Abstract
Piperine is a piperidine-ring containing alkaloid and a major constituent of Piper nigrum Linn. and Piper longum Linn. species, belonging to the Piperaceae family. The present study explored their mode of action in gastrointestinal disorders, such as diarrhea and colic. Piperine at the dose of 10 mg/kg provided complete protection from castor oil-induced diarrhea in mice, similar to that of loperamide. In isolated rabbit jejunum preparations, piperine exhibited concentration-dependent inhibition of spontaneous contractions with an EC50 value of 149.1 μM (89.26–249.20, 95% CI). When used to treat high K+ (80 mM)-induced sustained contractions, piperine inhibited such contractions with an EC50 value of 80.86 μM (56.10–116.50, 95% CI), which suggested a calcium channel blocking (CCB) effect. The CCB effect was further confirmed when pretreatment of the tissues with piperine (10–100 μM) caused a rightward shift in the Ca++ concentration–response curves (CRCs) in Ca++-free medium, similar to that caused by verapamil. Loperamide also caused the inhibition of spontaneous and high K+-induced contractions as well as shifted the Ca++ CRCs to the right at concentrations of 1–10 μM. These data indicate that piperine exhibits antidiarrheal and antispasmodic activities, mediated possibly through calcium channel blockade.
Introduction
Piperine is an alkaloid containing the piperidine ring found in Piper nigrum Linn. and Piper longum Linn. (CitationJohri & Zutshi, 1992), belonging to the Piperaceae family (CitationNadkarni, 1976). Peppers are common spices used worldwide, in addition to their medicinal use in antidiarrheal formulations (CitationBajad et al., 2001). Piperine was found to alter the intestinal motility of rabbit and guinea-pig ileum (CitationNeogi et al., 1971; CitationAnnamalai & Manavalan, 1990; CitationTakaki et al., 1990). It was also reported that piperine exhibited an inhibitory effect on gastrointestinal motility both in vivo (CitationTakaki et al., 1990) and in vitro (CitationIzzo et al., 2001). Efforts have been made to explore the possible mode of action in diarrhea and to rationalize the use of peppers in antidiarrheal formulations, whereas piperine was found to be a major component which provided protection against castor oil-, MgSO4-, and arachidonic acid-induced diarrhea (CitationBajad et al., 2001; CitationStohr et al., 2001). There are some studies which suggest that piperine reduces castor oil-induced diarrhea possibly through capsaicin-sensitive neurons (CitationIzzo et al., 2001; CitationCapasso et al., 2002). CitationMcNamara et al. (2005) suggested the involvement of the vanilloid receptor (TRPV1) in mediating the effects of piperine on gastrointestinal function. All these studies reflect that ambiguity prevails over the mode of action in inducing the antidiarrheal effect of piperine.
We have previously reported that piperidine- containing compounds suppress the spontaneous contraction of smooth muscle through calcium channel blockade (CitationTaqvi et al., 2006a). The present study reports the calcium channel blocking effect of piperine, being another possible mode of action for its antidiarrheal and antispasmodic activities. The antispasmodic and calcium channel blocking effects of piperine were found to be comparable to those of verapamil, a standard calcium antagonist (CitationTriggle, 1992) and loperamide, a known antidiarrheal agent, which also contains the piperidine ring (CitationReynolds et al., 1984).
Materials and methods
Drugs and chemicals
The following reference chemicals were obtained from the sources specified: verapamil hydrochloride, loperamide hydrochloride, potassium chloride, piperine (Sigma-Aldrich Chemie GmbH, Steinheim, Germany), dimethylsulfoxide (Sigma Chemical Company, St. Louis, MO, USA), and Tween 80 (Chemie S.S., Barcelona, Spain). Castor oil was purchased from Karachi Chemicals Labs, Karachi, Pakistan. All chemicals used were of the highest purity grade available. Stock solutions of physiological salt solution and verapamil were prepared in distilled water, whereas loperamide was dissolved in 10% dimethylsulfoxide (DMSO) and piperine was dissolved in Tween 80. Dilutions were made fresh on the day of the experiment.
Animals
Experiments complied with the rules of the Institute of Laboratory Animal Resources, Commission on Life Sciences, CitationNational Research Council (1996), and were approved by the Ethics Committee of the Aga Khan University, Karachi. Rabbits of local breed (Oryctolagus cuniculus) weighing 1.5–2.0 kg, belonging to the Leporidae family (CitationWilson & Reeder, 1993), and Balbc mice (20–25 g) of either sex used in this study were housed at the animal house of Aga Khan University under controlled conditions (23–25°C). Animals were fed a standard diet consisting of (g/kg): flour 380, choker 380, molasses 12, salt 5.8, nutrivetL 2.5, potassium metabisulfate 1.2, vegetable oil 38, fish meal 170, and powdered milk 150.
Castor oil-induced diarrhea
Mice (20–25g) of either sex were used as described earlier (CitationIzzo et al., 1994; CitationGilani et al., 2005). The animals were housed in individual cages and divided into five groups, five animals in each. Animals were fasted for 24 h prior to experiments. The first group received saline (10 mL/kg; p.o.) as vehicle and so acted as the negative control. The dose of test compound was selected on a trial basis, and then three increasing doses were given orally to the animals. A group of mice were treated with loperamide (10 mg/kg) as the positive control. One hour after treatment, each animal received 10 mL/kg of castor oil orally through a feeding needle. After 4 h cages were inspected for the presence of typical diarrhea droppings; their absence was noted as a positive result, indicating protection from diarrhea at that time.
Isolated tissue preparations
Isolated tissue experiments were carried out as described previously (CitationGilani et al., 2005). The animals were starved for 24 h prior to the experiment but had free access to water. The animals were sacrificed by cervical dislocation; the abdomen was cut open and the jejunum was isolated. Segments of 2 cm each were cut and mounted in a 10 mL tissue bath containing normal Tyrode’s solution, aerated with carbogen (95% O2 and 5% CO2). The composition of the Tyrode’s solution in mM was: KCl 2.7, NaCl 136.9, MgCl2 1.1, NaHCO3 11.9, NaH2PO4 0.4, glucose 5.6, and CaCl2 1.8 (pH 7.4). A preload of 1 g was applied and the tissues were allowed to equilibrate for 30 min. Then, control responses to a submaximal concentration of acetylcholine (0.3 μM) were recorded. Reproducible responses determined the stability of the tissues.
Under these experimental conditions, rabbit jejunum exhibits spontaneous rhythmic contractions, allowing testing of the relaxant (spasmolytic) activity directly without the use of an agonist (CitationGilani et al., 1994).
Determination of spasmolytic and calcium antagonist activity
To assess the spasmolytic activity of piperine, the tissues were treated with different concentrations of piperine in ascending order and in a cumulative fashion. To explore whether the spasmolytic effect was due to blockade of the calcium channel, high K+ (80 mM) was added to depolarize the rabbit jejunum (CitationFarre et al., 1991). K+ (80 mM) was added to the tissue bath, which produced sustained contraction. The test compound was then added in a cumulative fashion to obtain concentration-dependent inhibitory responses (Citationvan Rossum, 1963). Relaxation was expressed as a percent of the control response mediated by high K+.
To confirm the calcium antagonist activity of piperine, first the tissues were allowed to stabilize in normal Tyrode’s solution, and then the solution was replaced with Ca++-free Tyrode’s solution containing ethylenediaminetetraacetic acid (EDTA; 0.1 mM) for 30 min in order to remove calcium from the tissues. This solution was further replaced with K+-rich and Ca++-free Tyrode’s solution having the following composition in mM: KCl 50, NaCl 91.04, MgCl2 1.05, NaHCO3 11.90, NaH2PO4 0.42, glucose 5.55, and EDTA 0.1. Following an incubation period of 30 min, control concentration–response curves (CRCs) of Ca++ were constructed. When the control CRCs of Ca++ were found to be superimposable (usually after 2–3 cycles), the tissues were pretreated with test compound for 1 h to test the possible calcium channel blockade. The CRCs of Ca++ were reconstructed in the presence of an ascending concentration of test compound.
Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM), and median effective concentrations (EC50 values) are given with 95% confidence intervals (CIs). The statistical parameter applied was Student’s t-test, with p < 0.05 noted as significantly different. Concentration–response curves were analyzed by non-linear regression. Results for antidiarrheal activity were analyzed by χ2 test, where p < 0.05 was noted as significantly different. GraphPad program version 4 was used for all statistical analysis (GraphPad, San Diego, CA, USA).
Results and discussion
When tested against castor oil-induced diarrhea, piperine (1– 10 mg/kg) caused dose-dependent protection, similar to loperamide, which also provided 100% protection at 10 mg/kg (); this dose is in accordance with the dose range as previously reported (CitationBajad et al., 2001). Castor oil is known to induce diarrhea due to the action of ricinoleic acid formed as a result of hydrolysis of the oil (CitationIwao & Terada, 1962), which produces changes in the transport of water and electrolytes. This results in a hypersecretory response and the generation of huge contractions in the transverse and distal colon, observed as diarrhea in the animal. Thus, a potential antidiarrheal agent may exhibit its antidiarrheal effect by inhibiting the contractions and/or secretion, observed as the absence of diarrheal droppings (CitationCroci et al., 1997). These data suggest that piperine has an inhibitory effect on either gut motility and/or electrolyte outflux.
Table 1. Effect of piperine on castor oil-induceda diarrhea in mice (n = 5).
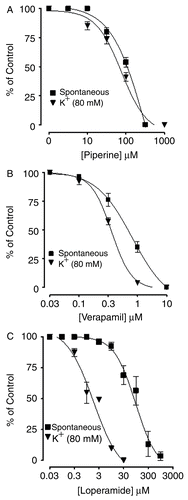
The effect of piperine on gut motility was evaluated in in vitro studies. For this purpose, isolated spontaneously contracting rabbit jejunum preparations were exposed to cumulative additions of piperine, which caused concentration-dependent inhibition (), with an EC50 value of 149.1 μM (89.26–249.20, 95% CI) (). Verapamil and loperamide also inhibited spontaneous contractions (). The contraction of smooth muscle is dependent on an increase in the cytoplasmic free Ca++, which activates the contractile elements (CitationKaraki & Wiess, 1983). The increase in intracellular Ca++ occurs via either influx through voltage-dependent Ca++ channels (VDCs) or its release from intracellular stores in the sarcoplasmic reticulum. Periodic depolarization and repolarization regulates the spontaneous movements of the intestinal smooth muscles. At the height of depolarization, the action potential induces a rapid influx of Ca++ via VDCs (CitationBrading, 1981).
Figure 1. A typical tracing of showing inhibitory effect of piperine, loperamide and verapamil on spontaneously contracting isolated rabbit jejunum preparations.
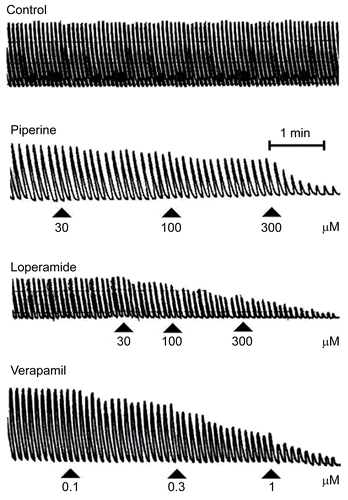
Figure 2. Inhibitory effect of piperine (A), verapamil (B) and loperamide (C) on spontaneous and high K+-induced contractions in isolated rabbit jejunum preparations. Values shown are mean ± SEM, n = 4.
Thus the inhibitory effect of piperine observed in this study may be due to interference of either Ca++ influx through VDCs or Ca++ release. Our previous studies (CitationTaqvi et al., 2006a, Citation2006b) reported a calcium channel blocking (CCB) effect of other piperidine derivatives, also selectively in smooth muscle. Therefore, further experiments were carried out to see whether piperine exhibits a CCB effect.
To determine the calcium channel blocking activity of piperine, high K+ was added in the tissue bath, which produced sustained contraction, followed by the cumulative addition of piperine. Piperine relaxed the high K+-induced sustained contractions in a concentration-dependent manner with an EC50 value of 80.86 μM (56.10–116.50, 95% CI), similar to verapamil or loperamide.
The sustained contractions induced by high K+ (>30 mM) are dependent on the influx of Ca++ into cells through VDCs (CitationBolton, 1979). A substance that can inhibit the high K+-induced contraction is therefore considered to be a CCB (CitationGodfraind et al., 1986). The CCB activity of piperine was further confirmed when pretreatment of tissues with piperine (10–100 μM) caused a rightward shift in the Ca++ CRCs (), constructed in Ca++-free medium, similar to verapamil, a standard CCB (CitationFleckenstein, 1977), or loperamide, which is also known to possess a CCB effect at antidiarrheal doses (CitationWang et al., 2005). These data indicate that piperine also possesses CCB activity, which may be responsible for its antidiarrheal activity, similar to loperamide. This activity may be attributed to the piperidine ring, which is shared by both loperamide and piperine. Moreover, this is in accordance with the known fact that CCBs such as verapamil possess antidiarrheal and antispasmodic activities (CitationPasricha 2006). The present data suggest that piperine mediates its antidiarrheal effect through CCB, though additional mechanism(s) cannot be ruled out.
Figure 3. Effect on the Ca++ concentration-response curves of piperine (A), loperamide (B) and verapamil (C) in isolated rabbit jejunum preparations in Ca++ free medium. Values shown are mean ± SEM, n=3.
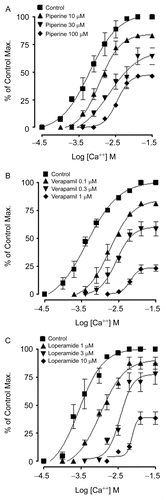
In summary, these data indicate that piperine possesses a CCB effect, which may be another possible mechanism involved in its antidiarrheal and antispasmodic activities in addition to those already reported. Further studies are recommended to evaluate the potential of piperine for development of a new antidiarrheal agent.
Declaration of interest: This study was partially supported by a research grant from the Pakistan Science Foundation.
References
- Annamalai AR, Manavalan R (1990): Effect of “Trikatu” and its individual components and piperine on gastrointestinal tract: “Trikatu”, a bioavailability enhancer. Indian Drugs 27: 595–604.
- Bajad S, Bedi KL, Singla AK, Johri RK (2001): Antidiarrheal activity of piperine in mice. Planta Med 67: 284–287.
- Bolton TB (1979): Mechanism of action of transmitters and other substances on smooth muscles. Physiol Rev 59: 606–718.
- Brading AJ (1981): How do drugs initiate contraction in smooth muscles? Trends Pharmacol Sci 2: 261–265.
- Capasso R, Izzo AA, Borrelli F, Russo A, Sautebin L, Pinto A, Capasso F, Mascolo N (2002): Effect of piperine, the active ingredient of black pepper, on intestinal secretion in mice. Life Sci 71: 2311–2317.
- Croci T, Landi M, Elmonds-Alt X, Le Fur G, Maffrand JP, Manara I (1997): Role of tachykinins in castor oil induced diarrhea in rats. Br J Pharmacol 121: 375–380.
- Farre AJ, Columbo M, Fort M, Gutierrez B (1991): Differential effects of various Ca++ antagonists. Gen Pharmacol 22: 177–181.
- Fleckenstein A (1977): Specific pharmacology of Ca++ in myocardium, cardiac pacemakers and vascular smooth muscle. Rev Pharmacol Toxicol 17: 149–166.
- Gilani AH, Janbaz KH, Lateef A, Zaman M (1994): Ca++ channel blocking activity of Artemisia scoperia extract. Phytother Res 8: 161–165.
- Gilani AH, Nabeel MG, Khalid A, Zaheer-ul Haq, Chaudhary MI, Rahman A (2005): Presence of antispasmodic, antidiarrheal, antisecretory, calcium antagonist and acetyl cholinesterase inhibitory steroidal alkaloids in Sarcococca saligna. Planta Med 71: 1–6.
- Godfraind I, Killer R, Wibo M (1986): Calcium antagonism and calcium entry blockade. Pharmacol Rev 38: 321–416.
- Iwao I, Terada Y (1962): On the mechanism of diarrhea due to castor oil. Jpn J Pharmacol 12: 137–145.
- Izzo AA, Mascolo N, Carlo GD, Capasso F (1994): NG-nitro-l-arginine methyl ester modulates intestinal secretion and motility produced by carbachol. Eur J Pharmacol 271: 31–35.
- Izzo AA, Capasso R, Pinto L, Giulia DC, Mascolo N, Capasso F (2001): Effect of vanilloid drugs on gastrointestinal transit in mice. Br J Pharmacol 132: 1411–1416.
- Johri RK, Zutshi U (1992): An Ayurvedic formulation “Trikatu” and its constituents. J Ethnopharmacol 37: 85–91.
- Karaki H, Wiess G (1983): Mini review: Calcium release in smooth muscles. Life Sci 42: 111–122.
- McNamara FN, Randall A, Gunthorpe MJ (2005): Effects of piperine, the pungent component of black pepper at the human vanilloid receptor (TRPV1). Br J Pharmacol 144: 781–790.
- Nadkarni AK (1976): Indian Materia Medica. Bombay, Popular Prakashan, pp. 965–969.
- National Research Council (1996): Guide for the Care and Use of Laboratory Animals. Washington, DC, National Academy Press, pp. 1–7.
- Pasricha PJ (2006): Treatment of disorders of bowel motility and water flux; antimemetics; agents used in biliary and pancreatic disease. In: Burton LL, Lazo JS, Parker KL, Gilman’s AG. (Eds.), Goodman and Gillman’s The Pharmacological Basis of Therapeutics, 11th edn. McGraw-Hill, New York, pp. 983–1008.
- Neogi NC, Haldar RK, Rathor RS (1971): Preliminary pharmacological studies on piperine. J Res Indian Med 6: 24–29.
- Reynolds IJ, Gould RJ, Synder SH (1984): Loperamide: Blockade of calcium channels as mechanism for antidiarrheal effects. J Pharmacol Exp Ther 231: 628–632.
- Stohr JR, Xiao PG, Bauer R (2001): Constituents of Chinese Piper species and their inhibitory activity on prostaglandin and leukotriene biosynthesis in vitro. J Ethnopharmacol 75: 133–139.
- Takaki M, Jin JG, Lu YF, Nakayama S (1990): Effects of piperine on the motility of the isolated guinea ileum: Comparison with capsaicin. Eur J Pharmacol 186: 71–77.
- Taqvi SIH, Nabeel MG, Gilani AH, Saify ZS, Tariq MA (2006a): Synthesis and smooth muscle selective relaxant activity of a piperidine analogue: 1-(4′-Fluorophenacy)-4-hydroxy-4-phenylpiperidinium chloride. Arch Pharmacol Res 29: 34–39.
- Taqvi SIH, Nabeel MG, Gilani AH, Saify ZS, Tariq MA (2006b): Synthesis of 1-(2′, s4′-dimethoxyphenacyl)-4-phenylpiperidinium bromide. Int J Pharmacol 2: 146–151.
- Triggle DJ (1992): Drugs affecting calcium regulation and actions. In: Smith GM, Reynard AM, eds., Text Book of Pharmacology. Philadelphia, WB Saunders Co., pp. 453–479.
- van Rossum JM (1963): Cumulative dose-response curves II. Techniques for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn 143: 299–330.
- Wang HH, Sheih MJ, Liao KF. (2005): A blind, randomized comparison of racecadotril and loperamide for stopping acute diarrhoea in adults. World J Gastroenterol 14: 1540–1543.
- Wilson D, Reeder D (1993): Mammal Species of the World: A Taxonomic and Geographic Reference. Washington, DC, The Smithsonian Institution.