Abstract
Lippia alba (Mill.) N.E. Brown (Verbenaceae), also known as Lippia geminata HBK or Lantana alba (Mill.), is a shrub about 3 m tall. In Brazilian traditional medicine, it is commonly known as Erva-Cidreira, Chá do Tabuleiro, and Salsa Limão. The present work was conducted in order to compare the phytochemistry of the roots and leaves and to determine the acute toxicity of the aqueous extracts from the roots of L. alba. The main components in the leaf extract were citral, β-citral, carvone, hemimelitene, germacrene D, and d-limonene. The main components in the root extract were durene, carvone, patchoulane and hemimellitene. To our knowledge, this is the first report of a phytochemical analysis of the root extract from Lippia alba. The LD50 for the aqueous root extract was calculated to be 1156.25 mgkg−1. The animals presented stimulant and depressive behaviors, showing moderate toxicity, most likely due to the presence of iridoids and phenylpropanoids.
Introduction
Lippia alba (Mill.) N.E. Brown, also known as Lippia geminata HBK or Lantana alba (Mill.), is a shrub about 3 m tall that belongs to the Verbenaceae family (CitationStashenko et al., 2004). In Brazilian traditional medicine it is commonly known as erva-cidreira, chá-do-tabuleiro and salsa limão (CitationBraga, 1976; CitationMatos et al., 1996). Its leaves are employed as an infusion or decoction for the treatment of gastric illnesses, diarrhea, fever, asthma, and cough, and as a tranquillizing remedy (CitationTavares et al., 2005; CitationMatos et al., 1996).
Large variations have been observed in the composition of L. alba essential oil extracts, depending on the part of the plant employed in the distillation, the state of development, the geographic location, and the characteristics of the soil, climate, and other local conditions (CitationAlea et al., 1997; Stashenko et al., 2003). Many reports exist on gas chromatography analyses of the different chemotypes of Lippia alba, with most chemical constituents belonging to the terpene class of hydrocarbons. Analyses of essential oil extracts from three chemotypes of L. alba in one study revealed the predominance of citral, β-myrcene, and limonene in chemotype I, citral and limonene for chemotype II, and carvone and limonene for chemotype III, as the main constituents (CitationMatos et al., 1996). Other research has reported the presence of iridoids and phenylpropanoids in the roots of Lippia alba, specifically theviridoside, mussaenoside, gardoside and shanzhiside methyl esther (CitationSena Filho et al., 2006, Citation2007). Chemotypes from the Brazilian Amazon were found to contain 1,8-cineole, limonene, carvone, and sabinene (from Santa Maria), limonene, carvone and myrcene (from Belterra), and neral, gernial, germacrene D, and β-caryophyllene (from Chaves) (CitationZoghbi et al., 1998). CitationSiani et al. (2002) isolated a new chemotype with a high content of linalool in the essential oil of L. alba leaves and explored the use of this chemotype for large-scale, industrial isolation of this compound, which is used as a substitute for bergamot or French lavender oil. Specimens from outside Brazil in French Guinea, Martinique and Guadeloupe have also been analyzed: that from French Guinea contained carvone and limonene; that from Martinique contained limonene and germacrene D; and those from Guadeloupe contained neral, geranial, α-guaiene and (E)-β-ocimene in one and α-guaiene, (E)-β-ocimene, β-caryophyllene, geranial, γ-terpinene, neral, β-myrcene and 6-methylhept-5-en-2-one in the other (CitationHennebelle et al., 2006). These authors also reviewed previous literature on component determination of L. alba chemotypes and proposed a classification of this species into seven main chemotypes based on major chemical constituents, minor but characteristic compounds which almost always occur in a given chemotype, data on seasonal influences causing compound variation and geographic data to establish a zone for a given chemotype.
Essential oil extracts from L. alba have shown antimicrobial activity against bacteria, fungi and viruses (CitationAlea et al., 1997; CitationDuarte et al., 2005; Oliveria et al., 2006; CitationRuffa et al., 2004). It has been hypothesized that the inhibitory effect of terpenoids on microbial oxygen uptake is most likely responsible for the inhibition of microbial growth (Oliveria et al., 2006). Rats given an infusion of L. alba demonstrated reduced gastric ulceration over both short and long term applications, lending credence to this plant’s use in gastric illnesses; however, the mechanism of action remains to be identified (CitationPascual et al., 2001). An evaluation of aqueous extracts from L. alba on the hearts of rats showed that it decreased cardiac rate significantly (CitationGazola et al., 2004), while another study demonstrated muscle relaxation and increased barbiturate sleeping time in mice after injection of citral, myrcene and limonene (Citationdo Vale et al., 2002), which may explain the sedative effects of this plant. Lastly, essential oil extracts from L. alba have demonstrated antioxidant activities, making it a promising source of natural antioxidants (CitationRamos et al., 2003; CitationStashenko et al., 2004).
Our research group evaluated a chemotype of Lippia alba for its chemical components and determined the acute toxicity of the aqueous extract from the roots to mice. Further, little toxicity data on this plant extract exists and is necessary if further suggestion of this plant as a medicinal application is to be pursued. We also performed a comparative phytochemical analysis of the leaves and roots from this specimen via TLC and GC-MS. To our knowledge, this is the first report of a chemical analysis of Lippia alba roots.
Materials and methods
Plant material
The plant specimens (Lippia alba) were collected in Timbaúba (7º35’S; 35º22’W), State of Pernambuco, Brazil, in January 2005. Plants were identified by Haroudo Sátiro Xavier. A voucher specimen was deposited under number 70003 at the Herbarium Dárdano de Andrade Lima, in Empresa Pernambucana de Pesquisa Agropecuaria (IPA)-Pernambuco State, Brazil.
Extraction
For GC analyses, air-dried, powdered roots and fresh leaves (60 g each) of Lippia alba were exhaustively extracted with petroleum ether (MERCK) (CitationSimões et al., 2003) for a yield of 1.6 and 2.2%, respectively.
For the acute toxicity assay, the air-dried and powdered roots (1.2 kg) of Lippia alba were extracted with distilled water for a yield of 3.22%.
Gas chromatography/mass spectrometry(GC/MS) analysis of the oil extracts
Dried petroleum ether extract was reconstituted in 0.5 ml dichloromethane and passed through a 13 mm 0.22 micron Teflon syringe filter before injection on to the GC/MS. The GC/MS analyses were made using a Perkin Elmer AutoSystem XL GC apparatus, equipped with an autosampler and a split-splitless injector, attached to a PE-5MS fused silica capillary 5% diphenyl/95% dimethylpolysiloxane column (30 m × 0.25 mm, 0.25 μm film thickness) using the following program: 60ºC held for 5.00 min then 60–280ºC at 4.0ºC/min with helium at 0.8 ml/min as the carrier gas. Line and injector temperature were both set at 220ºC. The sample (2 μl) was injected using a PSSI injector in the split mode (1:10). MS conditions were run through a Perkin Elmer TurboMass Upgrade mass spectrometer as follows: ionization energy 70 eV.; scan rate 1.6 scans/sec; interscan delay 0.01 sec; source temperature 200ºC; mass range 50 to 400 m/z; solvent delay 3.0 min.
Quantitative data were calculated using the TurboMass 5.1 software program (Perkin Elmer), while qualitative data were obtained from the Wiley NIST/EPA/NIH Mass Spectral Library 2005. Identification of individual components was made by computer searching and matching mass spectral data with those in the Wiley Library. Seven replicate injections were made and an average taken of the relative percentage for identified compounds.
Acute toxicity assay
Animals
Sixty day-old adult female Swiss mice weighing 22 ± 2 g were obtained from the Laboratory of Oncology of the Federal University of Pernambuco State-UFPE. The animals were kept 6 animals to a cage under standard laboratory conditions with a 12 h light/12 h dark photoperiod with a room temperature between 20–25°C. They received water ad libitum. Animal care and experimental procedures followed the principles and guidelines suggested by the Brazilian College of Animal Experimentation (COBEA).
Acute toxicity determination
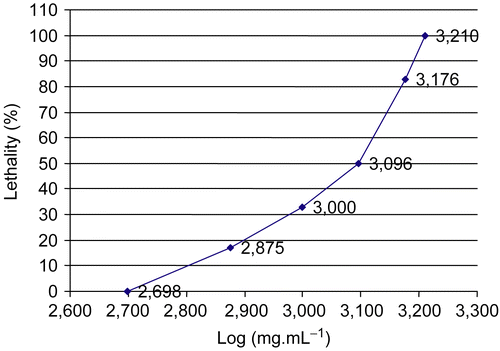
Different doses of the aqueous crude extract of Lippia alba (500, 750, 1000, 1250, 1375, 1500, 1625, and 1750 mg.kg−1 suspended in 0.9% physiologic serum) were administered intraperitoneally to mice. Each dose was given to a group of six animals (n = 6). The mice were observed for mortality rate at 1 and 48 h after extract administration. Evaluation of the acute toxicity and LD50 dose were determined according to the methodology described by Karber and Berhens (1964). The LD50 value was determined using two phases: a preliminary and a definitive phase. In the preliminary phase, increasing doses of aqueous Lippia alba extract were examined in order to define: 1) the highest dose which did not lead to lethality (D1) and 2) the dose which led to the death of 100% of the animals (D2). The data obtained for the preliminary phase allowed for the delineation of the dose range for the definitive phase. In the definitive phase the animals received doses in the range between D1 and D2, in order to determine the LD50. Using these data, we were able to calculate the LD50 (lethal dose of 50% of the animals) using the following equation:
LD50 = Df 2 ∑(a · b)/n
where Df = dose which lead to 100% lethality; a = difference in concentration between two consecutive doses; b = average number of deceased mice between two consecutive doses; n = number of mice used in the assay.
Results and discussion
The results of the GC/MS analysis of the leaf and root extracts are summarized in (leaves) and (roots). The major compounds found in the leaves were citral, β-citral, carvone, hemimelitene, germacrene D, and d-limonene. The main components found in the root extract were durene, carvone, patchoulane and hemimellitene. In regard to classifying this chemotype by the criteria set forth in CitationHennebelle et al. (2006), the leaf specimen would fall under chemotype Ia, as it contains citral as the main component. It is interesting to note that no chemical analyses have been performed previously on the roots of L. alba. To our knowledge, our study presents the first chemical determination of L. alba roots (), which shows a very different chemical profile than leaves from the same plant ().
Table 1. Percent composition of the oil extract from the leaves of Lippia alba.
Table 2. Percent composition of the oil extract from the roots of Lippia alba.
The LD50 dose was calculated to be 1156.25 mg.kg−1 (). The animals presented with stimulating behavior (increased respiratory frequency, agitation, exophthalmia and tail erection), depressive symptoms (somnolence and prostration), and additional observations including diarrhea, abdominal contortions, and fecal excretions.
Acknowledgements
The authors wish to thank CAPES, Brazil for their financial support.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alea JAP, Ortega AG, Rosado A, Rodriguez M, Baluja R (1997): Composición y propiedades antibacterianas del aceite essencial de Lippia alba (Mill.) N.E. Brown. Rev Cubana Farm 30: 1–8.
- Braga R (1976): Plantas do Nordeste, especialmente do Ceará. Fortaleza: Edição Mossoroeinse, pp. 186.
- do Vale TG, Furtado EC, Santos JG, Viana GSB (2002): Central effects of citral, myrcene and limonene, constituents of essential oil chemotypes from Lippia alba (Mill.) N.E. Brown. Phytomedicine 9: 709–714.
- Duarte MCT, Figueira GM, Sartoratto A, Rehder VLG, Delarmelina C (2005): Anti-Candida activity of Brazilian medicinal plants. J Ethnopharmacol 97: 305–311.
- Gazola R, Machado D, Ruggiero C, Singi G, Alexandre MM (2004): Lippia alba, Melissa officinalis and Cymbopogon citratus: Effects of the aqueous extracts on the isolated hearts of rats. Pharmacol Res 50: 477–480.
- Hennebelle T, Sahpaz S, Dermont C, Joseph H, Bailleul F (2006): The essential oil of Lippia alba: Analysis of samples from French Overseas Departments and review of previous works. Chem Biodivers 3: 1116–1125.
- Matos FJA, Machado MIL, Craveiro AA, Alencar JW (1996): The essential oil composition of two chemotypes of Lippia alba grown in Northeast Brazil. J Essent Oil Res 8: 695–698.
- Pascual ME, Slowing K, Carretero ME, Villar á (2001): Antiulcerogenic activity of Lippia alba (Mill.) N.E. Brown (Verbenaceae). Il Farmaco 56: 501–504.
- Ramos A, Visozo A, Piloto J, García A, Rodríguez CA, Rivero R (2003): Screening of antimutagenicity via antioxidant activity in Cuban medicinal plants. J Ethnopharmacol 87: 241–246.
- Ruffa MJ, Wagner ML, Suriano M, Vicente C, Nadinic J, Pampuro S, Salomón H, Campos RH, Cavallaro L (2004): Inhibitory effect of medicinal herbs against RNA and DNA viruses. Antivir Chem Chemother 15: 153–159.
- Sena Filho JG, Melo JGS, Saraiva AM, Gonçalves AM, Caetano N, Xavier HS (2006): Antimicrobial activity and phytochemical profile from the roots of Lippia alba (Mill.) N.E. Brown. Rev Bras Farmacogn 16: 506–509.
- Sena Filho JG, Duringer JM, Uchoa DEA, Xavier HS, Barbosa-Filho JM, Braz-Filho R (2007): Distribution of iridoid glucosides in plants from the genus Lippia (Verbenaceae): An investigation of Lippia alba (Mill.) N.E. Brown. Nat Prod Commun 2: 712–713.
- Siani AC, Tappin MRR, Ramos MFS, Mazzei, JL, Ramos MCKV, de Aquino Neto FR, Frighetto N (2002): Linalool from Lippia alba: Study of the reproducibility of the essential oil profile and the enantiomeric purity. J Agric Food Chem 50: 3518–3521.
- Simões CMO, Schenkel EP, Gosmann G, Mello JCP, Mentz LA, Petrovick PR (2003): Farmacognosia da Planta ao Medicamento. Porto Alegre/Florianópolis, Editora da UFRGS/Editora da UFSC, pp. 475–478.
- Stashenko EE, Jaramillo BE, Martinez JR (2004): Comparison of different extraction methods for the analysis of volatile secondary metabolites of Lippia alba (Mill.) N.E. Brown, grown in Colombia, and evaluation of its in vitro antioxidant activity, J Chromatogr A 1025: 93–103.
- Tavares ES, Julião LS, Lopes D, Bizzo HR, Lage CLS, Leitão SG (2005): Análise do óleo essencial de folhas de três quimiotipos de Lippia alba (Mill.) N.E.Br. (Verbenaceae) cultivados em condições semelhantes. Rev Bras Farmacogn 15: 1–5.
- Zoghbi M das GB, Andrade EHA, Santos AS, Silva MHL,andandMaia JGS (1998): Essential oils of Lippia alba (Mill.) N.E. Br growing wild in the Brazilian Amazon. Flavour Fragr J 13: 47–48.