Abstract
Pectins from the fruit peel of Citrus maxima (Burm. f.) Merr. or pomelo (Rutaceae) were composed of two main groups: 1) water-soluble pectin (WSP) [high-methoxyl pectin, degree of methoxylation (DM) = 69.32-78.68%], and 2) oxalate-soluble pectin (OSP) (low-methoxyl pectin, DM = 21.01-55.41%). Variation of plant cultivar and storing time of the fruits after harvesting did not significantly influence the content, percentage galacturonic acid, DM and neutral-sugar proportion of both WSP and OSP. The contents of WSP and OSP were 8.12-10.87% and 4.89-8.62%, respectively, whereas percentage galacturonic acid of WSP and OSP were 68.31-79.29% and 48.99-74.02%, respectively. Comparison between albedo (inner layer of the peel) and flavedo (outer layer of the peel), the content and percentage galacturonic acid of pectins in albedo were higher. After storing the fruits for 30 days, the molecular weight of WSP in albedo increased from 22-25 KDa to 41-50 KDa. Infrared (IR) spectra confirmed DM difference between WSP and OSP, and suggested more consistency character of WSP among cultivars and storing times. The proportion of neutral sugars in pectins was not influenced by cultivar and storing time, but it was different between WSP and OSP, and between pectins from albedo and flavedo.
Introduction
Pectins are polysaccharides consisting of polymerized units of methyl ester of d-galacturonic acid. They play an important role in the food industry as a gelling agent. For medical use, they are used as an ingredient of antidiarrheal and detoxicant preparations (CitationVoragen et al., 1995). Their anticancer, anticholesterol and hypoglycemic activities have been reported also (CitationYamada, 1996; CitationBehall & Reiser, 1986). In the pharmaceutical industry, pectins have been used as a suspending agent (CitationYokoi et al., 2002). Their potential applications on controlled-release drug delivery (CitationSungthongjeen et al., 1999; CitationSriamornsak, 2003) and colonic drug delivery (CitationAshford et al., 1993; CitationRubinstein et al., 1993) have also been investigated.
Raw materials of pectins presently employed in industrial practice are apple pomace and Citrus peel from juice manufacture (CitationVoragen et al., 1995). Among Citrus (Rutaceae), lime (C. aurantifolia Swing.) gives the highest molecular weight pectin whereas pectin of lemon (C. limon Burm. f.) possesses a higher degree of esterification. The quality of pectins from orange (C. reticulata Blanco, C. sinensis Osb.) is variable, depending on varieties and sources of orange (CitationMay, 1990). Possible use of some local Citrus as sources of pectins has been studied. For examples, they were Cheju mandarin (C. unshiu Marcow.) of Korea (CitationKim et al., 2000); galgal (C. pseudolimon Tan.) of India (CitationAttri & Maini, 1996); tonkan (C. tankan Hayata), wendun (C. grandis Osbeck) and kumquat (C. microcarpa Bunge) of Taiwan (CitationWang et al., 2008). Among Citrus, the fruit of pomelo or C. maxima (Burm. f.) Merr. is the largest. Therefore, a large amount of the peel could be obtained. In Thailand, C. maxima is a popular fruit widely grown in the central part of the country, especially in Nakhon-Pathom province, the so-called sweet pomelo town. Some of its peel is processed as dessert, e.g. jam. However, most of it is waste product. The objective of this study was to describe the amount and properties of pectins in the peel of three popular cultivars of C. maxima at different post-harvesting times. The use of the peel of C. maxima as another source of pectin is also discussed.
Materials and methods
Materials
Fruits of Citrus maxima (Burm. f.) Merr. were harvested from Nakhon-Pathom province, Thailand, during November 2003. Three cultivars, Khao-nam-phueng, Khao-paen and Khao-phuang, were identified. Each cultivar was divided into two groups. The first group was immediately examined within 10 days. The second group was stored at room temperature (30°C) for 30 days. Before extraction, the fruits were peeled and cut to separate the outer layer (flavedo) and the inner layer (albedo). The peel was chopped into small pieces of around 1 cm, dried at 45°C and kept at 4°C until use.
Sequential extraction of pectins
Pectin fractions were obtained by the method modified from CitationVoragen et al. (1995). C. maxima peel (20 g) was sequentially extracted with boiled water (1 h), 1% ammonium oxalate solution (pH 6.5, 30°C, 1 h) and diluted HCl solution (pH 3.5, 100°C, 1 h); 500 mL of each solvent was used. The extract was concentrated to 100 mL and it was dialyzed (D9527, Sigma-Aldrich, St. Louis, Missouri, USA) for 1 h, repeated for 8 times. Pectins were precipitated from the concentrated solution by adding 200 mL of 95% ethanol. The precipitate of pectins was collected by centrifugation at 3,500 rpm for 8 min, washed with 95% ethanol and dried at 45°C. For each extracted solvent, the peel was repeatedly extracted until no more pectins were precipitated. Pectins obtained from each extracted solvent were WSP, OSP and ASP, respectively.
Analysis of galacturonic acid, methoxyl group, DM, neutral sugar and molecular weight (MW)
Galacturonic acid content was determined by a modification of colorimetric m-hydroxybiphenyl method (CitationBlumenkrantz & Asboe-Hansen, 1973) using d- galacturonic acid (Fluka BioChemika, Switzerland) as standard. The content of methoxyl groups was determined by using the titration method (CitationUnited Stated Pharmacopeial Convention, 2002). The difference between the first end-point of free carboxylic groups and the second end-point of the amount of methyl- saponified carboxyl groups after alkaline de- esterification of pectins corresponded to the amount of methoxyl groups. DM was calculated as molar ratio from the contents of methoxyl group and galacturonic acid.
To analyze the neutral sugar composition, pectins were methanolyzed in 1 M methanol HCl at 80°C for 24 h (CitationSamuelsen et al., 1995). The released neutral sugars were analyzed as their trimethylsilyl derivatives by gas chromatography with mass selective detector (Agilent 6890N/5973N Series GC/MSD, Wilmington, Delaware, USA) using a DB-1 fused silica capillary column (30 m × 0. 25 mm internal diameter).
MW of pectins was estimated from applying the Mark-Houwink-Sakurada equation (CitationGarneir et al., 1993; Fried, 1995): [η] = 4.36 × 10−2 MW0.78, where [η] is the intrinsic viscosity. The viscosity of pectin solution (0.0002-0.0010 g/mL) in 0.1 M sodium chloride was measured using an Ostwald-type capillary viscometer (Schott-Geräte GmbH, Mainz, Germany) at 25°C. Ten readings with less than 0. 5 s differences were obtained for each sample. The intrinsic viscosity was calculated from the extrapolation of specific viscosity measurements to zero concentration.
IR analysis
Diffuse reflectance Fourier Transform IR spectra (DRIFTS) at 4000- 400 cm−1 of the isolated pectins were collected using a Nicolet Magna-IR spectrometer 750 at a resolution of 8 cm−1 and 32 co-added scans.
PCA analysis of neutral-sugar proportion
Triplicate data of neutral-sugar proportion were averaged and standardized. The principal component analysis (PCA) was carried out with The Unscrambler 9.5® (CAMO Process AS, Norway) to study the difference on the neutral-sugar proportion of the isolated pectins (ASP).
Results
The fruit peel of C. maxima was investigated for its pectin composition. Three groups of pectins were sequentially extracted (CitationVoragen et al., 1995). Low-MW high- methoxyl pectin or water-soluble pectin (WSP) was first extracted by water. Later, another group of low-MW pectin was liberated from the calcium complexation in plant tissues by oxalate chelating agent, producing oxalate-soluble pectin (OSP). Finally, protopectin, which had a large MW, was extracted from the cell wall by acid hydrolysis, producing acid-soluble pectin.
Three popular cultivars of C. maxima in Thailand, Khao-nam-phueng, Khao-paen, and Khao-phuang, were compared in this experiment. It was found that pectin content and pectin properties ( and ) among these cultivars did not significantly vary. Both albedo (white tissue, inner layer of the peel) and flavedo (green tissue, outer layer of the peel) were the sources of soluble pectins WSP and OSP. They possessed a very small amount of protopectin since a very small amount of ASP was extracted. The degree of methoxylation of WSP was 69.32-78.68%, whereas that of OSP was 21.01-55.41%. So WSP and OSP were clearly identified as high-methoxyl pectin and low-methoxyl pectin, respectively.
Table 1. Content (%), galacturonic acid (% GalA), methoxyl (% Methoxyl), degree of methoxylation (DM) and molecular weight (MW) of WSP from fruit peel (albedo and flavedo) of various cultivars of C. maxima at < 10 and 30 days after harvesting.
Table 2. Content (%), galacturonic acid (% GalA), methoxyl (% Methoxyl), degree of methoxylation (DM) and molecular weight (MW) of OSP from fruit peel (albedo and flavedo) of various cultivars of C. maxima at < 10 and 30 days after harvesting.
Comparing albedo and flavedo, the content of pectins in albedo was higher. The contents of WSP and OSP in albedo were 8.30-14.44% and 5.66-10.77%, respectively, whereas those in flavedo were 5.18-9.55% and 2.87-7.86%, respectively. OSP had low percentage galacturonic acid (48.99-74.02%) (). Percentage galacturonic acid of WSP from albedo was slightly higher than that from flavedo. They were 74.27-79.29% and 68.31-74.73%, respectively.
In practice, fruits of C. maxima were harvested before maturity and kept for quite a long period before consumption. Therefore, the effect of storing time on their pectin content and property was studied. It was found that the content, percentage galacturonic acid and DM of pectins were not greatly changed after storing for 30 days ( and ), but MW of WSP from albedo was significantly increased from 22026- 25283 Da to 41200- 49850 Da ().
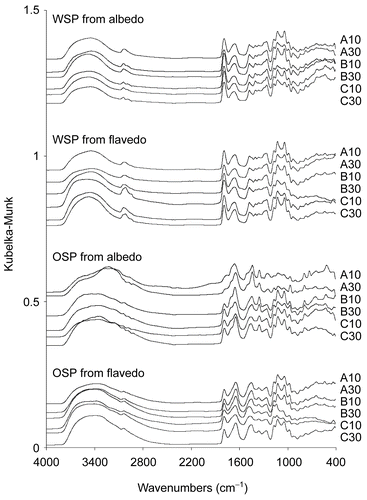
The diffuse reflectance FT-IR spectra of the extracted pectins are shown in . The existence of absorption bands at around 1650 and 1750 cm−1 were from free and esterified carboxyl groups, respectively. It was confirmed that the intensity of the esterified carboxyl band of WSP was higher than OSP. The broader band from 3800- 2500 cm−1 of O-H stretching supported the higher content of free carboxyl group of OSP, whereas C-H stretching and vibration bands of methyl group at around 3000 cm−1 supported a higher substitution of methyl ester of WSP. The fingerprint region of 1200- 1000 cm−1 contained C-O and C-C vibration bands of glycosidic bonds and a pyranoid ring (CitationSynytsya et al., 2003). Comparing with OSP, spectra in this region of WSP obtained from albedo and flavedo of various cultivars and storing times were not much different. This suggested that the character of WSP was more consistent than OSP.
Figure 1. Diffuse reflectance FT-IR spectra of pectins from C. maxima (A, B and C represent cultivar Khao-nam-phueng, Khao-paen, and Khao-phuang, respectively; 10 and 30 indicate the day of storage after harvesting).
Recently, most evidence indicated that the complex neutral sugar side-chain of pectins were important with regard to biological activities (CitationWillats et al., 2006). Compositions of neutral sugars in the pectins from C. maxima were then studied ( and ). Variation of neutral-sugar proportion in the isolated pectins was analyzed by principal component analysis (PCA). The score and loading plots of PC1 versus PC2 are shown in and , respectively. Clusters of WSP and OSP were clearly distinguished at the opposite side of the diagonal between the axes of PC1 and PC2 (). Their difference could be explained by the loading plot (). WSP was mainly located in the quarter between the positive side of PC1 and negative side of PC2, which corresponded to the relative proportion of xylose, glucose and mannose. This indicated that WSP possessed a higher proportion of these neutral sugars. By using the same analysis method it could be concluded that OSP contained a higher proportion of galactose and rhamnose. Comparing between albedo and flavedo within each group of pectins, a higher relative amount of arabinose was observed in pectins from flavedo. Vice versa, higher proportions of rhamnose, xylose, glucose and mannose were found for pectins from albedo. Score plots of pectins from different cultivars and storing times were not discriminated. This suggested that the proportion of neutral sugars was not influenced by these factors.
Figure 2. PCA discrimination of WSP and OSP from albedo and flavedo based on percentage relative mol of neutral sugars (A, B and C were cultivars. Khao-nam-phueng, Khao-paen, and Khao-phuang, respectively; 10 and 30 indicate the day of storage after harvesting).
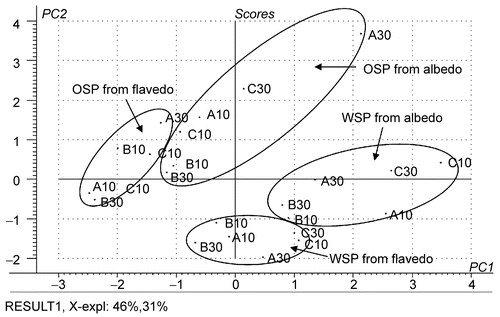
Figure 3. Projection of percentage relative mol of neutral sugars on the first two principal components. Ara, arabinose; Gal, galactose; Glc, glucose; Man, mannose; Rha, rhamnose; Xyl, xylose.
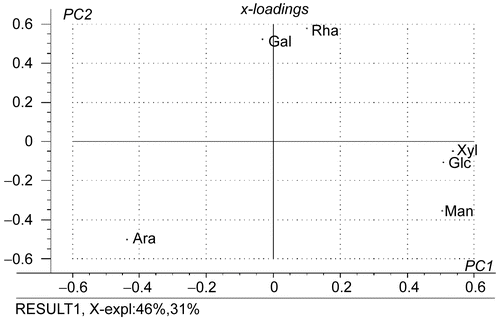
Table 3. Neutral sugar content (% relative mol) of WSP from fruit peel (albedo and flavedo) of various cultivars of C. maxima at < 10 and 30 days after harvesting.
Table 4. Neutral sugar content (% relative mol) of OSP from fruit peel (albedo and flavedo) of various cultivars of C. maxima at < 10 and 30 days after harvesting.
Discussion
This study was the first systematic investigation on the content and property of pectins in the fruit peel of C. maxima. Fruit peel of C. maxima is composed of WSP (or high-methoxyl pectin) and OSP (or low-methoxyl pectin). If albedo and flavedo were not separated, the contents of WSP and OSP in total peel would be 8.89-10.87% and 4.89-8.62%, respectively. Among the studied cultivars, Khao-nam-phueng gave the highest amount of the fruit peel (219.30 ± 58.96 g/fruit). Therefore, the contents of WSP and OSP of this cultivar would be 21.86 ± 2.39 and 12.09 ± 1.09 g per one fruit, respectively. OSP contained quite a low percentage of galacturonic acid which was not sufficient to qualify for pharmaceutical use (CitationUnited Stated Pharmacopeial Convention, 2002). The quality of OSP also was not consistent, whereas quality of WSP did not vary significantly among cultivars and fruit storing-time after harvesting. Between albedo and flavedo, some different characters on their WSP could be found. As an overview, the quantity and quality of WSP from albedo were better than that from flavedo. This observation was in agreement with the previous report of pectins from orange peel (CitationLiu et al., 2006). The predominant property of WSP from C. maxima was the high DM (69.32-78.68%). Even its MW was low and might cause some disadvantages on gelling and viscosity properties; the pectins with high DM and low MW have been reported for use to enhance the absorption of co-administered minerals, e.g., iron (CitationKim et al., 1996). Moreover, low MW pectins with low viscosity and high solubility demonstrated a better repression effect on liver lipid accumulation than high MW pectin, when taken as soluble dietary fiber (CitationFumihide et al., 1994). Therefore, WSP from C. maxima might be a potential neutraceutical, and its health benefit will be further studied.
Acknowledgements
This work was financially supported by Silpakorn University Research and Development Institute, under the research program “Production of pectin from pomelo (Citrus maxima) peel and application in pharmaceutical industry”.
References
- Ashford M, Fell J, Attwood D, Sharma H, Woodhead P (1993): An evaluation of pectin as a carrier for drug targeting to the colon. J Control Release 26: 213–220.
- Attri BL, Maini SB (1996): Pectin from galgal (Citrus pseudolimon Tan.) peel. Bioresour Technol 55: 89–91.
- Behall K, Reiser S (1986): Effects of pectin on human metabolism, in: Fishman ML, Ren JJ, eds, Chemistry and Functions of Pectins. Washington DC, American Chemical Society, pp. 248–265.
- Blumenkrantz N, Asboe-Hansen G (1973): New method for quantitative determination of uronic acids. Anal Biochem 54: 484–489.
- Fried JR (2003): Conformation, solutions, and molecular weight, in: Fried JR, ed., Polymer Science and Technology, 2nd edn. New Jersey, Prentice-Hall, pp. 87–152.
- Fumihide Y, Noriko S, Chitoshi H (1994): Preparation and physiological effect of low-molecular-weight pectin. Biosci Biotechnol Biochem 58: 679–682.
- Garneir C, Axelos MAV, Thibault JF (1993): Phase diagrams of pectin- calcium systems: Influence of pH, ionic strength, and temperature on the gelation of pectins with different degrees of methylation. Carbohydr Res 240: 219–232.
- Kim DH, Kim DG, Lee DY, Kim KE, Kim CW (2000): Physiological characterization of pectin extracted from cheju mandarin (Citrus unshiu) peels with citric acid. Food Sci Biotechnol 9: 95–98.
- Kim M, Atallah MT, Amarasiriwardena C, Barnes R (1996): Pectin with low molecular weight and high degree of esterification increases absorption of [58]Fe in growing rats. J Nutr 126: 1883–1890.
- Liu Y, Shi J, Langrish TAG (2006): Water-based extraction of pectin from flavedo and albedo of orange peels. Chem Eng J 120: 203–209.
- May CD (1990): Industrial pectins: sources, production and applications. Carbohydr Polym 12: 79–99.
- Robinstein A, Radai R, Ezra M, Pathak S, Rokem JS (1993): In vitro evaluation of calcium pectinate: Potential colon-specific drug delivery carrier. Pharm Res 10: 258–263.
- Samuelsen AB, Paulsen BS, Wold JK, Otsuka H, Yamada H, Espevik T (1995): Isolation and partial characterization of biologically active polysaccharides from Plantago major L. Phytother Res 9: 211–218.
- Sriamornsak P (2003): Chemistry of pectin and its pharmaceutical uses: A review. Silpakorn Univ Int J 3: 206–228.
- Sungthongjeen S, Pitaksuteepong T, Somsiri A, Sriamornsak P (1999): Studies of pectins as potential hydrogel matrices for controlled release drug delivery. Drug Dev Ind Pharm 25: 1271–1276.
- Synytsya A, Čopíková J, Matějka P, Machovič V (2003): Fourier transform Roman and infrared spectroscopy of pectins. Carbohydr Polym 54: 97–106.
- United States Pharmacopeial Convention (2002): The United States Pharmacopeia-The National Formulary (USP 26-NF21). Webcom Limited, Toronto, USA, pp. 1401–1402.
- Voragen AGJ, Pilnik W, Thibault JF, Axelos AAV, Renard CMGC (1995): Pectins, in: Stephen AM, ed., Food Polysaccharides and Their Applications. New York, Marcel Dekker, pp. 287–339.
- Wang YC, Chuang YC, Hsu HW (2008): The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan. Food Chem 106: 277–284.
- Willats WGT, Knox JP, Mikkelsen JD (2006): Pectin: New insights into an old polymer are starting to gel. Trends Food Sci Tech 17: 97–104.
- Yamada H (1996): Contribution of pectins on health care, in: Visser J, Voragen AGJ, eds, Pectins and Pectinases. Amsterdam, Elsevier, pp. 173–190.
- Yokoi H, Obita T, Hirose J, Hayashi S, Takasaki Y (2002): Flocculation properties of pectin in various suspensions. Bioresour Technol 84: 287–290.
