Abstract
Persea macrantha (Nees) Kosterm (Lauraceae) is a traditional medicinal plant being used in the treatment of asthma and rheumatism. The present study evaluated the anti-inflammatory and anti-arthritic properties of different extracts of stem bark of Persea macrantha in rats. Anti-inflammatory activity was determined in albino rats in a model of acute plantar inflammation induced by carrageenan, while the serial extracts were tested for anti-arthritic activity in adjuvant-induced arthritis in rats. The petroleum ether extract (PE) and aqueous extract (AE) caused a significant inhibition (43.90% and 39.02%, respectively) of the carrageenan-induced paw edema at a dose of 200 mg/kg, when compared with control and reference drug, diclofenac. PE (200 mg/kg) significantly decreased primary lesions (30.95%), secondary lesions (37.14%) and total radiological score (6.33 ± 0.42) in adjuvant induced arthritis. The findings of this experimental animal study indicate anti-inflammatory and anti-arthritic properties of Persea macrantha and thus provide pharmacological support to the traditional use of Persea macrantha in the treatment and management of painful, arthritic inflammatory conditions.
Introduction
Rheumatoid arthritis (RA) is a chronic, destructive inflammatory, polyarticular joint and systemic autoimmune disease. It is characterized by hyperplasia of synovial cells in multiple joints, which ultimately lead to the destruction of cartilage and bone. In particular, this joint degradation has long been explained by the over-production of inflammatory cytokines in synovial tissue, such as IL1, TNF-α and PGE2.
The treatment for pain and swelling in arthritis includes use of analgesics such as acetaminophen and opioids, NSAIDs, and intra-articular therapies such as glucocorticoids. In addition, disease modifying anti-rheumatic drugs (DMARDs) like methotrexate (MTX), sulfasalazine, leflunomide, hydroxychloroquine and newer therapies such as anti-tumor necrosis factor (TNF)-α therapy (etanercept, infliximab, and adalimumab), anti-CD20 therapy (rituximab) are used to modify the clinical and radiological course of RA. However, all of these agents are associated with numerous side effects. Although a number of drugs such as steroidal or non-steroidal anti-inflammatory agents used in the treatment of RA have been developed over the past few decades, there is still an urgent need for more effective drugs with lower side effects (CitationBadger & Lee, 1997). Agents derived from plants can modulate the painful-inflammatory conditions in arthritis. Several preclinical and clinical studies suggest that guggulsterone (guggul), boswellic acid (salai guggul), withanolides (ashwagandha), curcumin (from turmeric), and tea polyphenols are effective in the treatment of arthritis (CitationKhanna et al. 2007).
Persea macrantha (Nees) Kosterm (Lauraceae), also known as Machilus macrantha, has many folk uses in various states of India, the bark is used in asthma and rheumatism (CitationKirtikar & Basu, 1999), while the leaves are used externally to treat ulcers (CitationNadkarni, 2000). In order to establish scientific basis for ethnomedicinal uses of the stem bark of the plant, the present study evaluates its effect in acute inflammation and rheumatoid arthritis.
Materials and methods
Plant material
The fresh bark of Persea macrantha was collected in the month of April (2004) from Lonavala, Dist. Pune (MS), India. It was identified and authenticated by Dr. P.S.N. Rao of Botanical Survey of India, Pune, India. A voucher specimen (b-12) of the bark itself is deposited in the department for future reference.
Preparation of extracts
The bark was shade-dried and then coarsely powdered. The powdered bark (500 g) was successively extracted with petroleum ether, chloroform and methanol using Soxhlet extraction method. Aqueous extract was prepared by cold maceration method. The extracts were then filtered and evaporated in a rotary evaporator under reduced pressure to give petroleum ether extract (PE) (14.1 g), chloroform extract (CE) (12 g), methanol extract (ME) (45.1 g) and aqueous extract (AE) (93 g). The petroleum ether and chloroform extracts were suspended in gum acacia solution whereas methanol and water extracts were prepared in distilled water.
Animals
Inbred albino Wistar rats, 120-150 g in weight, were used for the experiments. The animals were maintained under standard husbandry conditions in the animal house of the institute (temperature 25° ± 2°C) in a natural light-dark cycle and had free access to food and water. All the experiments were approved by the Institutional Animal Ethics Committee (IAEC) and complied with the NIH guidelines on handling of experimental animals.
Toxicity studies
Acute toxicity studies were carried out according to OECD guidelines (Organization for economic co-operation and development). Mortality and general behavior of the animals were observed periodically for 48 h. The animals were observed continuously for the initial 4 h and intermittently for the next 6 h and then again at 24 and 48 h following drug administration. The parameters observed were grooming, hyperactivity, sedation, loss of righting reflex, respiratory rate and convulsions. The dosage of extracts was chosen such that they were 1/10th of their safe dose.
Anti-inflammatory activity
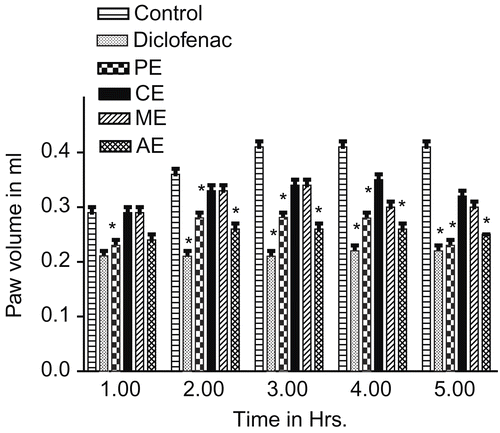
Pedal inflammation in male Wistar rats (120-150 g) was produced according to the method described earlier (CitationWinter et al., 1962). An injection (subcutaneous) was made of 0.1 mL of 1% carrageenan into the right paw of each rat under the subplantar aponeurosis. The test groups of rats were administered orally with 200 mg/kg of the various serial extracts, 1 h before carrageenan injection. At the same time, the control group received 5 mL/kg of 5% gum acacia and the reference group received 13.5 mg/kg diclofenac per oral (p.o.). The paw volume was measured up to 5 h after the injection by dipping the foot in the mercury bath of the plethysmograph-apparatus up to the anatomical hairline on lateral malleolus (CitationGoldenberg & Ilse, 1977) and was compared with the control animals, which received only the vehicle.
Anti-arthritic activity
The anti-arthritic activity was determined by using the method of CitationNewbould (1963). The rats were divided into six groups of six animals each. The animals of all the groups were injected with 0.1 mL of Freund’s complete adjuvant (DIFCO, MI, USA) into the sub-plantar portion of the hind paw. The rats of group I served as control while the rats of group II, III, IV, V and VI were treated with PE, CE, ME, AE (200 mg/kg each) and the reference drug diclofenac (13.5 mg/kg), respectively. The reference drug and serial extracts were administered daily from day 1 to day 12 of the experiment by oral route.
Assessment of primary and secondary lesions
Volumes of both hind paws were recorded on the day of injection. On day 5, the volume of the injected paw was measured again, indicating the primary lesion and the influence of therapeutic agents on this phase. The severity of the induced adjuvant disease was followed by measurement of the non-injected paw (secondary lesions) with a plethysmometer. On day 21, the severity of the secondary lesions was evaluated visually and graded according the scheme mentioned by CitationVogel (2002).
Assessment of radiological parameters
Radiographic evaluation of both injected and non-injected paw was carried out. Radiographs were taken on days 5, 12, 21 and 49 from the injection of the adjuvant with a Wipro GE Dx300 X-ray unit. All radiographs were scored using a previously described scale for the parameters like alignment, soft tissue swelling and periostitis (CitationNoguchi et al., 2005; CitationConway et al., 1995).
Statistical analysis
Data were statistically analyzed by analysis of variance (ANOVA) with the level of significance set at p < 0.05. Critical differences between means were evaluated by Dunnett’s multiple comparison test and Student’s t-test set at p < 0.05.
Results
Toxicity studies
Acute toxicity studies revealed that all extracts were practically non-toxic when administered orally; the LD50 value was higher than 2 g/kg. No mortality or any toxic reactions were found up to the end of the study. Even at this high dose there were no gross behavioral changes.
Anti-inflammatory activity
The anti-inflammatory effect of Persea macrantha against carrageenan-induced inflammation is shown in . PE (43.90%) and AE (39.02%) significantly inhibited the inflammation comparable to the reference drug diclofenac (46.34%). The anti-inflammatory effects of the CE and ME were not significant, when compared with the control. PE exhibited potent anti-inflammatory activity among the four extracts used for the studies.
Anti-arthritic activity
Assessment of primary and secondary lesions
AE, PE and the reference drug diclofenac significantly decreased the inflammation of the injected paw on day 5 with percentage inhibition of 16.66%, 30.95%, and 23.83%, respectively (). CE and ME failed to show any significant inhibition on the same day.
Table 1. Effect of various extracts of P. macrantha stem bark on primary lesions of adjuvant induced arthritis.
The effect of different extracts of Persea macrantha on secondary lesions is shown in . PE significantly reduced the secondary lesions than other three extracts used in the studies. The reference drug diclofenac failed to reduce secondary lesions caused due to injection of the adjuvant.
Table 2. Effect of various extracts of P. macrantha stem bark on secondary lesions of adjuvant induced arthritis in rats.
Assessment of radiological parameters
The total soft tissue radiological score in the arthritic control group was 2.16 ± 0.16 on day 5 () and it was decreased to 0.66 ± 0.21 (69.50%) and 0.83 ± 0.16 (61.57%) in PE and diclofenac treated groups respectively. The radiological score for CE and ME was 1.50 ± 0.22 (30.55%) and that for AE was 1.33 ± 0.21 (38.42%) on day 5.
Figure 2. Anti-arthritic activity of Persea macrantha Nees. in adjuvant induced arthritic rats during different time periods (based on radiological scores). ***p < 0.001, **p < 0.01 and *p < 0.05 versus control.
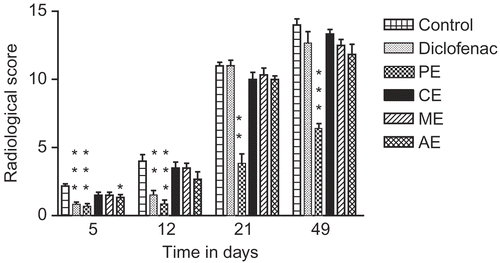
On day 12 soft tissue swelling remained consistent with that on day 5 in arthritic rats. The group treated with PE indicated a decrease in soft tissue swelling compared with that for groups treated with other extracts and the reference drug diclofenac. The rats in the control as well as in other groups showed slight changes in alignment of the bones in the injected and non-injected paw. Radiological score for PE treated group was minimum (0.83 ± 0.31) on day 12.
Radiological score for control group reached to 11.00 ± 0.26 on day 21. Soft tissue swelling of all groups except the PE treated group was increased. The groups treated with CE, ME and AE showed marked deformity in bones of both hind paws.
The PE treated group showed decreased loss of alignment compared with other groups. All groups indicated some degree of periostitic changes on day 21. Periostitis was mainly observed in the injected paw to varying degrees in different groups.
Rats in the control group showed intensely deformed bones and periostitis with a maximum radiological score of 14.00 ± 0.45 on day 49 (). Alignment of bones of both hind paws was considerably disturbed with varying degrees in the control, AE, CE, ME. The diclofenac treated group showed maximum loss of alignment. Intensity of periostitic inflammation and deformity of bones was least in the PE treated group with a minimum radiological score of 6.33 ± 0.42 ().
Thus, the studies of primary and secondary lesions and radiological analysis indicate that petroleum ether extract of Persea macrantha stem bark possesses significant anti-arthritic activity.
Discussion
Sub-plantar injections of carrageenan provoked marked time-related increase in the hind paw diameters of the “untreated” control rats. Maximal swelling and/or edema occurred approximately 90 min following carrageenan administration. It is known that the third phase of the edema induced by carrageenan, in which the edema reaches its highest volume, is characterized by the presence of prostaglandins (PGI2) and other compounds of slow reaction (CitationSpector, 1960). It has been found that the injection of carrageenan into the rat paw induces the liberation of bradykinin, which later induces the biosynthesis of prostaglandin and other autacoids, which are responsible for the formation of the inflammatory exudate (CitationUeno et al., 2000). Besides, in the carrageenan-induced rat paw edema model, the production of prostanoids has been through the serum expression of COX-2 by a positive feedback mechanism (CitationNantel et al., 1999). Therefore, it is suggested that the mechanism of action of PE may be related to prostaglandin synthesis inhibition.
Limb swelling, inflammatory cell filtration, proliferative synovitis and erosion of bone and cartilage structure are clinical findings common to human arthritis and adjuvant-induced arthritis in rats (CitationPearson & Wood, 1959). Due to these similarities in clinical and pathological features, the adjuvant-induced arthritis is a widely used model for rheumatoid arthritis in evaluating different anti-arthritic drugs.
Shortly after administration of adjuvant into one hind paw of the rats, a pronounced swelling appears in the injected paw which persists for days. This is usually considered as a primary lesion. AE and PE significantly inhibited the primary phase of adjuvant-induced arthritis along with the reference drug diclofenac.
A few days after the injection of the adjuvant, the contralateral paw, as well as the front paws, also becomes swollen and arthritic nodules appear on the various body parts such as ears, nose, and tail of the rats. This phase is considered as secondary lesions (delayed systemic response) in adjuvant induced arthritis (CitationSingh & Majumdar, 1996).
Parameters such as soft tissue swelling, alignment of bones and periostitis were considered for radiological analysis. Only petroleum ether extract was found to be effective in reducing radiological score.
From pharmacological investigations it may be concluded that Persea macrantha shows both anti-inflammatory and anti-arthritic effects. It is considered that investigations for these medicinal properties might give scientific authentication to the traditional claims. The preliminary phytochemical investigations of petroleum ether extract indicated presence of steroids. Further studies to isolate and characterize the active phyto-constituents responsible for medicinal properties are in progress as continuing research.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Badger AM, Lee JC (1997): Advances in antiarthritic therapeutics. Drug Discovery Today 2: 427–435.
- Conway JG, Wakefield JA, Brown RH, Marron BE, Sekut L, Stimpson SA, McElroy A, Menius JA, Jeffreys JJ, Clark RL, Gerard M McGeehan, Connollyll KM (1995): Inhibition of cartilage and bone destruction in adjuvant arthritis in the rat by a matrix metalloproteinase inhibitor. J Exp Med 182: 449–457.
- Goldenberg MM, Ilse AC (1977): Anti-inflammatory activity of EU-2972. Arch Int Pharmacodynamics 228: 153–161.
- Khanna D, Sethi G, Ahn KS, Pandey MK, Kunnumakkara A B, Sung B, Aggarwal A, Aggarwal BB (2007): Natural products as a gold mine for arthritis treatment. Curr Opin Pharmacol 7: 344–351.
- Kirtikar KR, Basu BD (1999): Indian Medicinal Plants, Vol. III, Dehradun, International Book Distributors, p. 2155.
- Nadkarni KM (2000). Indian Materia Medica. Vol. I, Mumbai, Popular Prakashan, p. 759.
- Nantel F, Denis D, Gordon R, Northey A, Cirino M., Metters KM, Chan CC (1999): Distribution and regulation of cyclooxygenase-2 in carrageenan-induced inflammation. Braz J Pharmacol 128: 853–859.
- Newbould BB (1963): Chemotherapy of arthritis induced in rats by mycobacterial adjuvant. Brit J Pharmacol 21: 127–136.
- Noguchi M, Kimoto A, Kobayashi S, Yoshino T, Miyata K, Sasamata M (2005): Effect of celecoxib, a cyclooxygenase-2-inhibitor, on pathophysiology of adjuvant induced arthritis in rat. Eur J Pharmacol 513: 229–235.
- OECD (Organization of Economic Co-operation Development) (2001): The OECD Guideline for Testing of Chemical: 420 Acute Oral Toxicity. OECD, Paris, pp. 1–14.
- Pearson CM, Wood FD (1959): Studies on polyarthritis and other lesions induced in rats by the injection of mycobacterial adjuvant: I. General clinical and pathological characteristics and some modifying factors. Arth Rheum 2: 440–459.
- Singh S, Majumdar DK (1996): Effect of fixed oil of Ocimum sanctum against experimentally induced arthritis and joint edema in laboratory animals. Int J Pharmacog 34: 218–222.
- Spector WG (1960): The inflammatory response. J Path Bact 84: 391–403.
- Ueno A, Naraba H, Ikeda Y, Ushikubi F, Murata T, Naramiya S, Ohishi S (2000): Intrinsic prostacyclin contributes to exudation induced by bradikinin or carrageenan: A study on the paw edema induced in ip-receptor-deficient mice. Life Science 66: 155–160.
- Vogel GH (2002): Drug Discovery and Evaluation: Pharmacological Assays, Berlin, Heidelberg, New York, Springer, pp. 696, 802–803.
- Winter CA, Risley EA, Nuss GW (1962): Carrageenan-induced edema in hind paw of the rats as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 111: 544–554.