Abstract
The growth promoting potential of alcohol and aqueous extracts of young prop roots of Ficus bengalensis Linn. (Moraceae), a medicinal plant widely used among the tribes of the western zone of Maharashtra state, India to increase height, was studied. Its growth promoting effect was evaluated in one-month-old immature female rats. Extracts were administered to young rats for 30 days. Significant (p < 0.05) increase in body weight was observed in alcohol and aqueous extract treated immature female rats. Animals treated with alcohol extract showed statistically significant difference (p < 0.05) in parameters such as mean food consumption, total body length and increase in alkaline phosphatase levels, a biochemical marker for bone formation. Significant results were not observed in other parameters such as feed efficiency, tail length, relative organ weight, bone density, tibial epiphyseal cartilage width and bone hydroxy proline levels. The results obtained establish the efficacy of the plant material as well as importance of chronic studies to justify the use of this plant in growth promotion.
Introduction
Ficus bengalensis Linn. (Moraceae) is widely distributed throughout the forests tracts of India, both in the sub-Himalayan region and in the deciduous forests of Deccan and south India. It is grown in gardens and roadsides for shade (CitationCSIR, 1956). All parts of the plant are astringent, acrid, sweet, refrigerant, anodyne, vulnerary, depurative, anti-inflammatory, ophthalmic, styptic, antiarthritic, diaphoretic, antidiarrheal, antiemetic and tonic (CitationPrajapati & Kumar, 2005).
The aerial roots are useful in obstinate vomiting and leucorrhea and are said to be used in osteomalacia of the limbs (CitationVarier, 1996). It is also used to treat intrinsic hemorrhage, bone fracture (externally), ulcers, and to enhance memory (CitationSingh & Prakash, 1994; CitationSivarajan & Balachandran, 1994; CitationSatapathy & Brahmam, 1996). According to tribal evidence, Katkari, Kokana, Mahadeo koli, Thakar, and Warli of the western zone of Maharashtra State in India, eat the pounded raw young prop roots of Ficus bengalensis to increase their height (CitationUpadhye et al., 1994). Findings from previous studies indicate that the milky sap of this plant exerts endocrine stimulating actions similar to those produced by somatotropin (CitationGupta, 1966, Citation1994).
With this in mind, the present study investigated the growth promoting potential of young prop roots of Ficus bengalensis using one-month-old intact (30 days) immature female rats.
Methods
Plant material
The young prop roots of the plant material Ficus bengalensis were collected in and around Manipal, Udupi, Karnataka, India, during the month of September 2004. The plant was authenticated by Gopalakrishna Bhat, Department of Botany, Poorna Prajna College, Udupi, Karnataka, India. The voucher specimen (pp-04/2004-05) has been deposited in the Department of Pharmacology, Manipal College of Pharmaceutical Sciences, Manipal, India.
Preliminary phytochemical screening
Preliminary phytochemical screening (CitationKokate, 1994) revealed the presence of alkaloids, steroids and tannins (CitationMukherjee et al., 1998).
Preparation of alcohol and aqueous extracts
About 100 g of coarse powder of the young prop roots were extracted in the Soxhlet extractor with ethanol as solvent. After complete extraction the solvent was recovered by distillation in vacuo and the yield was 5.7% w/w and for aqueous extract, the coarsely powdered drug was macerated in chloroform water for 7 days. After maceration, the residue (yield 7.2% w/w) was obtained by evaporation. The extracts obtained were then stored in a dessicator and used for subsequent experiments.
Animals
Healthy immature female Wistar albino rats one month of age (30 days) were used for the study. The animals were housed individually in polypropylene cages, maintained under standard conditions (12 h light; 12 h dark cycle; 25° ± 3°C; 35–60% humidity). They were fed with standard rat pellet diet (Hindustan Lever, Mumbai, India) and water ad libitum. The Institutional Animal Ethical Committee of KMC, Manipal, India (IAEC/KMC/06/2004–05) approved the study.
Acute toxicity study
An acute toxicity study was carried out by up and down method. Drugs were administered orally to overnight fasted animals. The rats were observed continuously for 2 h for behavioral, neurological and autonomic profiles and after 24 h and 72 h for any lethality (CitationGhosh, 1984). None of the animals died even at a dose of 3 g/kg of each extract. One tenth of this dose (300 mg/kg) was selected for the subsequent study.
Experimental design
One-month-old immature intact female rats, divided into three groups (n = 6), were tested with 1% Carboxy Methyl Cellulose (CMC), alcohol extract (300 mg/kg) and aqueous extract (300 mg/kg) respectively for 30 days, p.o., for evaluation of growth promoting potential. The following end points were evaluated.
Physical end points
Body weight was measured daily. Tail length, food intake and feed efficiency were measured on every fifth day during the 30 day period (CitationGroesbeck et al., 1987). Total body length was measured on day 31. Feed efficiency, the ratio of body weight (BW) gained to the amount of feed consumed, was estimated by measuring the amount of feed consumed during a 24 h period and the change in BW during the same 24 h period measured on every fifth day.
Morphological end points
On day 31 animals were sacrificed and organs such as liver, spleen, kidneys, heart and lungs were excised, blotted on a filter paper and weighed to determine relative organ weight. The relative organ weight (CitationChavalittumrong et al., 2004) was calculated using the formula:
The tibia and femurs were dissected out and cleaned of soft tissue. Bone weight of femurs was measured using an electronic balance. The density and volume of the right tibias were calculated from the mass in air and water by Archimedes principle (CitationBroulik et al., 2003).
Histological end point
Determination of tibial epiphyseal cartilage width
The proximal left tibiae were dissected free from soft tissues and split with a sharp razor in the mid-sagittal plane. Silver nitrate (2%) was used to stain the calcified portions of the bone. The width of the uncalcified epiphyseal cartilage was measured under a low power microscope using a micrometer eyepiece, calibrated with stage micrometer. A minimum of 8-10 readings was made across the section and mean value was calculated (CitationGreenspan et al., 1949).
Biochemical end points
Serum parameters
Blood was withdrawn under light ether anesthesia from retro-orbital sinus puncture. The serum was assayed for alkaline phosphatase (EC 3.1.3.1) (Nicholas Piramal India, Navi Mumbai). Biochemical analysis of serum samples was performed using semi-auto analyzer (STAR 21).
Bone parameters
The femur bones were dried to constant weight and hydrolyzed with 6 N HCl at 110°C for 24 h and aliquots of the hydrolysate were used to determine bone hydroxyproline (CitationNeuman & Logan, 1950).
Statistical analysis
Data were presented in mean ± SEM and evaluated by using one-way ANOVA, followed by post hoc Scheffe’s test using version 10.0 of SPSS computer software. The values were considered significant when p < 0.05.
Results
Physical endpoints
The alcohol and aqueous extracts used in these studies significantly stimulated body weight gain in normal immature rats (p < 0.05) at an average gain of 3.6 ± 0.09 g/day and 3 ± 0.14 g/day, respectively. This increase in body weight is comparable to that of an actively growing normal rat (2.5 ± 0.04 g/day), thus indicating maximum body growth. On day 30, treated animals weighed significantly more than the controls () and the differences in body weight between the groups persisted throughout the treatment period (). The BW difference was 42.6% greater than control for the alcohol extract treatment and 19.5% greater than control for the aqueous extract treatment group ().
Figure 1. Effects of extracts of young prop roots of Ficus bengalensis on body weight from day 0-30. Each point represents mean ± SE (Standard Error), n = 6.
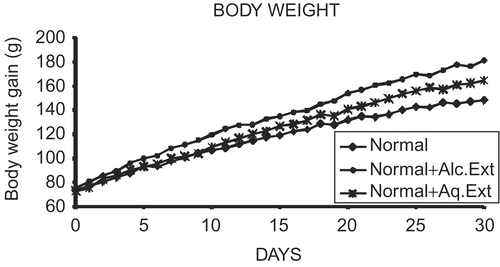
Table 1. Effects of extracts of young prop roots of Ficus bengalensis on body weight and tail length.
The mean food intake in the alcohol extract treatment group was elevated significantly (p < 0.05) based on the trial conducted on days 1, 5, 10, 15, 20, 25, 30 when compared with vehicle treated control rats whereas significant results were not observed in mean feed efficiency when treated group compared with the control group. Alcohol and aqueous extract showed an increased percentage in feed efficiency of 23.5% and 3.8%, respectively, when compared to control, but not significantly ().
Table 2. Effects of extracts of young prop roots of Ficus bengalensis on mean food intake and feed efficiency.
There was no significant difference in tail length among the treatment and control groups. The mean rate of tail elongation of control and treated rats during the experimental period is shown in . Total body length (i.e., overall growth) of an animal treated with alcohol extract increased significantly ().
Table 3. Effects of extracts of young prop roots of Ficus bengalensis on tail and total body length.
Morphological endpoints
The contribution of several organs to the increase in body weight after 30 days of treatment with extracts was studied. In relative organ weight, the statistical difference between the treatment and control groups was not significant (). Bone weight, density and volume did not differ significantly among the vehicle treated and extract treated groups ().
Table 4. Effects of extracts of young prop roots of Ficus bengalensis on relative organ weight (g).
Table 5. Effects of extracts of young prop roots of Ficus bengalensis on bone weight, density and volume.
Histological endpoint
The effect of extracts upon the proximal epiphyseal cartilage of the tibia of the immature rats did not show any statistically significant response in width of the cartilage plate (), and photographs of silver-stained tibiae from control and extract treated animals are shown in .
Figure 2. Effect of alcohol and aqueous extracts of young prop roots of Ficus bengalensis on the proximal epiphyseal cartilage of the tibia of one-month-old immature rats. (A) Normal rat, (B) normal + alcohol extract (300 mg/kg), (C) Normal + aqueous extract (300 mg/kg).
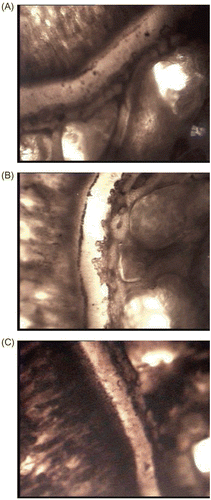
Table 6. Effects of extracts of young prop roots of Ficus bengalensis on tibial epiphyseal cartilage width.
Biochemical endpoints
Significant (p < 0.05) difference was observed in alkaline phosphatase (EC 3.1.3.1) levels of treated immature female rats when compared with normal animals. Bone hydroxyproline levels were not elevated and the results are presented in .
Table 7. Effects of extracts of young prop roots of Ficus bengalensis on Alkaline Phosphatase (ALP) and hydroxypraline.
Discussion
Growth is a complex process and involves the interaction of multiple, diverse factors – the “cumulative sum of millions of unsynchronized cell replications”. Growth is common to all multicellular organisms and occurs by cell replication and enlargement along with the non-homogenous process of cell and organ differentiation (CitationReiter & Rosefeld, 2003). Growth is a sensitive indicator of health status, and deviation from the normal range both for height and for rate of growth may indicate an underlying congenital or acquired problem (CitationCowell, 1995).
The pituitary gland is the main regulator of the endocrine system and it plays a major role in regulation of growth. Growth hormone is the major stimulus of postnatal growth (CitationWidmaier et al., 2004) and main regulator of longitudinal growth and exerts a multitude of effects on several tissues (CitationFlores Morales et al., 2001).
In our present investigation, one-month-old immature female rats were used for the assessment of growth promotion by extracts. Both alcohol and aqueous extracts promote significant increase in body weight. Increase in food consumption may be in part associated with body weight gain in animals treated with alcohol extract, and without significantly affecting the feed efficiency implies that food utilization is not the factor for the stimulation of growth rate. The results can also be supported by the effect of ghrelin, a new member of growth hormone (GH) axis, which stimulates growth hormone secretion, body weight gain and food intake (CitationUeno et al., 2005). It causes a positive energy balance by stimulating food intake through a GH independent mechanism (CitationCaminos et al., 2005).
Body weight gain in the immature rats is accompanied by significant increase in skeletal growth (CitationGroesbeck et al., 1987). Skeletal growth is normally exemplified by elongation of tail length. The aquous extract did not cause any increase in tail length of the animals. The alcoholic extract, on the other hand, significantly promoted the overall growth of the animals.
Growth hormone is essential in the development and growth of the skeleton and for the maintenance of bone mass and density (CitationYamanouchi et al., 2004). The extract did not cause any significant change in bone mass and density of the animals.
The growth of the long bones is driven by the cellular activity of chondrocytes within the growth plate. Chondrocytes represent a target tissue for a number of molecules that are integrated into the developmental program of the skeleton. The major regulation of the growth plate is due to systemic hormones mainly those related to the Growth hormone-insulin-like growth factor-I axis (GH-IGF-I axis) and locally produced peptide factors play an important autocrine and/or paracrine role in ensuring skeletal development and growth (CitationCaminos et al., 2005). Ghrelin potently stimulates GH release present also in the growth plate and promote the longitudinal bone growth.
There was found to be no significant increment in the width at the proximal epiphyseal cartilage of tibia of intact immature rats, and cartilage plate plays a major role in longitudinal growth. The result clearly shows that extract did not have much effect on the promotion of longitudinal growth and this fact is also supported from the results obtained in tail growth. The other factor contributing to this, is the dose of extract (300 mg/kg), because very large doses of rat growth hormone are required to induce a supranormal growth rate in prepubertal rats when compared to hypophysectomized and plateaued rats (CitationGroesbeck et al., 1987). Hypophysectomized animals are more sensitive to growth hormone treatment (CitationMarx et al., 1942).
Administration of alcohol extracts in immature rats showed a significant elevation in alkaline phosphatase levels which signifies that the extract plays a significant role in bone formation. The result can be correlated to elevation of alkaline phosphatase levels in growth hormone treatment as well as to ghrelin. Ghrelin directly stimulates bone formation and its synthetic analogs also stimulate longitudinal bone growth in rats as well as increase the biochemical markers of bone formation (CitationFukushima et al., 2005; CitationCaminos et al., 2005).
In the present investigation, the alcohol extract of young prop roots of Ficus bengalensis showed growth hormone and ghrelin-like activity in a few parameters like body weight, food consumption, in total body length and increase in the biochemical markers of bone formation at a dose of 300 mg/kg within the short duration of experimental period. Further study is necessary to ascertain the growth promoting activity of these extracts.
The GH endocrine axis mainly involves the hypothalamus, the hypophysis and the liver, its regulation is as complex as that of other endocrine axes. As a consequence, there are many potential sites for the pharmacological manipulation of this axis.
Further pharmacological studies will be focused in the near future in hypophysectomized, normal plateaued and in adult female rats. Growth hormone induction and inhibitor of negative regulator somatostatin to release GH can also be investigated.
Acknowledgement
The authors sincerely thank Manipal Academy of Higher Education (MAHE), India, for providing all the facilities to carry out this study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Broulik PD, Haluzik M, Skrha J (2003): The influence of nitric oxide synthase inhibitor L-NAME on bones of male rats with streptozotocin induced diabetes. Physiol Res 52: 729–739.
- Caminos JE, Gualillo O, Lago F, Otero M, Blanco M, Gallego R, Garcia-Caballero T, Goldring MB, Casanueva FF, Gomez-Reino JJ, Dieguez C (2005): The endogenous growth hormone secretagogue (ghrelin) is synthesized and secreted by chondrocytes. Endocrinology 146: 1285–1292.
- Chavalittumrong P, Chivapat S, Attawish A, Bansiddhi J, Phadungpat S, Chaorai B, Butraporn R (2004): Chronic toxicity study of Portulaca grandiflora Hook. Ed. Brook CGD. London. J Ethnopharmacol 90: 375–380.
- Cowell CT (1995): Short stature. In: Clinical Pediatric Endocrinology. Blackwell Science Ltd, pp. 136–141.
- CSIR (1956): The Wealth of India: Raw Materials. New Delhi, Council of Scientific and Industrial Research, pp. 24–25.
- Flores Morales A, Stahlberg N, Tollet-egnell P, Lundeberg J, Malek RL, Quackenbush J, Lee NH, Norstedt G (2001): Micro array analysis of the in vivo effects of hypophysectomy and growth hormone treatment on gene expression in the rat. Endocrinology 142: 3163–3176.
- Fukushima N, Hanada R, Teranishi H., Fukue Y, Tachibana T, Ishikawa H, Takeda S, Takeuchi Y, Fukumoto S, Kangawa K, Nagata K, Kojima M (2005): Ghrelin directly regulates bone formation. J Bone Miner Res 20: 790–798.
- Ghosh MN (1984): Toxicity studies. In: Fundamentals of Experimental Pharmacology. Calcutta, Scientific Book Agency, pp. 153–158.
- Greenspan FS, Hao Li C, Simpson, Evans HM (1949): Bioassay of hypophyseal growth hormone: The tibia test. Endocrinology 45: 455–463.
- Groesbeck MD, Parlow AF, Daughaday WH (1987): Stimulation of supra normal growth in prepubertal, adult plateaued, and hypophysetomized female rats by large doses of rat growth hormone: Physiological effects and adverse consequences. Endocrinology 120: 1963–1975.
- Gupta SS (1966): Experimental studies on pituitary diabetes. Effects of Shilajit, Ficus bengalensis and anterior pituitary extract on glucose tolerance in rats. Ind J Med Res 54: 354–362.
- Gupta SS (1994): Prospects and perspectives of natural plants products in medicine. Indian J Pharmacol 26: 1–12.
- Kokate CK (1994): Phytochemical screening. In: Practical Pharmacognosy. New Delhi, Vallabha Prakshan, pp. 103–107.
- Marx W, Simpson E, Evans HM (1942): Bioassay of the growth hormone of the anterior pituitary. Endocrinology 30: 1–10.
- Mukherjee PK, Saha K, Murugesan T, Mandal SC, Pal M, Saha BP (1998): Screening of anti-diarrhoeal profile of some plant extracts of a specific region of West Bengal, India. J Ethnopharmacol 60: 85–89.
- Neuman RE, Logan MA (1950): The determination of collagen and elastin tissue. Biol Chem 186: 549–552.
- Prajapati ND, Kumar U (2005): Agro’s dictionary of medicinal plants. New Delhi, Agro Publications, p. 35.
- Reiter EO, Rosefeld RG (2003): Normal and aberrant growth. In: Williams’s Textbook of Endocrinology. Elsevier Science, Philadelphia: Saunders, pp. 1003–1079.
- Satapathy KB, Brahmam M (1996): Some medicinal plants used by tribals of Sundargarh District, Orissa, India. In: Ethnobiology in Human Welfare. New Delhi, Deep Publications, pp. 153–158.
- Singh KK, Prakash A (1994): Indigenous phytotherapy among the Gond tribe of Uttar Pradesh. India. Ethnobotany 6: 37–41.
- Sivarajan VV, Balachandran I (1994): Ayurvedic drugs and their plant sources, Delhi, Oxford and IBH Publishing, pp. 333–334.
- Ueno H, Yamaguchi H, Kangauk H, Nakazato M (2005): Ghrelin: A gastric peptide that regulates food intake and energy homeostasis. Regulatory Peptides 126: 11–9.
- Upadhye AS, Vartak VD, Kumbhojkar MS (1994): Ethno-medico-botanical studies in Western Maharashtra, India. Ethnobotany 6: 25–31.
- Varier PS (1996): Indian Medicinal Plants, Compendium of 500 species, Aryavaidya sala, Kottakkal. Hyderabad, Orient longman Limited, pp. 20–26.
- Widmaier EP, Raff H, Strang KT (2004): Endocrine control of growth. In: VanderAJ, Sherman JH, and Luciano DS, Human Physiology - The Mechanisms of Body Function. New York, McGraw-Hill Companies, pp. 366–373.
- Yamanouchi K, Yada E, Hozumi H, Ueno C, Nishihara M (2004): Analyses of hind leg skeletons in human growth hormone transgenic rats. Exp Gerontol 39: 1179–1188.