Abstract
The genus Hypericum (Hypericaceae) contains a variety of structurally diverse natural products which possess a wide array of biological properties. The present study examined quantitative variations in total hypericin, phenols, and flavonoids in Hypericum triquetrifolium Turra. in relation to phenological development. Plant material was harvested at different phenological stages (vegetative, full flowering, and mature fruiting stages). The amounts of bioactive compounds in whole shoots were assayed. The highest total hypericin, phenolic, and flavonoid contents were observed at the full flowering stage.
Introduction
Hypericum (Hypericaceae) species are herbaceous plants, naturally found in the temperate zones of Europe, Asia, and Africa (CitationCampbell & Delfosse, 1984). The genus is well known for its anti-inflammatory, diuretic, and sedative properties and as a healing agent (CitationDias et al., 1998). Extracts of the crude drug are widely used in the treatment of depression and have also been identified as a possible anticancer agent (CitationHubner et al., 1994; CitationLinde et al., 1996; CitationAlecu et al., 1998; CitationKamuhabwa et al., 2000).
The genus Hypericum is traditional medicinal containing a broad spectrum of secondary metabolites (CitationPatocka, 2003). Many pharmacological activities appear to be attributable to their hypericin derivatives and phenolic constituents (CitationAgostinis et al., 2002; CitationKim et al., 2002).
Hypericin and pseudohypericin are photodynamic pigments, produced from dimerized emodin anthrone (CitationFalk, 1999). Photodynamic hypericin activities displayed under the influence of light are used for therapy in various diseases. Photoactivated hypericin generates reactive oxygen species and its photocytotoxicity properties have been proposed as photochemotherapeutic (CitationVandenbogaerde et al., 1997). These properties allow hypericin to act as an antiviral agent. Attention has been focused on its use against human immunodeficiency virus type 1 (CitationMeruelo et al., 1988).
Recent studies have focused on the health functions of phenolics, including flavonoids and anthocyanins. Phenolic compounds are widely distributed in the plant kingdom. External stimuli such as microbial infections, ultraviolet radiation, and chemical stressors induce their synthesis. The phenolic compounds, flavonoids and furanocoumarins, have many ecologic functions and affect human health. Ecologic functions include defense against microbial pathogens and herbivorous animals. The synthesis of phenolic compounds in plants can be modulated by the application of herbicides and, to a lesser extent, insecticides and fungicides.
Phenolics possess a wide spectrum of biochemical activities such as antioxidant, antimutagenic, and anticarcinogenic, as well as the ability to modify gene expression (CitationCarbanaro et al., 2002; CitationFloridi et al., 2003).
Hypericum triquetrifolium, widespread in warm temperate areas throughout the world, is a perennial usually growing on dry, stony, and sandy places (CitationRobson, 1975). The aerial parts of H. triquetrifolium contain more hypericin than Hypericum perforatum L., the main and traditional source of hypericin (CitationKitanov, 2001; CitationAlali et al., 2004). There are some literature reports on phytochemical analyses of hypericin (CitationApaydın et al., 1999; CitationYeşilada et al., 1999) and on the antioxidant effects of ethanol and methanol extracts of H. triquetrifolium (CitationCouladis et al., 2002; CitationConforti et al., 2007; CitationKızıl et al., 2008).
Based on the strong biological activity of hypericin and phenolic compounds, the aim of present study was focused on determination of the total hypericin, phenolics, and flavonoid contents of H. triquetrifolium during its phenological cycle.
Materials and methods
Plant material
H. triquetrifolium was collected in Diyarbakır in the area of south east of Turkey from May to September 2006 at the stages of vegetative, full flowering, and mature fruiting. Voucher specimens have been deposited at the herbarium of the Department of Biology, Faculty of Science and Art, Dicle University (voucher no. DUF-2512). They were identified by Dr A. Selçuk Ertekin from the same institution.
Preparation of extracts
Aerial parts were dried for 10 days at room temperature. Each sample was extracted with 50 mL of 99% ethanol for 72 h at room temperature. The extracts were kept dark in a deep freezer until used for the determination of total phenolic and flavonoid contents.
Determination of total hypericin content
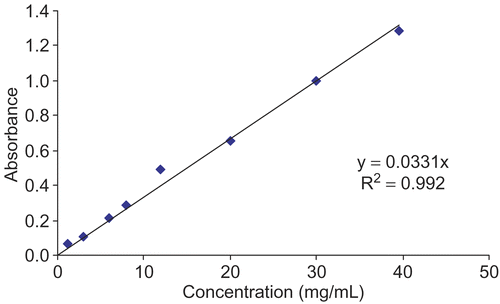
Dried plant material (200 mg) was placed in vials containing chloroform (10 mL) and extracted in a sonicator to remove chlorophyll. Next, chloroform was removed under vacuum. After removing chloroform, the samples were re-extracted with methanol (10 mL) in a sonicator. The methanol extraction process was repeated three times, and then the methanol was removed under vacuum. The final samples were dissolved in methanol and 1 mL of the supernatant was placed in a test tube. Absorbance was measured at 589 nm (CHEBIOS T80+UV/VIS spectrometer). Spectrophotometric analysis was performed by using an eight-point calibration curve generated with pure hypericin (Sigma, Italy) as standard (). The hypericin content in H. triquetrifolium was calculated and expressed as micrograms of hypericin per gram of dry material.
Determination of total phenolic content
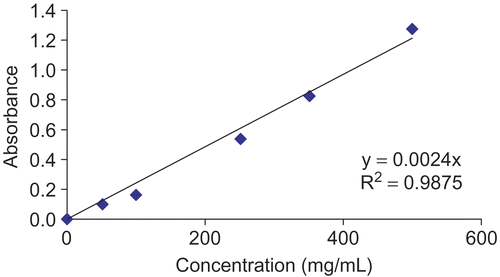
The content of total phenolic compounds in the ethanol extract of H. triquetrifolium was determined using Folin–Ciocalteus reagent according to the method of CitationSingleton et al. (1999). Crude ethanol extract (40 μL) of H. triquetrifolium (1 mg/mL) was mixed with 200 μL Folin–Ciocalteus reagent (Sigma Aldrich, Germany) and 1160 μL of distilled water, followed by 600 μL of 20% (v/v) Na2CO3 three minutes later. The mixture was shaken for 2 h at room temperature and absorbance was measured at 765 nm. Spectrophotometric analysis was performed by using a five-point calibration curve generated with pure gallic acid (Sigma Aldrich) as standard (). The concentration of total phenolic compounds in H. triquetrifolium was determined as micrograms of gallic acid equivalents per milligram of extract using the following equation obtained from the standard gallic acid graph (R2=0.9875):
Absorbance=0.0024 × gallic acid (μg).
Determination of total flavonoid content
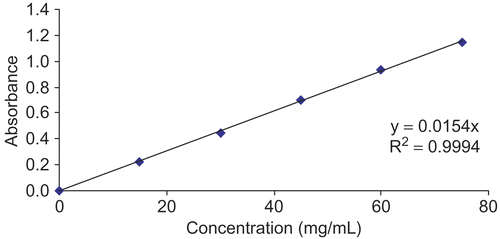
Measurement of flavonoid concentration of the extract was based on the method described by CitationPark et al. (1997) with a slight modification. An aliquot of 1 mL of the solution (containing 1 mg of extract in methanol) was added to a test tube containing 0.1 mL of 10% aluminum nitrate, 0.1 mL of 1 M potassium acetate, and 3.8 mL of methanol. After 40 min at room temperature, the absorbance was determined at 415 nm. Spectrophotometric analysis was performed by using a five-point calibration curve generated with pure quercetin (Sigma) as standard (). The flavonoid content in H. triquetrifolium was expressed in milligrams of quercetin equivalents per gram fresh weight of plant material.
Statistical analyses
All analyses were performed in triplicate, and the results expressed as mean±standard deviation. Significant differences among the groups were determined by one-way ANOVA using the SPSS 12.0 software package. The results were considered significant if the value of p was less than 0.05.
Results
The sampled plant material included leaves and stems at the vegetative stage; reproductive parts, leaves, stems, and flowers at the full flower stage; and stems and brown capsules with seeds at the mature fruiting stage.
The total hypericin contents, expressed as μg of hypericin in a given dry material, of the H. triquetrifolium methanol extracts (1 mg each) from the vegetative, full flowering, and mature fruiting stage were 140.0, 242.3, and 125.0, respectively.
The total amounts of phenolic compounds, determined as mg gallic acid equivalents/mg extract, of the H. triquetrifolium ethanol extracts (1 mg each) from the vegetative, full flowering, and mature fruiting stage were 172.3, 212.6, and 133.3, respectively.
The total flavonoid contents, expressed as mg quercetin equivalents/g fresh weight of plant material, from the vegetative, full flowering, and mature fruiting stage were 173.3, 217.3, and 168.3, respectively.
The highest levels of total hypericin, total flavonoids, and total phenolic compounds were observed at the full flowering stage. The concentration of these compounds decreased during fruit development. These differences were statistically significant (p<0.005) ().
Table 1. Variation in total hypericin, total flavonoid and total phenolic contents (μg/g dry weight) of Hypericum triquetrifolium during its phenological cycle.
Discussion and conclusions
Concentrations of secondary metabolites in plants may vary substantially with developmental stage. Therefore, investigations on the ontogenic variation of secondary metabolites are worthwhile. In the present study, we investigated the variation in total hypericin, flavonoid, and phenolic contents of H. triquetrifolium during its phenological cycle. The highest hypericin content was obtained from the full flowering stage. This finding is in agreement with previous published results (CitationAlali et al., 2004; CitationAyan & çırak, 2008). Similarly, the highest content of hypericin in H. perforatum L. (CitationKazlauskas & Bagdonaite, 2004; CitationCouceiro et al., 2006), Hypericum prunatum, Hypericum avicularifolium (Citationçırak et al., 2006), Hypericum perfoliatum (Citationçırak et al., 2007b) and Hypericum origanifolium Willd. (Citationçırak et al., 2007b) was determined during flower ontogenesis. The antioxidant activity of herbs results mainly from phenolic compounds (CitationMalenčić et al., 2007; CitationRadulovic et al., 2007). In the present work, the total phenolic and flavonoid contents of H. triquetrifolium were found to change during the phenological cycle. The highest total phenolic and flavonoid contents were also obtained from the full flowering stage.
The present results therefore suggest that, for medicinal purposes, plant material of H. triquetrifolium should be collected during the full flowering stage, in which bioactive compounds reach their highest level.
Acknowledgements
The work was supported by research grants from the Dicle University Research Council (DUAPK; project numbers DUAP-2000-FF-410).
Declaration of interest: The authors report no conflicts of interest.
References
- Agostinis P, Vantieghem A, Merlevede W, De Witte D (2002): Hypericin in cancer treatment: more light on the way. Int. J Biochem Cell Biol 34: 221–241.
- Alali F, Tawaha K, Al-Eleimat T (2004): Determination of hypericin content in Hypericum triquetrifolium Turra (Hypericaceae) growing wild in Jordan. Nat Prod Res 18: 147–151.
- Alecu M, Ursacıuc C, Halalau F, Coman G, Merlevede W, Waelkens E, De Witte P (1998): Photodynamic treatment of basal cell carcinoma and squamous cell carcinoma with hypericin. Anticancer Res 18: 4651–4654.
- Apaydın ş, Zeybek U, İnce İ, Elgin G, Karamenderes C, öztürk B, Tuğlular I (1999): Hypericum triquetrifolium Turra. extract exhibits anticiceptive activity in the mouse. J Ethnopharmacol 67: 307–312.
- Ayan AK, çirak C (2008): Hypericin and pseudohypericin contents in some Hypericum species growing in Turkey. Pharm Biol 46: 288–291.
- Campbell MH, Delfosse ES (1984): The biology of Australian weeds. 13. Hypericum perforatum L. J Aust Inst Agric Sci 50: 63–73.
- Carbanaro M, Mattera M, Nicoli S, Bergamo P, Cappelloni M (2002): Modulation of antioxidant compounds in organic vs conventional fruit (peach, Prunus persica L., and pear, Pyrus communis L.). J Agric Food Chem 50: 5458–5462.
- çırak C, Sağlam B, Ayan AK, Kevseroğlu K (2006): Morphogenetic and diurnal variation of hypericin in some Hypericum species from Turkey during the course of ontogenesis. Biochem Syst Ecol 34: 1–13.
- çırak C, Radusiene J, Janulis V, Ivanauskas L (2007a): Secondary metabolites in Hypericum perfoliatum variation among plant parts and phenological stages. Botanica Helvatica 117: 29–36.
- çırak C, Radusiene J, Ivanauskas L, Janulis V (2007b): Variation of bioactive secondary metabolites in Hypericum origanifolium during its phenological cycle. Acta Physiol Plant 29: 197–203.
- Conforti F, Loızzo MR, Statti AG, Menichini F (2007): Cytotoxic activity of antioxidant constituents from Hypericum triquetrifolium Turra. Nat Prod Res 21: 42–46.
- Couceiro MA, Afreen F, Zobayed SMA, Kozai T (2006): Variation in concentrations of major bioactive compounds of St. John’s wort: effects of harvesting time, temperature and germplasm. Plant Sci 170: 128–134.
- Couladis M, Bazıou P, Verykokıdeu E, Loukıs A (2002): Antioxidant activity of polyphenols from Hypericum triquetrifolium Turra. Phytother Res 16: 769–770.
- Dias ACP, Francisco A, Barberan T, Ferreria F, Ferreres F (1998): Unusual flavonoids produced by callus of Hypericum perforatum. Phytochemistry 48: 1165–1168.
- Falk H (1999): From the photosensitizer hypericin to the photoreceptor stentorian: the chemistry of phenanthroperylene quinines. Angew Chem Int Ed Engl 38: 3116–3136.
- Floridi S, Montanari L, Ombretta M, Fantozzi P (2003): Determination of free phenolic acids in wort and beer by coulometric array detection. J Agric Food Chem 51: 1548–1554.
- Hubner WD, Lande S, Podzuweit H (1994): Hypericum treatment of mild depression with somatic symptoms. J Geriatr Psychiatry Neurol 7: 12–14.
- Kamuhabwa AR, Agostinis P, Kasran A, De Witte PA (2000): Photodynamic activity of hypericin in human urinary bladder carcinoma cell. Anticancer Res 20: 2579–2584.
- Kazlauskas S, Bagdonaite E (2004): Quantitative analysis of active substances in St. John’s wort (Hypericum perforatum L.) by the high performance liquid chromatography method. Medicina (Kaunas) 40: 975–981.
- Kim DO, Lee KW, Lee HJ, Lee CY (2002): Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J Agric Food Chem 50: 3713–3717.
- Kitanov GM (2001): Hypericin and pseudohypericin in some Hypericum species. Biochem Syst Ecol 29: 171–178.
- Kızıl G, Kızıl M, Yavuz M, Emen S, Hakimoğlu F (2008): Antioxidant activities of ethanol extracts of Hypericum triquetrifolium and Hypericum scabroides. Pharm Biol 46: 231–242.
- Linde K, Ramirez G, Mulrow CD, Pauls A, Weidenhammer W, Melchart D (1996): St John’s wort for depression: an overview and meta-analysis of randomised clinical trials. BMJ (Clin Res Ed) 313: 253–258.
- Malenčić D, Popović M, Miladinović J (2007): Phenolic content and antioxidant properties of soybean (Glycine max (L.) Merr.) seeds. Molecules 12: 576–581.
- Meruelo D, Lavie G, Lavie D (1988): Therapeutic agents with dramatic antiretroviral activity and little toxicity and effective doses, aromatic polycyclic diones hypericin and pseudohypericin. Proc Natl Acad Sci U S A 85: 5230–5234.
- Moreno MIN, Isla MI, Sampietro AR, Vattuone MA (2000): Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J Ethnopharmacol 71: 109–114.
- Park YK, Koo MH, Ikegaki M, Contado JL (1997): Comparison of the flavonoid aglycone contents of Apis melliferapropolis from various regions of Brazil. Arq Biol Technol 40: 97–106.
- Patocka J (2003): The chemistry, pharmacology, and toxicology of the biologically active constituents of the herb Hypericum perforatum L. J Appl Biomed 1: 61–73.
- Radulovic N, Stankov-Jovanovic V, Stojanovic G, Melcerovic AS, Spiteller M, Asakawa Y (2007): Screening of in vitro antimicrobial and antioxidant activity of nine Hypericum species from the Balkans. Food Chem 103: 15–21.
- Robson NKB (1975): Hypericum L. In: Davis PH, editor. Flora of Turkey and the East Aegean Islands. Edinburgh: Edinburgh University Press. pp 355–401.
- Singleton VL, Orthofer R, Lamuela-Raventos RM (1999): Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. In: Packer L, editor. Methods in Enzymology. Vol. 299: Oxidants and antioxidants (Part A). San Diego, CA: Academic Press. pp 152–178.
- Yeşilada E, Gürbüz I., Shitabo H (1999): Screening of Turkish anti-ulcergenic folk remedies for anti-Helicobacter pylori activity. J Ethnopharmacol 66: 289–293.
- Vandenbogaerde AL, Cuveele JF, Proot P, Himpens BE, Merlevede W, and de Witte PA (1997): Differential cytotoxic effects induced after photosensitization by hypericin. J Photochem Photobiol B 38: 136–142.