Abstract
From the fruits of Ferula badrakema (Kos.-Pol.) (Umbelliferae), five known sesquiterpene coumarins (conferone, mogoltacin, feselol, ferocaulidin and ligupersin A) were isolated for the first time, using silica gel column chromatography and preparative thin-layer chromatography. The structures were characterized by 1D and 2D NMR experiments.
Introduction
The exclusively old world genus Ferula (Umbelliferae), with about 130 species, is distributed throughout the Mediterranean area and central Asia, especially in the former USSR and neighboring countries such as Iran. This genus is well documented as a good source of biologically active compounds such as sesquiterpene derivatives (CitationAhmed et al., 2001; CitationAhmed, 1999; CitationValle et al., 1987). Ferula badrakema (Kos.-Pol.) (CitationRechinger et al., 1994), similar to other species of the genus Ferula, is a rich source of sesquiterpene coumarins (CitationBukreeva & Pimenov, 1991). Ferula badrakema (Syn. = F. gumosa) is a resinous plant and has a strong odor. To our knowledge, no study has been done on the chemistry of the fruits of this species. Previously, some sesquiterpene coumarins have been isolated from the roots of the plant (CitationKir’yalov, 1967; CitationBukreeva & Pimenov, 1991). In the present study, we report the isolation and the structure elucidation of five sesquiterpene coumarins from Ferula badrakema fruits for the first time (1-5).
Materials and methods
Plant material
Fruits of Ferula badrakema were collected in Hezarmasjed Mountains, north east of Iran, in August 2005, and identified by Mohammadreza Joharchi, Ferdowsi University of Mashhad Herbarium (FUMH). A voucher specimen (1002) is deposited at the Herbarium of the Department of Pharmacognosy, Faculty of Pharmacy, Mashhad University of Medical Sciences.
General experimental procedures
The 1H, gCOSY, ROESY, gHSQC, gHMBC NMR experiments were run under standard conditions on a Bruker DRX-600 spectrometer at 300 K. NMR samples were prepared by dissolving each sample in CDCl3 (99.8% D) (Carlo Erba). The spectra were calibrated using the solvent signal as internal standard (1H, δ: 7.27 ppm; 13C, δ 77.0 ppm). The ROESY spectra were executed with a mixing time of 400 ms. The NMR data were processed on a Silicon Graphic Indigo2 Workstation using UXNMR software. Column chromatography was conducted with silica gel 230–400 mesh (Merck). Preparative TLC was performed on Silica gel 60 GF254 plates (Merck) and observation of plates was carried out under UV CAMAG spectrometer (254 nm).
Extraction and isolation
The air-dried fruits (500 g) were ground into powder, defatted by petroleum ether and extracted exhaustively by maceration with dichloromethane at room temperature. After filtration, the extract was concentrated under vacuum to yield 20 g of a brown residue.
Part of the extract (15 g) was subjected to column chromatography on silica gel (5 × 50 cm) using petroleum ether with increasing volumes of acetone (petrol-Me2CO (20:1), (15:1), (10:1), (9:1), (8:1), (7:1), (6:1), (5:1), (4:1), (3:1), (2:1), (1:1) and (0:1)). The fractions were compared by TLC (Silica gel using petrol-Me2CO as solvent), and those giving similar spots were combined. Five fractions were finally obtained. Fractions 1-3 afforded compounds 1 (15 mg), 2 (770 mg) and 3 (48.5 mg) as white crystals, respectively. Fractions 4 and 5 were subjected to silica gel PTLC (petrol-Me2CO, 2:1) to give compounds 4 (11.2 mg) and 5 (54.5 mg), respectively.
Results and discussion
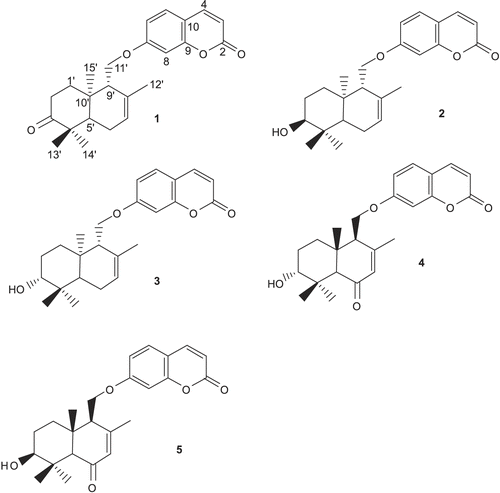
Normal-phase column chromatography of the dichloromethane extract of fruits of F. badrakema, followed by preparative TLC, afforded five sesquiterpene coumarins including conferone (1), mogoltacin (2), feselol (3), ferocaulidin (4), and ligupersin A (5). The isolated coumarins () were identified by comparison of their NMR with those previously described in the literature (CitationVandyshev et al., 1972; CitationNabiev et al., 1978; CitationKuliev et al., 1980; CitationMurray et al., 1982; CitationLee & Mabry, 1985; CitationAbd El-Razek et al., 2003). This is the first report of sesquiterpene coumarins from F. badrakema fruits.
The structure of compound 1 was established from analysis of the 1H and 13C NMR spectra ( and ). Compound 1 displayed 24 carbon signals, 9 being typical of an umbelliferone skeleton and the other 15 signals were ascribable to a sesquiterpene moiety. The downfield signal at δC 161.5 was assigned to the carbonyl carbon of the coumarin moiety, whereas the downfield signal at δC 216.4 was indicative of a ketone group belonging to the sesquiterpene unit. HSQC spectrum classified the carbon signals to four aliphatic methylenes at δC 38.8 (C-1′), 34.8 (C-2′), 24.3 (C-6′), and a primary alcoholic carbon at δC 67.0 characteristic for C-11′, to nine methines, five of them for umbelliferone moiety at δC 113.4 (C-3), 143.7 (C-4), 129.2 (C-5), 113.6 (C-6) and 101.7 (C-8) and to four methyls at δC 25.6 (C-12′), 22.7 (C-13′), 25.6 (C-14′), and 14.9 (C-15′). The 1H NMR spectrum of compound 1 showed resonances characteristic for four methyl singlets at δH 1.75 (H-12′),1.17 (H-13′),1.13 (H-14′) and 1.18 (H-15′), and three olefinic resonances at δH 6.28 (H-3), 7.66 (H-4) and 5.62 (H-7′). Three aromatic protons at δH 7.39 (H-5), 6.86 (H-6) and 6.85 (H-8) suggested the presence of a 7, 9, 10-trisubstituted benzene ring, which was supported by the 13C NMR spectrum.
Table 1. 1H-NMR data obtained with compounds 1-5 (600 MHz, δ ppm).
Table 2. 13C-NMR data obtained with compounds 1-5 (125.7 MHz, δ ppm).
The NMR spectral data of compound 2 were similar to those of compound 1, except a slight difference in the signals assigned to the sesquiterpene unit which was due to the replacement of the ketone group at C-3′ with an oxymethine. Regarding this portion and with respect to conferone, HMBC spectrum showed the correlations of the secondary alcoholic H-3′ (δH 3.52) to δC 25.2 (C-2′), δC 37.6 (C-4′), and to δC 43.8 (C-5′). Other NMR spectral data of compound 2 were closely comparable to those of compound 1. Hence, the structure of compound 2 was determined as mogoltacin (or conferol).
The NMR spectral data of compound 3 were very similar to those of compound 2, except for the stereochemistry of C-3′. Thus, the differences between the 1H NMR of compounds 2 and 3 appeared in the signals due to protons on C-2′, C-3′ and methyl groups of 13′ and 14′. In compound 3, the signals for the H-2′ and H-3′ appeared at δH 1.67 and δH 3.31, respectively, and those for the methyl groups of 13′ and 14′ at δH 1.05 and 0.93, respectively. In compound 2, the corresponding signals appeared at δH 1.69 (H-2′), 3.52 (H-3′), 1.02 (H-13′) and 0.97 (H-14′). ROESY experiments confirmed the stereochemistry of 39-OH as β and α conformers for compounds 2 and 3, respectively. Therefore, compound 3 was determined as feselol.
13C NMR spectrum of compound 4 showed the presence of a ketone group at δC 199.8. In the HMBC spectrum, correlations between H-5′ and H-7′ with C-6′, confirmed the location of carbonyl group at C-6′. On the other hand, no resonances were observed for the protons of C-6′ in the 1H NMR spectrum of compound 4. 1H and 13C NMR spectral data of compound 4 were similar to those of compound 3, except for the C-6′ and chemical shifts of protons and carbons adjacent to C-6′ ( and ). Compound 4 was assigned to be ferocaulidin according to the mentioned references.
The only difference between structures of compounds 4 and 5 was in the stereochemistry of 39-OH. In compound 5, the stereochemistry of 3′-OH was determined by ROESY experiment. In ROESY spectrum of compound 5, a cross-peak between H-3′ and H-5′, indicated the conformation of 3′-OH as β, while in compound 4, a cross-peak between H-3′ and Me-15′ was observed, indicating α conformer of H-3′. Compound 5 was determined as the known compound, ligupersin A.
Acknowledgement
This research was supported by a grant from Mashhad University of Medical Sciences Research Council.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abd El-Razek MH, Ohta S, Hirata T (2003): Terpenoid coumarins of the genus Ferula. Heterocycles 60: 689–716.
- Ahmed AA (1999): Sesquiterpene coumarins and sesquiterpenes from Ferula sinaica. Phytochemistry 50: 109–112.
- Ahmed AA, Abd El-Razek MH, Nassar MI, Izuma S, Ohta S, Hirata T (2001): Sesquiterpene coumarins from the roots of Ferula assa-foetida. Phytochemistry 58: 1289–1295.
- Bukreeva TV, Pimenov MG (1991): Coumarins from the roots of Ferula badrakema. Khim Prir Soedin 27: 718.
- Kir’yalov NP (1967): The coumarin, badrakemin from the roots of Ferula badrakema. Khim Prir Soedin 3: 363.
- Kuliev ZA, Khasanov TK, Malikov VM (1980): Terpenoid coumarin glycosides of Ferula conocaula. Chem Nat Comp 15: 414–416.
- Lee E, Mabry TJ (1985): Sesquiterpene coumarin ethers of Ferula tingitana. J Nat Prod 48: 326–327.
- Murray RDH, Mendez J, Brown SA (1982): The Natural Coumarins. New York, John Wiley, 555, 559.
- Nabiev AA, Khasanov TK, Melibaev S (1978): Coumarins of Ferula diversivittata. Chem Nat Comp 14: 441.
- Rechinger KH, Lemond JM, Hedge IC (1994): Flora Iranica (Umbelliferae), Vol. 162. Graz, Austria, Akademischev Druk-u. Verlagsanstalt, p. 411.
- Valle GM, Appendino G, Nano GM, Picci V (1987): Prenylated coumarins and sesquiterpenoids from Ferula communis. Phytochemistry 26: 253–258.
- Vandyshev VV, Sklyar YE, Perel’son ME, Moroz MD, Pimenov MG (1979): Terpenoid coumarin glycosides of Ferula conocaula. Chem Nat Comp 15: 414–416.