Abstract
Five known compounds were isolated from the aerial parts of Colliguaja integerrima Gillies & Hook (Euphorbiaceae). A pharmacological evaluation of the diuretic activity of the plant was carried out in isotonic saline-loaded rats. The data reported in the present work indicate that the aqueous extract of the aerial parts from C. integerrima showed a moderate diuretic activity. The urinary levels of sodium and potassium are increased by treatment with C. integerima aqueous extract. This activity could be due, in part, to the presence of betulin isolated as the major metabolite of C. integerrima.
Introduction
The Euphorbiaceae family, with some 300 genera and over 7,000 species, is one of the largest and most diverse families of flowering plants. The great majority of Euphorbiaceae are tropical or subtropical. Although some are tall trees, the majority of taxa in the family are short-lived, understory trees, shrubs, or herbs that grow characteristically in secondary vegetation or open arid regions. Biochemically, the Euphorbiaceae are considerably diversified and are especially rich in alkaloids and terpenoid compounds (CitationWebster, 1986).
Colliguaja integerrima Gillies & Hook (Euphorbiaceae), commonly known as “duraznillo”, “duraznillo patagónico”, “coliguay” or “colihuai”, is a plant that grows in Chile and Argentina, from La Rioja to Santa Cruz (CitationPinto Vitorino et al., 2004). Several species of this family have been used as folk medicine for diuretic activity, e.g., Euphorbia ovalifolia (Klotzsch & Garcke) Boiss. (Euphorbiaceae), Euphorbia serpens H. B. K. var. mycrophylla J. Mueller (Euphorbiaceae), Euphorbia lathyrus L. (Euphorbiaceae), Phyllantus niruri L. (Euphorbiaceae), and Phyllantus sellowianus Mïller Arg. (Euphorbiaceae) (CitationDel Vitto et al., 1997; CitationRoig, 2002; CitationHnatyszyn et al., 2002).
This study was designed to determine the effects of 5% infusion and betulin from Colliguaja integerrima on urinary excretion, sodium and potassium in isotonic saline-loaded Wistar rats. Betulin, isolated from the extract, was detected by capillary electrophoresis in the infusion. Comparison was made with furosemide, a high-ceiling diuretic agent.
Materials and methods
Plant material
The authentic samples of aerial parts of Colliguaja integerrima used in this study were collected in December 2004, in Departamento Luján de Cuyo: Potrerillos, El Salto, Mendoza, Argentina. They were identified by L. A. Del Vitto et al., Universidad Nacional of San Luis, Argentina. A voucher specimen (L. A. Del Vitto et al. no. 9391) was deposited at the Herbarium of the Universidad Nacional of San Luis.
Extraction and isolation
Powered, dried aerial parts of C. integerrima (4 kg) were extracted with methanol (3 × 5 L), at room temperature, yielding 175 g of crude extract. Similar plant material (50 g) was extracted by infusion with distilled water (1 × 1 L) and filtered after 20 min to give an extractive solution. This solution was later lyophilized.
The methanol extract was purified by “flash” chromatography (CitationColl & Bowden, 1986), eluted with n-hexane-EtOAc mixtures having increasing polarity to afford 6 fractions. Betulin, 3β, 28-diol-20(29)-lupene (1) (), 3β-OAc-20(29)-lupene (2), and lanosterol (8,24-lanostadien-3β-OAc) (3) were isolated and identified from the n-hexane-AcOEt (90-10) fraction; epi-lupeol (9(11)-fernen-3α-ol) (4) was isolated form n-hexane-AcOEt (80-20) fraction; glucoside de 24-etil-colestan-5-en-3β-ol (5), purified as acethyl derivative, was identified from the fraction AcOEt (100%). Compounds 1, 2, 3, 4, and 5 were identified by IR, 1H NMR, 13C NMR and GC-MS. The characteristics signals were coincident with previously reported data (CitationOgunkoya et al., 1977; CitationLugemwa et al., 1990; CitationRowan & Russell, 1992; CitationKeller et al., 1996). Retention times and mass spectral data were confirmed comparing with those obtained from authentic samples and/or from the GC-MS instrument library. GC-MS experiments were performed on an ion tramp GCQ-Plus (Finnigan, Termo Scientific, Austin, TX) instrument with MS/MS program using a silica capillary column Restek Rtx®-5MS (30 m × 0.25 mm ID, 0.25 μm df), the gas carrier was helium ( 40 cm s−1).
Chemical compounds
Compound 1 (3β, 28-diol-20(29)-lupene): The pure compound exhibited the following spectral properties: IR (KBr) 3450, 3432, 1631 cm−1; 1H NMR (CDCl3, 300 mHz) δ 4.68 (m, 1 H, H-29), 4.58 (m, 1 H, H-29), 3.80 (d, J = 12 Hz, 1H, H-28) 3.33 (d, J = 12 Hz, 1aH, H-28), 3.18 (m, 1H, H-3), 2.38 (m, 1H, H-19), 1.68 (m, 3H, H-30), 1.02, 0.98, 0.97, 0.82, 0.76 (all s, 15 H, 5 × CH3); MS (EI) m/z (relative intensity) 442 [M+] (27), 424 (15), 411 (59), 393 (11), 381 (5), 234 (18), 207 (62), 191 (25), 189 (100), 187 (29), 175 (27), 121 (31), 107 (47). 13C NMR: δ 38.6 (t, C-1), δ 27.2 (t, C-2), δ 78.9 (d, C-3), δ 38.79 (s, C-4), δ 55.2 (d, C-5), δ 18.2 (t, C-6), δ 34.1 (t, C-7), δ 40.8 (s, C-8), δ 50.3 (d, C-9), δ 37.0 (s, C-10), δ 20.7 (t, C-11), δ 25.1 (t, C-12), δ 37.2 (d, C-13), δ 42.6 (s, C-14), δ 26.9 (t, C-15), δ 29.1 (t, C-16), δ 47.7 (s, C-17), δ 48.6 (d, C-18), δ 47.7 (d, C-19), δ 150.4 (s, C-20), δ 29.6 (t, C-21), δ 33.9 (t, C-22), δ 27.9 (q, C-23), δ 15.3 (q, C-24), δ 16.0 (q, C-25), δ 15.9 (q, C-26), δ 14.7 (q, C-27), δ 60.3 (t, C-28), δ 109.6 (t, C-29), δ 19.0 (q, C-30).
Compound 2 (3β-OAc-20(29)-lupene): is a colorless crystalline solid. 1H NMR (CDCl3, 300 mHz) δ 5.35 (d, 2H, H-11), 4.48 (m, 1H, H-3) and δ 2.04 (s, -CO-CH3). MS (EI) m/z (relative intensity) 442 [M+] (27), 468 (16.5), 453 (47.49), 408 (36.02), 393 (100), 301 (50.95), 241 (67.13). 13C NMR: δ 36.7 (t, C-1), δ 24.0 (t, C-2), δ 81.1 (d, C-3), δ 30.6 (s, C-4), δ 50.8 (d, C-5), δ 20.1 (t, C-6), δ 18.9 (t, C-7), δ 54.1 (d, C-8), δ 145.2 (s, C-9), δ 41.5 (s, C-10), δ 115.9 (d, C-11), δ 36.4 (t, C-12), δ 35.2 (s, C-13), δ 37.6 (s, C-14), δ 30.2 (t, C-15), δ 32.2 (t, C-16), δ 42.8 (s, C-17), δ 47.8 (d, C-18), δ 24.1 (t, C-19), δ 28.2 (t, C-20), δ 59.5 (d, C-21), δ 30.6 (q, C-22), δ 29.6 (q, C-23), δ 21.3 (q, C-24), δ 25.2 (q, C-25), δ 15.7 (q, C-26), δ 15.3 (d, C-27), δ 14.0 (q, C-28), δ 22.1 (q, C-29), δ 22.9 (q, C-30), δ 171.0 (CH3-CO-), δ 21.0 (CH3-CO-).
Compound 3 (8,24-lanostadien-3β-OAc): 1H RMN (CDCl3, 300 mHz) δ 0.75 (d, 3H, H-18), δ 0.85 (s, 3H, H-29), δ 0.89 (s, 3H, H-28), δ 0.90 (s, 3H, H-30), δ 0.97 (s, 3H, H-19), δ 1.56 (s, 6H, H-26 and H-27), δ 3.25 (m, 1H, H-3) and δ 5.34 (d, 1H, H-24). MS (EI) m/z (relative intensity) 426 [M+] (15), 411 (100), 393 (75), 259 (40).
Compound 4 (9(11)-fernen-3α-ol): 1H NMR (CDCl3, 300 mHz) δ 4.58 (dd, 2H, H-29), δ 3.40 (m, J = 2, 1H, H-3α), δ 2.38 (m, 1H, H-18). 13C NMR δ 38.7 (t, C-1), δ 27.3 (t, C-2), δ 78.6 (d, C-3), δ 38.8 (s, C-4), δ 55.2 (d, C-5), δ 18.2 (t, C-6), δ 35.7 (t, C-7), δ 40.7 (s, C-8), δ 50.0 (d, C-9), δ 37.5 (s, C-10), δ 21.2 (t, C-11), δ 25.8 (t, C-12), δ 38.2 (d, C-13), δ 41.5 (s, C-14), δ 24.7 (t, C-15), δ 35.7 (t, C-16), δ 43.2 (s, C-17), δ 48.4 (d, C-18), δ 46.62 (d, C-19), δ 150.67 (s, C-20), δ 29.84 (t, C-21), δ 39.98 (t, C-22), δ 21.76 (q, C-23), δ 21.76 (q, C-24), δ 16.45(q, C-25), δ 17.59 (q, C-26), δ 14.65 (q, C-27), δ 18.01 (q, C-28), δ 109.3 (t, C-29), δ 19.3 (q, C-30).
Compound 5 (glucoside of 24-etil-colestan-5-en-3β-OAc): 1H NMR (CDCl3, 300 mHz) δ 5.35 (d, 1H, H-6), δ 3.5 (m, 1H, H-3) δ 4.6 (d, 1H, H-19), δ 4.95 (dd, 1H, H-29), δ 5.10 (m, 1H, H-39), δ 5.01 (m, 1H, H-49), δ 3.65 (m, 1H, H-59), δ 4.05 (dd, 1H, H-69a), δ 4.29 (dd, 1H, H-69b), δ 2.01, δ 2.02, δ 2.05, δ 2.07 (s, -CO-CH3 of sugar). 13C NMR δ 99.5 (d, C-19), δ 71.6 (d, C-29), δ 71.4 (d, C-39), δ 72.8 (d, C-59), δ 65.9 (t, C-69), δ 170.4, δ 170.1, δ 169.8, δ 169.2 (CH3-CO-), δ 20.5, δ 20.6, δ 20.7, δ 20.9 (CH3-CO-).
Capillary electrophoresis
The employed CE system consisted of a Beckman P/ACE MDQ instrument (Beckman Instruments, Fullerton, CA) equipped with a diode array detector and a data handling system comprising an IBM personal computer and P/ACE System MDQ software. Detection was performed at 208 and 265 nm. The optimized experimental conditions were: background electrolyte (BGE), sodium tetraborate buffer 20 mM, pH 9.3; voltage applied 30 kV; capillary and sample temperature 25°C; injection sample at 0.5 psi during 5 s.
Animals
The experiments were conducted on male Wistar rats (200-250 g). The animals were randomly assigned to three groups (n = 5-8) and were housed in colony cages at room temperature of 25ºC with a 12/12 h light-dark cycle, deprived of food for 18 h prior to starting the experiments. They were provided with water ad libitum. All experiments were in compliance with the ANMAT no. 6344/96 for animal care guidelines.
Diuretic activity
The method described by CitationLipschitz et al. (1943) was followed. The treated rats received the substance to be tested: 5% infusion of the aerial parts of C. integerrima (250 mg/kg, p.o.) and betuline (100 mg/kg, p.o.). Furosemide as a standard drug (10 mg/kg), administered at a volume of 50 mL/kg by intragastric route. The control group received only the NaCl isotonic solution (50 mL/kg). The animals were then placed in individual metabolism cages in which the feces cannot contaminate the urine samples and the environmental temperature was kept constant at 25ºC. Urine was collected into a graduated cylinder and its volume was recorded at 15 min intervals for 3 h. The urinary volumetric excretion was calculated as follows:
The urinary concentrations of sodium and potassium were determined by flame atomic emission spectroscopy-FAES.
Flame atomic emision spectroscopy-FAES
The measurements were performed with a sequential FAES (METROLAB,Buenos Aires, Argentina). PTFE tubing (Ismatec, Cole-Parmer, Vernon Hills, IL) was employed to propel the reagents. The 589 nm and 766.5 nm spectral lines were used for Na+ and K+, respectively, which were corrected against the reagent blank.
Chemicals
Furosemide (Sigma) was used as a diuretic reference drug. All other chemicals used were of reagent grade.
Statistical analysis
All values were expressed as the mean ± SEM. Student’s t-test was performed to evaluate the statistical differences between the control and the experimental samples, and p values of 0.05 or less were considered significant.
Results and discussion
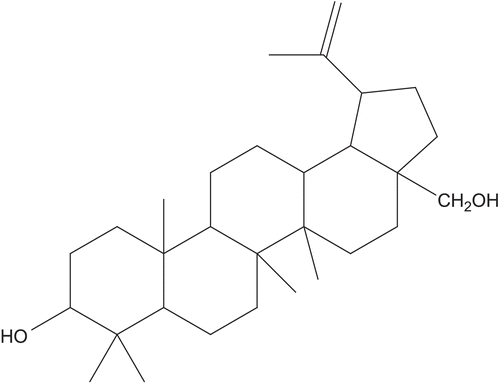
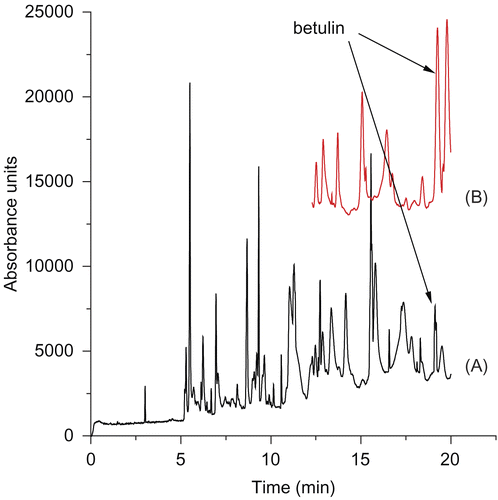
The phytochemical investigation of the aerial parts of C. integerrima led to the isolation and characterization of five known compounds. The lupane triterpene betulin was isolated as the major metabolite of C. integerrima () and characterized by espectroscopical means IR, 1H NMR, 13C NMR and GC-MS. The presence of betulin in the aqueous extract from the plant was confirmed by capillary electrophoresis (). Peak purity was assessed analyzing the UV spectra from the diode array detector at 19 min.
Figure 2. A) Electropherogram of the Colliguaja integerrima infusion; B) Electropherogram of the Colliguaja integerrima infusion spiked with betulin. Conditions: buffer tetraborate 20 mM, pH 9.3; injection: hydrodynamic mode, 0.5 psi, 5 seconds; capillary temperature: 25ºC; Diode array detection: 208 nm.
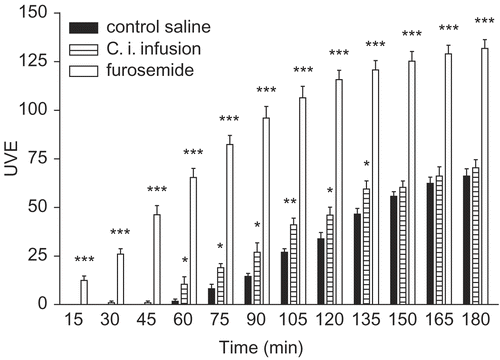
Diuretic activity of 5% infusion and betulin from aerial parts of C. integerrima was evaluated. C. integerrima infusion at a dose of 50 mL/kg (p. o.) produced significant increases in urinary volumetric excretion (UVE) compared with the saline treated control group (). The statistical analysis was compared for each time: saline solution versus furosemide showed a significant difference starting from 15 min (p < 0.001); infusion versus saline solution showed significant difference from 60 at 135 min (p < 0.05 or p < 0.01). The diuretic activity of the infusion of C. integerrima was moderate (p < 0.05 versus furosemide).
Figure 3. Effect of the Colliguaja integerrima 5% infusion (50 mL/kg, p. o.), furosemide (l0 mg/kg) and saline solution (50 mL/kg) on urinary volumetric excretion (UVE) recorded at 15 min intervals for 3 h in saline loaded rats. Statistical analysis was carried out by unpaired Student´s t-test. All values were expressed as mean ± SEM. Asterisks indicate significant differences at the levels of *p < 0.05, **p < 0.01 and ***p < 0.001 versus saline control.
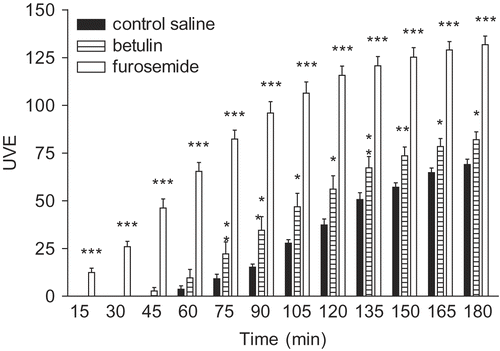
Our results show that betulin increased the UVE 75 min after administration (p < 0.01 or p < 0.05) (). The difference in the mean values of furosemide and betulin groups indicate that betulin showed moderate diuretic activity (p < 0.05).
Figure 4. Effect of betulin (100 mg/kg, p. o.), furosemide (10 mg/kg) and saline solution (50 rnL/kg) on urinary volumetric excretion (UVE) recorded at 15 min intervals for 3 h in saline loaded rats. Statistical analysis was carried out by unpaired Student´s t-test. All values were expressed as mean ± SEM. Asterisks indicate significant differences at the levels of *p < 0.05, **p < 0.01 and ***p < 0.001 versus saline control.
As shown from the data in , urinary excretion of sodium (UNaV) was significantly increased in rats treated with furosemide (10 mg/kg), 5% infusion of C. integerrima (250 mg/kg) or betulin (100 mg/kg) (p < 0.001, p < 0.05 and p < 0.01, respectively). The effects on urinary potassium excretion (UKV) are shown in . UKV was increased significantly by furosemide (p <0.001) and infusion or betulin from C. integerrima (p <0.05).
Table 1. Effect of the 5% infusion of Colliguaja integerrima (50 mL/kg, p.o.), betulin, saline solution (50 mL/kg, p.o.) and furosemide (10 mg/kg) on urinary excretion of sodium (UNaV) and potassium (UKV) in saline-loaded rats. All values were expressed as mean ± SEM (mEq/3h/kg). Statistical analysis was carried out by unpaired Student´s t-test. Saline served as the control.
The data reported in the present work indicate that the infusions of the aerial parts of C. integerrima showed moderate diuretic activity, in comparison with furosemide, a high-ceiling diuretic agent. This activity could be due, at least in part, to the presence of betulin in this plant. Many species of this family has been reported for has been used as a diuretic (CitationDel Vitto et al., 1997; CitationRoig, 2002; CitationHnatyszyn et al., 2002).
Further work is suggested to know the exact mechanism of action. Although evidence on diuretic efficacy of the tested plant has been obtained, further investigations are necessary prior to their recommendation for use as safe and effective diuretics.
Acknowledgements
This work was supported by INTEQUI-CONICET (PIP 2429) and Universidad Nacional de San Luis (Project 22-Q505). Thanks are also due to Ing. Luis A. Del Vitto for his collaboration in the collection of plants specimens and for the botanical identification and to Tec. Mario Soloa for his technical assistance. This paper is a part of the Alvarez ME doctoral thesis.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Coll JC, Bowden BF (1986): The application of vacuum liquid chromatography to the separation of terpene mixtures. J Nat Prod 49: 934–936.
- Del Vitto LA, Petenatti EM, Petenatti ME (1997): Recursos herbolarios de San Luis (República Argentina). Primera parte: plantas nativas. Multequina 6: 49–66.
- Hnatyszyn O, Miño J, Ferraro G, Acevedo C (2002): The hypoglycemic effect of Phyllantus sellowianus fractions in streptozotocin- induced diabetic mice. Phytomedicine 9: 556–559.
- Keller AC, Maillard MP, Hostettmann K (1996): Antimicrobial steroids from the fungus Fomitopsis pinicola. Phytochemistry 41: 1041–1046.
- Lipschitz WL, Haddian Z, Kerpscar A (1943): Bioassay of diuretics. J Pharmacol Exp Ther 79: 110–116.
- Lugemwa FN, Huang FY, Bentley MD, Mendel M, Alford AR (1990): A Heliotis zea antifeedant from the abundant birchbark triterpene betulin. J Agr Food Chem 38: 493–496.
- Ogunkoya L, Olubajo OO, Sondha DS (1977): A new triterpenoid alcohol from Trema orientalis. Phytochemistry 16: 1606–1608.
- Pinto Vitorino G, Toledo IB, Córdoba OL, Flores ML, Cabrera JL (2004): Análisis fitoquímico de Colliguaja integerrima (Hook.) Gill. et Hook. (Euphorbiaceae), una planta de la Patagonia Argentina. Acta Farm Bonaerense 23: 459–465.
- Roig FA (2002): Flora medicinal mendocina. Las plantas medicinales y aromáticas de la provincia de Mendoza (Argentina) Primera reimpresión. Editorial de la Universidad Nacional de Cuyo (EDIUNC), 104–107.
- Rowan DD, Russell GB (1992): 3β-Methoxyhop-22(29)-ene from Chionochloa chessemanii. Phytochemistry 31: 702–703.
- Webster GL (1986): Irritant plants in the spurge family (Euphorbiaceae). Clin Dermatol 4: 36–45.