Abstract
Herb formula FBD is composed of fu ling [or poria, or the sclerotium of the fungus of Poria cocos (Schw.) Wolf (Polyporaceae)], bai zhu [white atractylodes rhizome, or the rhizome of Atractylodes macrocephala Koidz.(Compositae)], and dang gui [or dong quai, Chinese angelica root, or the root of Angelica sinensis (Oliv.) Diels (Umbelliferae)], the three Chinese medicinal plants used traditionally to improve cognitive disorders. The present study aimed to observe the effects of dual doses of aqueous extracts of FBD (200 and 600 mg/kg) administered orally for 6 days on memory deficit induced by scopolamine, 4 mg/kg intraperitoneally (i.p.) in male imprinting control region (ICR) mice. Aqueous extracts of FBD markedly ameliorated scopolamine induced memory deficit in passive step-through avoidance test and passage water maze test, significantly inhibited acetylcholinesterase (AChE) activity in cortex and hippocampus, and circulating butyrylcholinesterase activity. Moreover, cortical AChE activity in vitro was suppressed in a dose-dependent manner by aqueous extracts of FBD at 50–200 μg crude herb/mL, in which dang gui extract exerted a stronger anti-AChE activity than that of fu ling or bai zhu. In contrast, no acute toxicity was observed within 14 days, after administration with FBD extracts at the maximal tolerable dose of 69 g/kg. These results suggested that FBD might be a potential and safe herbal agent for Alzheimer’s disease, and its nootropic action was mediated, in part, via enhancing the cholinergic nervous system.
Introduction
Alzheimer’s disease (AD) is the principal type of dementia among the elderly. In AD patients, there is a decreased level of acetylcholine (ACh) in some brain regions, such as the cerebral cortex and hippocampus, correlated critically to learning and memory (CitationDavies & Maloney, 1976). Based on the cholinergic hypothesis that cognitive impairments in AD patients result from a defect in the cholinergic system, an important approach to treat AD is to enhance ACh level in brain by inhibiting acetylcholinesterase (AChE) (CitationLeonard, 2004). AChE inhibitors have been widely used for the treatment of AD, such as donepezil (CitationLahiri et al., 2002). However, their applications were limited owing to the short half-life and peripheral adverse effects, as well as hepatotoxicity (CitationFarlow et al., 1992; CitationKnapp et al., 1994; CitationRogers et al., 1998). Recently, natural botanical AChE inhibitors indicated potential as alternative and safe therapies for AD (CitationChattipakorn et al., 2007; CitationKim et al., 2007; CitationRubio et al., 2007).
As documented in Pharmacopeia of the People’s Republic of China, fu ling [Poria, or the sclerotium of the fungus of Poria cocos (Schw.) Wolf (Polyporaceae)], bai zhu [white atractylodes rhizome, or the rhizome of Atractylodes macrocephala Koidz.(Compositae)], and dang gui [Chinese angelica root, or the root of Angelica sinensis (Oliv.) Diels (Umbelliferae)] are three essential Chinese medicinal plants that are ordinarily used to modulate psychoneural, gastrointestinal, and gynecological disorders, respectively (CitationAdams et al., 2007). Recently, several traditional formulae comprising the three herbs have been successfully applied to treat AD, such as yi-gan-san (yokukan-san) (CitationIwasaki et al., 2005; CitationShinno et al., 2007), and danggui-shaoyao-san (toki- shakuyaku-san) (CitationLu, 2001; CitationToriizuka et al., 2000). Moreover, the three herbs were beneficial to cognitive function, whether used alone (CitationHsieh et al., 2000; CitationSmriga et al., 1995; CitationOh et al., 2004; CitationHatip-Al-Khatib et al., 2004), or in combination (fu ling (F) - bai zhu (B) - dang gui (D), FBD) (CitationLin et al., 2005; CitationDong, et al., 2006). All these data suggest that FBD has a clear potential for AD treatment.
However, it remained unclear whether FBD is targeting AChE to prevent memory deficits. In this study, we examined the effect of aqueous extracts of FBD on scopolamine induced memory disruption in ICR mice, and its anti-AChE activity in vivo and in vitro, to elucidate its underlying anti-AD pathway. Furthermore, we preliminarily evaluated its acute toxicity for its potential therapeutic applications.
Materials and methods
Preparation of aqueous extracts of FBD
Three herbal materials used in this work were purchased from Nanjing herbal materials company (Nanjing, China). The three voucher specimens, CPU 000701, 000702, 000703, were conserved at the herbarium, and authenticated by Boyang Yu, Department of Pharmacognosy, China Pharmaceutical University, Nanjing. Clinically, a single formula of FBD consisted of 10 g poria, 5 g white atractylodes rhizome and 3 g Chinese angelica root. Aqueous extract powders of FBD and F, B, and D were prepared as previously, with yields 20%, 1.5%, 40%, and 30.0%, respectively (w/w) (CitationLin et al., 2005).
Reagents and chemicals
Scopolamine and donepezil were purchased from Sigma Chemical Co. (St. Louis, MO). Commercial kits for determinations of protein, AChE, and butyrylcholinesterase (BChE) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Group and protocol
Male ICR mice were provided by Shanghai Institute of Pharmaceutical Industry (SIPI), Shanghai, China, and randomized into five groups. Saline or drugs were administered daily for 6 consecutive days. Group a: normal control group, receiving saline ( 20 ml/kg, per oral (p.o) p.o./i.p.); group b: saline treated group, receiving saline ( 20 ml/kg, p.o.) and scopolamine ( 4 mg/kg, i.p.); group c: FBD treated groups, receiving FBD aqueous extracts (200, 600 mg/kg, p.o.) and scopolamine ( 4 mg/kg, i.p.); group d: donepezil treated group, receiving donepezil ( 4 mg/kg, p.o.) and scopolamine ( 4 mg/kg, i.p.). The animal handling procedures were in compliance with the China National Institutes of Healthy Guidelines for the Care and Use of Laboratory Animals.
Passive avoidance step-through test
A passive avoidance reflex apparatus (National Academy of Traditional Chinese Medicine (TCM), Beijing, China) was separated into light and dark chambers with a connecting hole and copper grids on the floor. On day 5, ICR mice were placed into the light chamber. If they stepped through the hole into the dark chamber they would suffer 30 V-electric stimulation and escape out of the dark chamber as trained. Mice were placed 24 h later into the light chamber again, the latency to firstly enter the dark chamber and the error numbers of entering the dark chamber within 5 min were recorded (CitationLin et al., 2005).
Passage water maze test
The passage water maze apparatus was a double-layer opaque plastic box ( 80 cm × 50 cm × 20 cm) including three start points, a terminal platform and four non-exits (National Academy of TCM, Beijing, China). The maze was filled with water to a depth of 12 cm and the temperature was kept at 25 ± 1°C. ICR mice were placed on the terminal platform for 20 s before placing into the water. Each mouse was trained daily to find the terminal platform within 3 min for 2 days. There were two non-exits on day 1, three on day 2 and four on days 3–5. If mice arrived at the terminal platform within 2 min, they were allowed to remain there for 20 s. Mice were injected with scopolamine (4 mg/kg, i.p.) or saline (20 mL/kg, i.p.) 1 h after the sixth administration. The swimming time to find the terminal platform, and the error numbers of entering non-exits within 2 min, were recorded 30 min later (CitationZhang et al., 2006).
AChE and BChE assay in vivo
Another batch of mice was injected with identical scopolamine or saline 1 h after the sixth administration. Blood from the mice was rapidly collected 30 min later, to separate serum for BChE assay. Mice were sacrificed rapidly by cervical dislocation. Cortical cortexes and hippocampus were dissected out, rinsed and homogenized with ice-cold 0.85% saline (containing 0.1% triton), centrifuged at 3000 g for 15 min to obtain supernatant for AChE assay in compliance with specification of kits (CitationRubio et al., 2007). Protein concentrations were determined by the Lowry method (CitationLowry et al., 1951), using bovine serum albumin as a standard. All samples were run in parallel to avoid between-assay variation.
AChE assay in vitro
The homogenates of cortical cortexes and hippocampus from normal littermates were prepared in buffer (100 mM sodium phosphate buffer, pH 8), and used as source of enzyme for AChE assay in vitro (CitationKim et al., 2007). FBD or F/B/D aqueous extracts were initially dissolved in buffer and diluted to various concentrations (0–200 μg/mL, shown by crude herb) immediately before use. An aliquot of diluted FBD, or F/B/D solution (10 μL) was then incubated with 90 μL brain supernatant in triplicate for 1 h at 4°C, with donepezil ( 1 nM-10 μM) as a positive control. AChE activity in supernatant was detected as mentioned.
Acute toxic test
After fasting for 4 h, 34.5 g/kg aqueous extracts of FBD were administrated orally twice to 20 ICR mice (half of sex) at an interval of 4 h, with administration volume 30 mL/kg. Body weight, toxic actions and death were recorded for 14 consecutive days.
Statistical analysis
Statistical software SPSS 12.0 (SPSS, Chicago, IL) and Origin 7.0 (OriginLab, Northampton, MA) were applied to analyze experimental data, and results were expressed as means ± SD, with time data (latency or swimming time) transformed into logarithm. All data were evaluated with ANOVA followed by two-sided Dunnett’s t-test for multiple comparisons with p <0.05 indicating significant statistical difference.
Results
Nootropic action of FBD in vivo
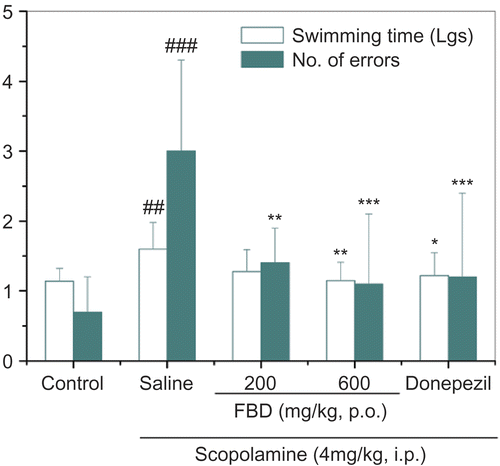
In the step-through test, one-way ANOVA showed an overall significant effect of drug pretreatment on latency and the number of errors - F (4, 45) = 7.047, p <0.001; F (4, 45) = 8.046, p <0.001, respectively. In comparison with the saline treated group, aqueous extracts of FBD prolonged the latency by 36.4-54.5% ( 200 mg/kg, p >0.05; 600 mg/kg, p <0.05), and curtailed errors by 43.5-65.2% ( 200 mg/kg, p <0.01; 600 mg/kg, p <0.01). At 600 mg/kg, FBD exerted a stronger nootropic action than that of donepezil ().
Figure 1. Effects of aqueous extracts of FBD on memory dysfunction in mice induced by scopolamine in step-through passive avoidance test. Data represent means ± SD of 10 mice per group. ##p < 0.01 compared with control group; *p < 0.05, **p < 0.01 compared with saline-treated group.
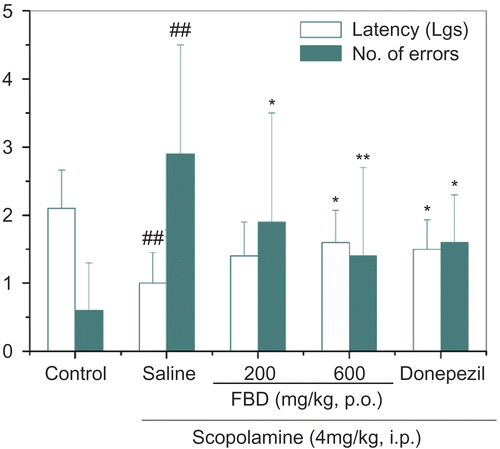
Likewise, significant differences among groups were observed in the water maze test, as for both swimming time, F (4, 45) = 4.014, p <0.01, and error numbers, F (4, 45) = 9.081, p <0.001. Compared with the saline treated group, errors rather than swimming time were lowered by FBD extracts at 200 mg/kg (p <0.001 and p >0.05), while FBD extracts ( 600 mg/kg, p <0.001 and p <0.01) and donepezil ( 4 mg/kg, p <0.001 and p <0.05) markedly cut down both, protected against scopolamine induced spatial memory disruption ().
Anti-AChE and anti-BChE activity of FBD in vivo
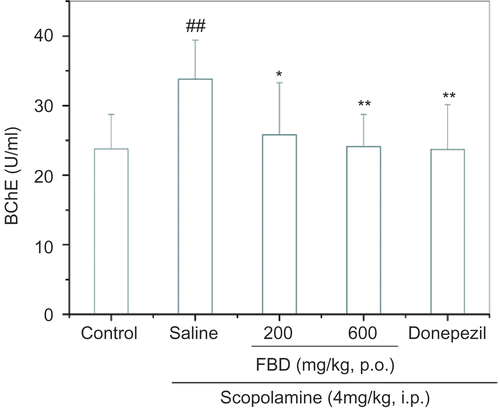
The overall analysis revealed significant differences among groups in either cortical AChE activity, F (4, 45) = 7.453, p <0.001, or in circulating BChE activity, F (4, 45) = 5.313, p <0.001. Scopolamine injection markedly elevated AChE activity from 0.1 ± 0.1 U/mg to 0.2 ± 0.1 U/mg (p <0.001) and BChE activity from 23.8 ± 4.9 U/mL to 33.8 ± 5.6 U/mL (p <0.01). Dual doses of FBD extracts ( 200 mg/kg, p <0.01; 600 mg/kg, p <0.001) significantly inhibited AChE by 71.4–85.7%, in analog to donepezil ( 4 mg/kg, p <0.001, ). Meanwhile, rise in circulating BChE activity was also significantly restrained by two doses of FBD extracts ( 200 mg/kg, p <0.05; 600 mg/kg, p <0.01) or donepezil (p <0.01, ).
Figure 3. lnhibitory activities of FBD aqueous extracts on cortical and hippocampal acetylcholinesterase activity and circulating butyrylcholinesterase activity in mice treated with scopolamine in vivo. AChE, acetylcholinesterase; Data represent means ± SD of 10 mice per group. ###p <0.001 compared with control group; **p < 0.01 compared with saline-treated group.
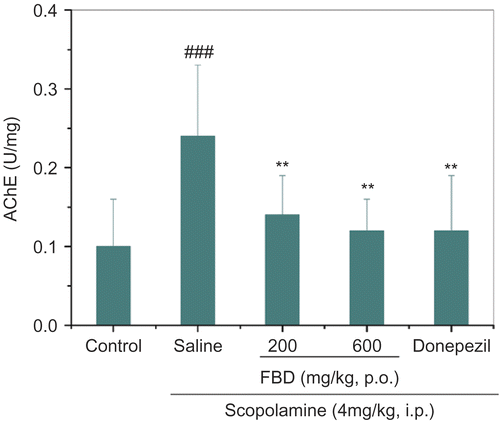
Figure 4. lnhibitory activities of FBD aqueous extracts on circulating butyrylcholinesterase activity in mice treated with scopolamine in vivo. BChE, butyrylcholinesterase. Data represent means ± SD of 10 mice per group. ##p <0.01 compared with control group; *p < 0.05, **p < 0.01 compared with saline-treated group.
Anti-AChE activity of FBD in vitro
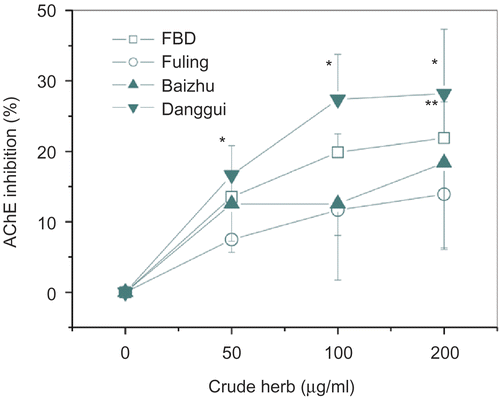
Aqueous extracts of FBD and F, B, and D were found to inhibit AChE activity in a dose-dependent manner, within 0-200 μg crude herb/mL. Of the three herbs, the aqueous extract of dang gui exerted a stronger inhibition on AChE activity than that of fu ling or bai zhu. At 200 μg crude herb/mL (or 60 μg extract/mL), dang gui extract inhibited obviously AChE activity by 28.2% (p <0.05), whereas fu ling extract (3 μg extract/mL) or bai zhu extract (80 μg extract/mL) attained only inhibitions 13.9 and 18.4%, respectively (). The IC50 value of donepezil was 1.5 μM (0.62 μg/mL).
Figure 5. Inhibitory activities of FBD or F/B/D aqueous extracts on acetylcholinesterase activity in cortical and hippocampal homogenates in vitro. Inhibitory activities were expressed as percentage inhibition of AChE activity compared to the control value (100%). AChE, acetylcholinesterase; F, Fuling; B, Baizhu; D, Danggui. Data represent means ± SD in triplicate. *p < 0.05, **p < 0.01 compared with the control.
Acute toxicity of FBD
No reduced locomotor activity or feed intake was found in either female or male ICR mice following acute administration of 2 FBD extracts, and no mice died during the period of observation. The maximal tolerable dose was 69 g/kg, equal to 1500-fold of the clinical dose (46 mg/kg).
Discussion
Traditional Chinese medicine (TCM) has a long history of clinical application, and has become a vital complementary AD therapy (CitationIwasaki et al., 2005; CitationShinno et al., 2007; CitationLu, 2001; CitationToriizuka et al., 2000; CitationZhu & Hu, 2007; CitationHowes & Houghton, 2003). Our present study further demonstrates the protective potential of the TCM formula FBD against scopolamine induced memory disruption in mice. Its nootropic action appears at least in part to inhibit AChE.
It is well documented that scopolamine interferes with cognitive function in humans and experimental animals by blocking muscarinic receptors (CitationKopelman & Corn, 1988). Our results indicated that pretreatment with FBD extracts offered protection against scopolamine induced learning acquisition deficit and spatial memory deficit in ICR mice, because FBD extracts elongated latency and reduced the errors in the step-through test, as well as shortened escape latency and lessened the errors in the water maze test ( and ), in harmony with other studies (CitationBejar et al., 1999; Citationde Angelis & Furlan, 1995).
Further in vivo and in vitro studies testified that the nootropic action of FBD resulted in part from its anti-AChE activity. To our knowledge, ACh is a crucial neurotransmitter involved in learning and memory. Clinically, augmented AChE activity degraded ACh, resulting in ACh shortage and cognitive deficit in patients with AD (CitationDavies & Maloney, 1976). FBD extracts cut down the increased hippocampal AChE activity for over 70% of scopolamine treated mice (), was consistent with our previous findings that FBD prevented cognitive disruption, and inhibited cortical AChE activity in mice chronically exposed to d-galactose (CitationLin et al., 2002). FBD might be an unselective anti-AChE agent, because it also had obvious influence on BChE (). Although the potential differs from donepezil, FBD extracts inhibited AChE activity in vitro, where activity appeared most dominant in the dang gui extracts ().
The compounds in FBD, especially dang gui, pertinent to its anti-AChE activity have not been elucidated. However, it was reported that dang gui antagonized scopolamine induced memory deficit, due to its active constituents N-methyl-beta-carboline-3-carboxamide and amines from butanol fraction and n-hexane fraction (CitationHsieh et al., 2000; CitationHatip-Al-Khatib et al., 2004), and Z-Ligustilide from essential oil (CitationKuang et al., 2008). In addition, 27.8% inhibition on AChE at 200 μg/mL was observed by methanol extract from fu ling, suggesting that multiple components were attributed to the whole actions of the herbal formula (CitationSmriga et al., 1995; CitationOh et al., 2004).
For thousands of years, the three herbs of FBD have been applied widely as medicinal plants, especially in Asia, since their clinical uses were first recorded in the Shen Nong Ben Cao Jing during the first century AD. Up to now no obvious toxic report was available for the three herbs. In the present acute toxicology evaluation, the maximal tolerable dose of FBD was equal to 1500 times the clinical dose, and no abnormal physical appearance, body weight and feed intake, were observed, so FBD was safe to some extent, and reasonable for long-term traditional folk application in the past.
In summary, the work demonstrated that FBD may improve scopolamine induced memory deficit, by enhancing the cholinergic system. Preliminary evidence on safety pertinent to FBD was also offered in an acute toxicity test. It had an implication in the pharmaceutical industry for use as a natural therapy against AD.
Acknowledgements
We thank Min Chen (Ph.D.) from the Department of Pharmacology and Pharmaceutical Sciences, University of Southern California, and Domenic Caravetta (Ph.D.) from Unilever Research, China, for reviewing the manuscript. This work was supported by the research program of the National New Drug Foundation of China (969010538), and the general programs of the National Natural Science Foundation of China (30271604 & 30500683).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adams M, Gmünder F, Hamburger M (2007): Plants traditionally used in age related brain disorders – A survey of ethnobotanical literature. J Ethnopharmacol 113: 363–381.
- Bejar C, Wang R, Weinstock M (1999): Effect of rivastigmine on scopolamine-induced memory impairment in rats. Eur J Pharmacol 383: 231–240.
- Chattipakorn S, Pongpanparadorn A, Pratchayasakul W, Pongchaidacha A, Ingkaninan K, Chattipakorn N (2007): Tabernaemontana divaricata extract inhibits neuronal acetylcholinesterase activity in rats. J Ethnopharmacol 110: 61–68.
- Davies P, Maloney AJF (1976): Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 2: 1403.
- de Angelis L, Furlan C (1995): The effects of ascorbic acid and oxiracetam on scopolamine- induced amnesia in a habituation test in aged mice. Behav Brain Res 64: 119–124.
- Dong WX, Ruan KF, Gu FH, Shen PN, Li PY, Lin ZH (2006): FBD used to improve learning and memory function of animals. Zhong Cao Yao 37: 1831–1835.
- Farlow M, Gracon SI, Hershey LA, Lewis KW, Sadowsky CH, Dolan-Ureno J (1992): A controlled trial of tacrine in Alzheimer’s disease The Tacrine Study Group. JAMA 268: 2523–2529.
- Hatip-Al-Khatib I, Egashira N, Mishima K, Iwasaki K, Iwasaki K., Kurauchi K, Inui K, Ikeda T, Fujiwara M (2004): Determination of the effectiveness of components of the herbal medicine Toki- Shakuyaku-San and fractions of Angelica acutiloba in improving the scopolamine-induced impairment of rat’s spatial cognition in eight-armed radial maze test. J Pharmacol Sci 96: 33–41.
- Howes MJ, Houghton PJ (2003): Plants used in Chinese and Indian traditional medicine for improvement of memory and cognitive function. Pharmacol Biochem Behav 75: 513–527.
- Hsieh MT, Lin YT, Lin YH, Wu CR (2000): Radix Angelica Sinensis extracts ameliorate scopolamine- and cycloheximide-induced amnesia but not p-chloroamphetamine-induced amnesia in rats. Am J Chin Med 28: 263–272.
- Iwasaki K, Satoh-Nakagawa T, Maruyama M, Monma Y, Nemoto M, Tomita N, Tanji H, Fujiwara H, Seki T, Fujii M, Arai H, Sasaki H (2005): A randomized observer-blind controlled trial of the traditional Chinese medicine Yi-Gan San for improvement of behavioral and psychological symptoms and activities of daily living in dementia patients. J Clin Psychiatry 66(2): 248–252.
- Kim DH, Kim DY, Kim YC, Jung JW, Lee S, Yoon BH, Cheong JH, Kim YS, Kang SS, Ko KH, Ryu JH (2007): Odakenin, a coumarin compound ameliorates scopolamine-induced memory disruption in mice. Life Sci 80: 1944–1950.
- Knapp MJ, Knopman DS, Solomon PR, Pendlebury WW, Davis CS, Gracon SL (1994): A 30-week randomized controlled trial of highdose tacrine in patients with Alzheimer’s disease. JAMA 271: 985–991.
- Kopelman MD, Corn TH (1988): Cholinergic blockade as a model of cholinergic depletion. Brain 111: 1079–1110.
- Kuang X, Du JR, Liu YX, Zhang GY, Peng HY (2008): Postischemic administration of Z-Ligustilide ameliorates cognitive dysfunction and brain damage induced by permanent forebrain ischemia in rats. Pharmacol Biochem Behav 88: 213–221.
- Lahiri DK, Farlow MR, Greig NH, Sambamurti K (2002): Current drug targets for Alzheimer’s disease treatment. Drug Dev Res 56: 267–281.
- Leonard BE (2004): Pharmacotherapy in the treatment of Alzheimer’s disease: An update. World Psychiatry 3(2): 84–88.
- Lin ZH, Zhu DN, Yan YQ, Yu BY (2002): Study II on substance basis and mechanism of action of Danggui Shaoyao San in the prevention and treatment of senile dementia-similar efficacy in the prescription. Chin J Exp Traditional Med Formula 8(4): 19–21.
- Lin ZH, Zhu DN, Yan YQ, Yu BY, Wang QJ (2005): Protective effects of FBD-an experimental Chinese traditional medicinal formula on memory dysfunction in mice induced by cerebral ischemia-reperfusion. J Ethnopharmacol 97: 477–483.
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951): Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275.
- Lu MC (2001): Danggui Shaoyao San improve colchichine-induced learning acquisition impairment in rats. Acta Pharmacol Sin 22: 1149–1153.
- Oh MH, Houghton PJ, Whang WK, Cho JH (2004): Screening of Korean herbal medicines used to improve cognitive function for anti-cholinesterase activity. Phytomedicine 11: 544–548.
- Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT (1998): A 24-week double-blind placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Neurology 50: 136–145.
- Rubio J, Dang H, Gong M, Liu X, Chen S, Gonzales GF (2007): Aqueous and hydroalcoholic extracts of Black Maca (Lepidium meyenii) improve scopolamine-induced memory impairment in mice. Food Chem Toxicol 45: 1882–1890.
- Shinno H, Utani E, Okazaki S, Kawamukai T, Yasuda H, Inagaki T, Inami Y, Horiguchi J (2007): Successful treatment with Yi-Gan San for psychosis and sleep disturbance in a patient with dementia with Lewy bodies. Prog Neuropsychopharmacol Biol Psychiatry 31: 1543–1545.
- Smriga M, Saito H, Nishiyama N (1995): Hoelen (Poria cocos Wolf) and ginseng (Panax Ginseng C A Meyer) the ingredients of a Chinese prescription DX-9386 individually promote hippocampal long-term potentiation in vivo. Biol Pharm Bull 18: 518–522.
- Toriizuka K, Hou PH, Yabe T, Iijima K, Hanawa T, Cyong JC (2000): Effects of Kampo medicine Toki- shakuyaku-san (Tang-Kuei- Shao-Yao-San) on choline acetyltransferase activity and norepinephrine contents in brain regions and mitogenic activity of splenic lymphocytes in ovariectomized mice. J Ethnopharmacol 71: 133–143.
- Zhang D, Liu GT, Shi JG, Zhang JJ (2006): Coeloglossum viride var bracteatum extract attenuates d-galactose and NaNO2 induced memory impairment in mice. J Ethnopharmacol 104: 250–256.
- Zhu W, Hu H (2007): A survey of TCM treatment for Alzheimer’s disease. J Tradit Chin Med 27: 226–232.