV subgenomic replicon via cytokine secretion.
It has been observed that IFN-α at 5000 IU/ml is much less potent at inhibiting replication of the entire HCV genome than at inhibiting the replication of subgenomic HCV RNA (CitationHwang et al., 2004). In our investigation, IFN-α at 5-100 IU/ml demonstrated dose-dependent inhibition of subgenomic HCV RNA replication, and a substantial suppression effect was observed at a concentration of 100 IU/ml. Triterpenoids Enterprise (Shuang Hor Lingzhi®), Polysaccharides Enterprise (Shuang Hor Supreme Lingzhi®), C. aromatica extract and C. indica extract at 1 mg/ml were able to enhance the anti-HCV efficacy of IFN-α in combined treatments.
At a concentration of 1 mg/ml, none of the water extracts, including Triterpenoids Enterprise (Shuang Hor Lingzhi®), Polysaccharides Enterprise (Shuang Hor Supreme Lingzhi®), C. aromatica or C. indica produced obvious cell toxicity (). Only the combination treatment of IFN-α and Triterpenoids Enterprise (Shuang Hor Lingzhi®) or Polysaccharides Enterprise (Shuang Hor Supreme Lingzhi®) at 1 mg/ml reduced the cell survival rates to less than 75%. It may cause some side effects for patients.
It has been reported C. aromatica on hydrodistillation-containing essential oil with the major compounds are 1,8-cineole and linalool (CitationBehura et al., 2002), the root extract of C. indica L. with distilled water presenting several groups of flavonoids and phenolic compounds (CitationPurintrapiban et al., 2006). Isolation and analyses of the active ingredients of these herbal medicines are in progress and may lead to new treatments for HCV infection.
Acknowledgments
The subgenomic HCV RNA replicon was kindly provided by Professor J. Ou, of the Department of Molecular Microbiology and Immunology, University of Southern California, Los Angeles, CA. Triterpenoids Enterprise (Shuang Hor Lingzhi®) or Polysaccharides Enterprise (Shuang Hor Supreme Lingzhi®) were kindly supported by Double crane Group Bio-Tec. Research and Development Institute. This work was supported by the Graduate Institute of Chinese Medical Science, China Medical University. This work was supported by the Graduate Institute of Chinese Medical Science, China Medical University and grants from National Taiwan University, Yunlin Branch (NTHUHYL96.8001)
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alter HJ, Sanchez-Pescador R, Urdea MS, Wilber JC, Lagier RJ, Di Bisceglie AM, Shih JW, Neuwald PD (1995): Evaluation of branched DNA signal amplification for the detection of hepatitis C virus RNA. J Viral Hepat 2: 121–132.
- Behura S, Sahoo S, Srvastava VK (2002): Major constitutents in leaf essential oils Curcuma aromatica Salisb. Cur Sci 83: 1312–1313.
- Butler T, Kariminia A, Levy M, Kaldor J (2004): Prisoners are at risk for hepatitis C transmission. Eur J Epidemiol 19: 1119–1122.
- Choi J, Xu Z, Ou JH (2003): Triple decoding of hepatitis C virus RNA by programmed translational frameshifting. Mol Cell Biol 23: 1489–1497.
- Choi J, Lee KJ, Zheng Y, Yamaga AK, Lai MM, Ou JH (2004): Reactive oxygen species suppress hepatitis C virus RNA replication in human hepatoma cells. Hepatology 39: 81–89.
- Crofts N, Stewart T, Hearne P, Ping XY, Breshkin AM, Locarnini SA (1995): Spread of bloodborne viruses among Australian prison entrants. BMJ 310: 285–288.
- Di Bisceglie AM (1998): Hepatitis C. Lancet 351: 351–355.
- Donadel E, Pontisso P, Ruvoletto MG, Gerotto M, De Salvo G, Chemello L, Casarin C, Alberti A (1998): Characteristics of hepatitis C virus before and after interferon treatment in patients with ongoing viraemia but sustained biochemical response. J Med Virol 54: 7–11.
- Feinman SV, Berris B, Bojarski S (1988): Posttransfusion hepatitis in Toronto, Canada. Gastroenterology 95: 464–469.
- Glue P, Rouzier-Panis R, Raffanel C, Sabo R, Gupta SK, Salfi M, Jacobs S, Clement RP (2000): A dose-ranging study of pegylated interferon alfa-2b and ribavirin in chronic hepatitis C. The Hepatitis C Intervention Therapy Group. Hepatology 32: 647–653.
- Huang SP, Shieh GJ, Lee L, Teng HJ, Kao ST, Lin JG (1997): Inhibition effect of shengma-gegen-tang on measles virus in Vero cells and human peripheral blood mononuclear cells. Am J Chin Med 25: 89–96.
- Huang SPSG, Lu HC, Lin JG, Chen JC (2003): Effect of Xaio Chai Hu Tang in peripheral blood mononuclear cells of HCV patients in Taiwan. The National Union of Chinese Medical Associations (ROC) 2: 80–92.
- Hwang DR, Tsai YC, Lee JC, Huang KK, Lin RK, Ho CH, Chiou JM, Lin YT, Hsu JT, Yeh CT (2004): Inhibition of hepatitis C virus replication by arsenic trioxide. Antimicrob Agents Chemother 48: 2876–2882.
- Hwang DR, Wu YS, Chang CW, Lien TW, Chen WC, Tan UK, Hsu JT, Hsieh HP (2006): Synthesis and anti-viral activity of a series of sesquiterpene lactones and analogues in the subgenomic HCV replicon system. Bioorg Med Chem 14: 83–91.
- Kim DH, Shim SB, Kim NJ, Jang IS (1999): β-Glucuronidase-inhibitory activity and hepatoprotective effect of Ganoderma lucidum. Biol Pharm Bull 22: 162–164.
- Komoda Y, Shimizu M, Sonoda Y, Sato Y (1989): Ganoderic acid and its derivatives as cholesterol synthesis inhibitors. Chem Pharm Bull (Tokyo) 37: 531–533.
- Lemon S, Brown E (1994): Hepatitis C virus and chronic liver disease. In: eds. Swartzand MN, Remington JS, Current Clinical Topics in Infection Disease. New York, Vol. 14, pp. 121–141.
- Li YQ, Wang SF (2006): Anti-hepatitis B activities of ganoderic acid from Ganoderma lucidum: Biotech Lett: 837–841.
- Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R (1999): Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285: 110–113.
- Martin J, Quiroga JA, Navas S, Pardo M, Carreno V (1999): Modulation by biologic response modifiers of hepatitis C virus antigen-independent cytokine secretion in blood mononuclear cells. Cytokine 11: 267–273.
- Mondelli MU, Manns M, Ferrari C (1988): Does the immune response play a role in the pathogenesis of chronic liver disease? Arch Pathol Lab Med 112: 489–497.
- Neumann AU, Lam NP, Dahari H, Gretch DR, Wiley TE, Layden TJ, Perelson AS (1998): Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science 282: 103–107.
- Park EJ, Ko G, Kim J, Sohn DH (1997): Antifibrotic effects of a polysaccharide extracted from Ganoderma lucidum, glycyrrhizin, and pentoxifylline in rats with cirrhosis induced by biliary obstruction. Biol Pharm Bull 20: 417–420.
- Pawlotsky JM, Bouvier-Alias M, Hezode C, Darthuy F, Remire J, Dhumeaux D (2000): Standardization of hepatitis C virus RNA quantification. Hepatology 32: 654–659.
- Purintrapiban J, Suttajit M, Forsberg N (2006): Differential activation of glucose transport in cultured muscle cells by polyphenolic compounds from Canna indica L. root. Biol Pharm Bull 29: 1995–1998.
- Rino Y, Tarao K, Morinaga S, Ohkawa S, Miyakawa K, Hirokawa S, Masaki T, Tarao N, Yukawa N, Saeki H, Takanashi Y, Imada T (2006): Reduction therapy of alanine aminotransferase levels prevent HCC development in patients with HCV-associated cirrhosis. Anticancer Res 26: 2221–2226.
- Shields PL, Mutimer DJ, Muir D, Skidmore S, Britnell T, Roberts A, Wilde JT (2000): Combined alpha interferon and ribavirin for the treatment of hepatitis C in patients with hereditary bleeding disorders. Br J Haematol 108: 254–258.
- Shindo M, Di Bisceglie AM, Cheung L, Shih JW, Cristiano K, Feinstone SM, Hoofnagle JH (1991): Decrease in serum hepatitis C viral RNA during alpha-interferon therapy for chronic hepatitis C. Ann Intern Med 115: 700–704.
- Simmonds P (1995): Variability of hepatitis C virus. Hepatology 21: 570–583.
- Sydiskis RJ, Owen DG, Lohr JL, Rosler KH, Blomster RN (1991): Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrob Agents Chemother 35: 2463–2466.
- Szabo G, Dolganiuc A (2006): HCV immunopathogenesis: Virus-induced strategies against host immunity. Clin Liver Dis 10: 753–771.
- Takada N, Takase S, Enomoto N, Takada A, Date T (1992): Clinical backgrounds of the patients having different types of hepatitis C virus genomes. J Hepatol 14: 35–40.
- Taylor DR, Shi ST, Lai MM (2000): Hepatitis C virus and interferon resistance. Microbes Infect 2: 1743–1756.
- Tsuboi H, Hossain K, Akhand AA, Takeda K, Du J, Rifa’i M, Dai Y, Hayakawa A, Suzuki H, Nakashima I (2004): Paeoniflorin induces apoptosis of lymphocytes through a redox-linked mechanism. J Cell Biochem 93: 162–172.
- Varaklioti A, Vassilaki N, Georgopoulou U, Mavromara P (2002): Alternate translation occurs within the core coding region of the hepatitis C viral genome. J Biol Chem 277: 17713–17721.
- Verbeeck J, Maes P, Lemey P, Pybus OG, Wollants E, Song E, Nevens F, Fevery J, Delport W, Van der Merwe S, Van Ranst M (2006): Investigating the origin and spread of hepatitis C virus genotype 5a. J Virol 80: 4220–4226.
- Walewski JL, Keller TR, Stump DD, Branch AD (2001): Evidence for a new hepatitis C virus antigen encoded in an overlapping reading frame. RNA 7: 710–721.
- Wang SY, Hsu ML, Hsu HC, Tzeng CH, Lee SS, Shiao MS, Ho CK (1997): The anti-tumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int J Cancer 70: 699–705.
- Wang YY, Khoo KH, Chen ST, Lin CC, Wong CH, Lin CH (2002): Studies on the immuno-modulating and antitumor activities of Ganoderma lucidum (Reishi) polysaccharides: Functional and proteomic analyses of a fructose-containing glycoprotein fraction responsible for the activities. Bioorg Med Chem 10: 1057–1062.
- Wohnsland A, Hofmann WP, Sarrazin C (2007): Viral determinants of resistance to treatment in patients with hepatitis C. Clin Microbiol Rev 20: 23–38.
- Wu WY, Xu Q, Shi LC, Zhang WB (2000): Inhibitory effects of Curcuma aromatica oil on proliferation of hepatoma in mice. World J Gastroenterol 6: 216–219.
- Xu Z, Choi J, Yen TS, Lu W, Strohecker A, Govindarajan S, Chien D, Selby MJ, Ou J (2001): Synthesis of a novel hepatitis C virus protein by ribosomal frameshift. Embo J 20: 3840–3848.
- Yao E, Tavis JE (2005): A general method for nested RT-PCR amplification and sequencing the complete HCV genotype 1 open reading frame. Virol J 2: 88.
- Zhu M, Chang Q, Wong LK, Chong FS, Li RC (1999): Triterpene antioxidants from Ganoderma lucidum. ` 13: 529–531.