Abstract
This study examined the gastroprotective potential of a black tea brew (BTB) of Camellia sinensis Linn. O. Kuntze (Theaceae) using Sri Lankan high grown Dust grade No: 1 tea in a rat ethanol-induced gastric lesion model. Three oral doses of BTB (84, 167, or 501 mg/mL) were used in evaluation of the gastroprotective activity. The results showed a strong dose dependent and significant (p < 0.05) gastroprotective activity (in terms of number, length, and area of hemorrhagic lesions). The gastroprotective activity of BTB was superior to that of the reference drug cimetidine. The high dose of BTB (only dose tested) also offered gastroprotection in rat indomethacin- and serotonin-induced gastric lesion models. Intraperitoneal treatment of BTB and oral treatment of BTB following decaffeination suppressed its gastroprotective potential. However, indomethacin pretreatment did not reduce the gastroprotective potential of BTB in the ethanol-induced gastric lesion model. BTB also increased the gastric mucus content (by Alcian blue test), thickness of the gastric mucus layer (by histopathology), pH of the gastric contents, and possibly the gastric mucosal blood flow, and reduced the gastric acid output of the stomach. BTB also had antihistamine (by wheal test) and antioxidant activity (by the DPPH method) and impaired the gastric transit (by charcoal plug test). It is concluded that BTB of C. sinensis possesses strong oral gastroprotective action, which is mediated via multiple mechanisms. The results also justify the claim made by Sri Lankan traditional practitioners that BTB of C. sinensis has gastroprotective action.
Introduction
Camellia sinensis Linn. O. Kuntze (Theaceae), commonly known as the tea plant, is an evergreen shrub or tree (if not pruned) with alternate, chartaceous or coriaceous leaves. The leaves are typically 5–9 × 2– 3 cm, obtuse or with a short, rounded point. The flowers are bisexual and solitary or 2–3 in a cluster (CitationDassanyake & Forsberg, 1981). Phytochemically, C. sinensis shoots contain flavanols (epigallocatechin gallate, epigallocatechin, epicatechin gallate, epicatechin, gallocatechin, and catechins), flavonols and their glycosides, leucoanthocyanins, caffeine, amino acids including theanine, carbohydrates, organic acids, and volatile compounds (CitationModder & Amarakoon, 2002). C. sinensis is native to Southeast Asia and is extensively cultivated in tropical countries such as Sri Lanka, India, Java, China, Japan, Bangladesh, Indonesia, Kenya, and Turkey for the manufacture of tea (CitationModder & Amarakoon, 2002). Tea is produced from freshly harvested tender shoots, comprising two or three of the topmost immature leaves and buds of the C. sinensis plant. Depending on the manufacturing technique there are three main types of tea: black (fully aerated or fermented), green (unaerated or unfermented), and oolong (partially aerated or semi-fermented) (CitationModder & Amarakoon, 2002).
Sri Lankan traditional native physicians, who are reputed for treating disorders of the gastrointestinal tract, claim that drinking a black tea brew (BTB) of C. sinensis has a protective effect against the development of gastritis and peptic ulcer disease (Native Doctor Abraham Jayasekera, personal communication). Further, experimental studies have shown that triterpene saponins of the seeds of C. sinensis exert gastroprotective effects on ethanol- and indomethacin-induced gastric lesions in rats (CitationMorikawa et al., 2006). However, saponins are absent in BTB of C. sinensis (CitationModder & Amarakoon, 2002). On the other hand, in Ayurvedic medicine, no mention has been made of the gastroprotective action of C. sinensis. However, the claim of the Sri Lankan traditional practitioners that BTB of C. sinensis is gastroprotective seems to be valid, since herbal extracts with a bitter taste (Ticktarasa) are usually recommended in the Ayurvedic system of medicine for gastritis (CitationJayasinghe, 1976), and tea has a slightly bitter taste.
The aim of this study was to investigate the gastroprotective potential of BTB of C. sinensis. This was tested in rats using Sri Lankan high grown Dust grade No: 1 black tea. Dust grade is the most commonly consumed tea in Sri Lanka.
Materials and methods
Experimental animals
Healthy adult Wistar rats (males 200–220 g and females 180–200 g) purchased from the Medical Research Institute, Colombo, Sri Lanka, were used. These animals were acclimatized for 1–2 weeks and were housed in raised, wide mesh bottom cages (to prevent coprophagy) under standard animal house conditions (temperature: 28–31°C; photoperiod: approximately 12 h natural light per day; relative humidity: 50–55%) at the animal house of the Department of Zoology, University of Colombo. All rats had free access to pelleted food (Ceylon Grain Elevators, Colombo, Sri Lanka) and domestic tap water. All animal experiments were conducted in accordance with internationally accepted laboratory animal use and care (based on the Helsinki convention), and guidelines and rules of the Faculty of Science, University of Colombo, for animal experimentation.
Manufacture of tea samples
Black tea belonging to the Dust No: 1 grade was manufactured at the St. Coombs estate tea factory of the Tea Research Institute, Talawakelle, Sri Lanka, from its own green leaves (1382 m above mean sea level) using the orthodox-rotorvane manufacturing technique. The C. sinensis leaves used to manufacture the black tea samples were identified and authenticated by Professor (Mrs.) A. S. Senaviratna, Department of Plant Science, University of Colombo. A voucher specimen (wdr/tspf 200) was deposited at the museum of the Department of Zoology, University of Colombo. Decaffeinated black tea was made as described by CitationPavia et al. (1976). Tea samples were packed in triple laminated, aluminum foil bags (1 kg each) and stored at −20°C until use.
Preparation of black tea brew (BTB)
BTB was made according to the ISO standard (CitationInternational Organization for Standardization, 1980), by adding 2 g of black tea to 100 mL water and brewing for 5 min. This contains 43.7% (w/w) tea solids in water (CitationJayakody & Ratnasooriya, 2008). Based on these data, 501 mg/mL (equivalent to nine cups; 1 cup = 170 mL) of BTB in 2 mL was made by adding 8 g of black tea to 20 mL boiling water and brewing for 5 min; 167 mg/mL (equivalent to three cups) and 84 mg/mL (equivalent to 1.5 cups) concentrations of BTB were then made by appropriately diluting with boiling water. The doses of BTB selected were identical to those used previously for the investigation of bioactivities of C. sinensis leaves in Sri Lanka (CitationRatnasooriya, 2008). All doses of BTB for oral administration (via gastric intubation) were made in 2 mL aliquots.
Evaluation of gastroprotective activity using ethanol-induced acute gastric lesions
Fifty-four rats were randomly divided into six equal groups (n = 9/group). Food was withheld for 36 h and water for 24 h, and then rats were treated orally in the following manner: rats in group 1 with 2 mL of water; group 2 with 84 mg/mL of BTB; group 3 with 167 mg/mL of BTB; group 4 with 501 mg/mL of BTB; group 5 with 501 mg/mL of decaffeinated BTB; and group 6 with 20 mg/kg body weight (b.w.) of cimetidine (CitationFernandopulle et al., 1996). Thirty minutes later, gastric hemorrhagic lesions were induced in these rats by oral administration of 1 mL of absolute ethanol (Fluka Chemicals Co., Buchs, Switzerland). One hour later, these rats were killed with an overdose of ether (BDH Chemicals, Poole, UK). Their stomachs were excised and instilled with 5 mL of 10 % formalin solution (v/v) and immersed in the same solution to fix the outer layer of the stomach. Each stomach was slit opened along the greater curvature, and rinsed with tap water to remove gastric contents and blood clots. The number of macroscopic mucosal hemorrhagic lesions in the glandular portion of the stomach was counted and their lengths were measured with a vernier caliper and summed up per stomach (CitationRatnasooriya et al., 1995; CitationFernandopulle et al., 1996). The outline of each lesion was taken on a transparency sheet and the area was determined using graph paper.
The stomachs of rats treated with water (control) and 501 mg/mL of BTB were preserved in 10% buffered formalin; 5 μm sections were cut using a rocking microtome (LR-85; Osaka, Japan), stained with hematoxylin and eosin, and examined microscopically (× 400) for histopathological changes. The thickness of the mucus layer was determined at 20 random points on each microscope slide using an ocular micrometer.
In another study, 18 rats were subjected to a similar pretreatment procedure. Nine rats were then intraperitoneally injected with 501 mg/mL of BTB and the other nine with 2 mL of water. Gastric lesions were induced and the same hemorrhagic lesion parameters were determined as described previously.
In yet another parallel study, 12 rats were pretreated with 10 mg/kg b.w. of indomethacin (State Pharmaceutical Corporation, Colombo, Sri Lanka) given subcutaneously (CitationRatnasooriya et al., 1995). One hour later, six rats were orally treated with 501 mg/mL of BTB and the other six with 2 mL of water. Gastric lesions were then induced with ethanol and parameters of gastric lesions were determined as described previously.
Serotonin-induced acute gastric lesions
Twelve rats were randomly divided into two equal groups (n = 6/group) and fasted for 24 h with free access to water. The rats in one group were orally administered with 501 mg/mL of BTB and the others with 2 mL of water. Thirty minutes later, a 20 mg/kg b.w. dose of serotonin (Fluka Chemicals Co.) (CitationHemamalini & Varma, 2006) was injected subcutaneously to each of these rats. Eighteen hours later, the animals were sacrificed, and parameters of gastric lesions were determined as described previously.
Indomethacin-induced acute gastric lesions
Twelve rats were randomly divided into two equal groups (n = 6/group) and fasted for 24 h with free access to water. Those in one group were orally treated with 501 mg/mL of BTB and the others with 2 mL of water. After 30 min, all the rats were orally administered with 20 mg/kg b.w. of indomethacin (CitationHemamalini & Varma, 2006). Six hours later, the animals were killed, and parameters of gastric lesions were recorded as described previously.
Measurement of gastric juice volume, pH, acidity, and acid output of gastric secretion
Twelve rats were randomly distributed into two equal groups (n = 6/group). One group was orally administered with 501 mg/mL of BTB and the other with 2 mL of water. One hour later, the rats were anesthetized with ether; their upper abdominal regions were opened with a mid-ventral incision using aseptic precautions. The pylori were ligated using silk ligatures. The animals were then sutured, polymycin antibiotic cream (Astron Ltd., Ratmalana, Sri Lanka) was applied, and they were allowed to regain consciousness, and kept for 4 h without access to water. The animals were then killed with ether, the abdomen opened, and another ligature placed around the esophagus close to the diaphgram. The gastric contents were aspirated using a plastic syringe, and their volumes were recorded. These were centrifuged at 1500 rpm for 15 min, and the supernatants were removed (CitationRatnasooriya et al., 1995). The pH of the supernatants was measured using a pH meter (TOH Electronics, Tokyo, Japan). Free acidity (using phenol red as the indicator) and total acidity (using methyl orange as the indicator) of supernatants were determined by titrating against 0.1 N NaOH as described by CitationVarley (1962). The basal acid output was calculated as the product of total acidity and volume of gastric juice and expressed in terms of μEq/100 g b.w. (CitationRatnasooriya et al., 1995). The protein content of the supernatant was then determined using a Randox assay kit (Randox Laboratories, Antrim, UK).
Assessment of carbohydrate content of gastric juice
The carbohydrate content was estimated using part of the supernatant obtained in the previous part of the experiment, as described by CitationMunasinghe et al. (2002).
Assessment of quantity of mucus adhered to gastric mucosa
Six rats were orally treated with 501 mg/mL of BTB and another six with 2 mL of water. After 1 h, all rats were sacrificed with ether and their stomachs excised, opened along the lesser curvature, inverted, and rinsed with 0.25 M sucrose solution. The quantity of mucus adhered to gastric mucosa was then monitored using the technique described by CitationCorn et al. (1974).
Assessment of pepsin content of gastric juice
Twelve rats were randomly distributed into two equal groups (n = 6/group). One group was orally administered with 501 mg/mL of BTB and the other with 2 mL of water. One hour later, gastric juice was collected and centrifuged, the supernatant separated as described earlier, and the pepsin content determined as described by CitationMunasinghe et al. (2002).
Evaluation of antihistamine activity
Eighteen rats were randomly assigned to two equal groups (n = 9/group). The left posterior lateral side of their skin was clean-shaved under aseptic conditions. One group was orally treated with 501 mg/mL of BTB and the other with 2 mL of water. After 1 h, 50 μL of 200 μg/mL of histamine (Fluka Chemicals Co.) in normal saline was subcutaneously injected to the shaved area of the skin and the area of the wheal formed was determined after 1.5 min (CitationSpector, 1956).
Evaluation of transit time in the gut
Twelve rats were starved for 24 h but water was provided ad libitum. These rats were randomly divided into two equal groups (n = 6/group). One group was orally treated with 501 mg/mL of BTB and the other with 2 mL of water. One hour later, the rats in both groups were orally administered with 0.5 mL of 10% charcoal suspension in distilled water (DW). After 20 min, these rats were sacrificed with ether, and their small intestines (pylorus to cecum) were removed gently, blotted free of blood, unfolded, and placed on white paper. The total length of the small intestine and the distance along which the charcoal plug had moved were measured. The results are presented as percentage distance traveled (CitationRatnasooriya et al., 1995).
Evaluation of the osmotic pressure
BTB with a concentration of 501 mg/mL was made and was allowed to cool to 37°C. The osmotic pressure of the BTB was determined in triplicate using an osmometer (Wescor 5500; Osaka, Japan).
Evaluation of conductivity
The conductivity of the BTB (501 mg/mL) and DW was measured in triplicate at 37°C using a conductivity meter (COND 330i; Weilheim, Germany).
Evaluation of viscosity
The viscosity of BTB (501 mg/mL) was measured in triplicate at 37°C using a viscometer.
Evaluation of antioxidant activity (DPPH assay)
This was done using 750 μL of freshly prepared 20 ppm of 1-1-diphenyl-2-picrylhydrazyl (DPPH) solution as described in detail by CitationAbeywickrama et al. (2005). Briefly, three concentrations of BTB (84, 167, 501 mg/mL) were made, and 750 μL of each sample was added to 750 μL of DPPH solution (in triplicate) and incubated at 30°C for 5 min. The absorbance was then measured at 517 nm using a spectrophotometer. The percentage of the DPPH radical scavenged by the tea extracts was calculated, and the antioxidant activity was expressed as the Trolox equivalent in μg L−1.
Statistical analysis
The results are expressed as mean ± SEM. Statistical comparisons were made using the Mann–Whitney U test. Dose dependency was determined using Pearson’s correlation and trend test. Significance was set at p ≤ 0.05.
Results
Ethanol-induced gastric lesions
The results obtained are summarized in . As shown, the two higher doses, but not the 84 mg/mL dose, of BTB significantly (p < 0.05) impaired the number (167 mg/mL by 58% and 501 mg/mL by 97%), the length (167 mg/mL by 72% and 501 mg/mL by 97%), and the area (167 mg/mL by 79% and 501 mg/mL by 75%) of hemorrhagic lesions. This effect was dose-dependent (number r2 = −0.96, length r2 = −0.96, and area r2 = −0.88; p < 0.05). On the other hand, 100% of rats on 84 mg/mL and 167 mg/mL of BTB exhibited, at least, small lesions, while with the 501 mg/mL dose the number was markedly and significantly (p < 0.05) reduced. The reference drug cimetidine also significantly (p < 0.05) reduced the number (by 67%), length (by 85%), and area (by 93%) of ethanol-induced gastric lesions. In contrast, 501 mg/mL of decaffeinated BTB did not significantly (p > 0.05) reduce any of these three parameters investigated.
Similarly, intraperitoneal administration of the 501 mg/mL dose of BTB failed to alter any of these parameters significantly (p > 0.05) (control vs. treatment: number 10.2 ± 0.03 vs. 12.3 ± 0.02; length 86.5 ± 3.6 vs. 93.5 ± 0.6 mm; and area 139.6 ± 0.8 vs. 142.5 ± 0.9 mm2).
Table 1. The effect of oral treatment of black tea brew and decaffeinated black tea brew of Camellia sinensis on ethanol-induced gastric lesions in rats (mean ± SEM; n = 9/group).
The effectiveness of 501 mg/mL of BTB in inhibiting ethanol-induced gastric lesions with indomethacin pretreatment is shown in . As shown, this dose of BTB significantly (p < 0.05) inhibited the number of lesions (by 92%), the length of lesions (by 96%), and the area of lesions (by 96%) in rats pretreated with indomethacin.
Table 2. The effect of oral treatment of high dose (501 mg/mL) black tea brew of Camellia sinensis on ethanol-induced gastric lesions of rats pretreated with indomethacin (mean ± SEM; n = 6/group).
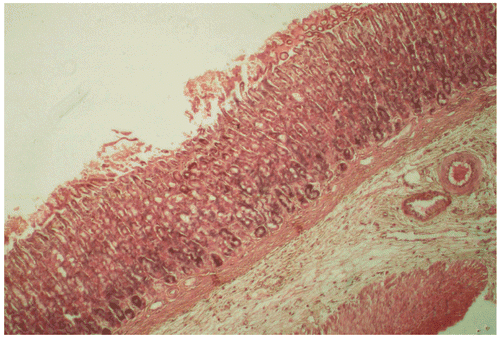
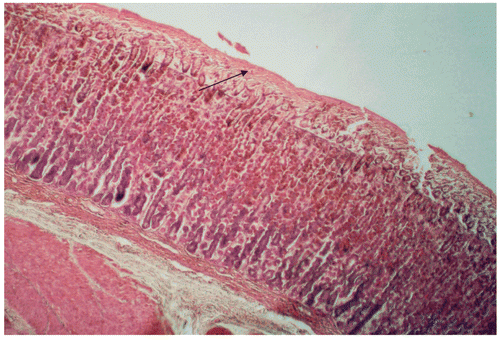
Microscopic examination of sections of the stomachs of control rats revealed disruption of the integrity of the mucosal surface, with deep necrotic penetrations and congestions. In contrast, in the stomachs of rats treated with 501 mg/mL of BTB, the integrity of the endothelium was essentially preserved, with no necrotic damage or congestion. The thickness of the mucus gel of BTB treated rats was significantly (p < 0.05) higher (83%) than that of controls (control vs. treatment: 2.83 ± 0.09 vs. 16.41 ± 0.51 μm) (see also and ).
Serotonin-induced gastric lesions
As shown in , 501 mg/mL of BTB significantly (p < 0.05) and markedly impaired the number (by 64%), the length (by 77%), and the area (by 82%) of gastric lesions induced by subcutaneous administration of serotonin.
Table 3. The effect of oral treatment of high dose (501 mg/mL) black tea brew of Camellia sinensis on serotonin-induced gastric lesions in rats (mean ± SEM; n = 6/group).
Indomethacin-induced gastric lesions
As depicted in , 501 mg/mL of BTB significantly (p < 0.05) inhibited the number (by 80.3%), the length (by 85.1%), and the area (by 88.6%) of gastric lesions induced by intraperitoneal administration of indomethacin.
Table 4. The effect of oral treatment of high dose (501 mg/m) black tea brew of Camellia sinensis on indomethacin-induced gastric lesions in rats (means ± SEM; n = 6/group).
Evaluation of gastric juice volume, pH, acidity, and acid output of gastric secretion
As shown in , 4 h following ligation, 501 mg/mL of BTB significantly (p < 0.05) and markedly reduced the gastric juice volume (by 67%) and acid output (by 69%), and increased the pH of gastric juice (by 76.8%). However, the total protein content was not significantly (p > 0.05) altered (control vs. treatment: 0.75 ± 0.001 vs. 0.77 ± 0.001 mg/dL).
Table 5. The effect of oral treatment of high dose (501 mg/mL) black tea brew of Camellia sinensis on some parameters of gastric content in pyloric ligated rats (mean ± SEM).
Assessment of carbohydrate content of gastric juice
BTB at 501 mg/mL concentration did not significantly (p > 0.05) alter the carbohydrate content of the gastric juice (control vs. treatment: 0.48 ± 0.001 vs. 0.40 ± 0.001 mg/dL).
Assessment of quantity of mucus adhered to gastric mucosa
BTB at 501 mg/mL concentration significantly (p < 0.05) increased (by 120%) the gastric mucus content (control vs. treatment: 0.029 ± 0.002 vs. 0.121 ± 0.001 μg/stomach).
Assessment of pepsin content
BTB at 501 mg/mL concentration reduced the pepsin content (by 33%), although the effect was not significant (p > 0.05) (control vs. treatment: 1.81 ± 0.1 vs. 1.20 ± 0.1 mg/mL).
Evaluation of antihistamine activity
The 501 mg/mL concentration of BTB significantly (p < 0.05) reduced (by 33.5%) the area of the wheal formed following subcutaneous injection of histamine (control vs. treatment: 48.77 ± 1.12 vs. 32.44 ± 0.59 mm2).
Evaluation of transit time in the gut
The 501 mg/mL concentration of BTB significantly (p < 0.05) and markedly impaired (by 54%) the percentage distance that the charcoal plug moved in the small intestine (control vs. treatment: 71.7 ± 1.28 vs. 32.8 ± 1.19%).
Evaluation of osmotic pressure
The osmotic pressure of 501 mg/mL of BTB was 257.2 ± 7.49 mmol.
Evaluation of conductivity
The conductivity of 501 mg/mL of BTB was three-fold and significantly (p < 0.05) higher than that of DW (DW vs. BTB: 3.2 ± 0.1 vs. 9.2 ± 0.1 μs/cm).
Evaluation of viscosity
The viscosity of 501 mg/mL of BTB was not significantly different (p > 0.05) from that of DW (control vs. treatment: 27.5 ± 0.8 vs. 28.5 ± 1.3 Redwood/s).
Antioxidant activity (DPPH assay)
As shown in , the BTB exhibited dose-dependent (r2 = 0.83, p < 0.05) antioxidant activity in vitro.
Table 6. In vitro antioxidant activity of Sri Lanka black tea brew as determined by DPPH assay (mean ± SEM).
Discussion
In this study, we examined the gastroprotective potential of BTB of C. sinensis made from Sri Lankan high grown Dust grade No: 1 black tea. The results showed that BTB has strong oral gastroprotective activity (in terms of number, length, and area of mucosal hemorrhagic lesions). This effect was dose-dependent and had a rapid onset. The presence of a dose–response relationship suggests that the gastroprotective effect is genuine and treatment related. Further, the gastroprotective activity of BTB was superior to that of the reference drug cimetidine.
Usually, a drug, whether natural (CitationRatnasooriya et al., 1995, Citation2005; CitationFernandopulle et al., 1996; CitationAl-Qarawi et al., 2005; CitationHemamalini & Varma, 2006) or synthetic (CitationRang et al., 1995), offers gastroprotection by multiple mechanisms. This was the case with BTB of C. sinensis too. In this study, oral but not intraperitoneal administration of BTB induced gastroprotection. This indicates that local contact with the gastric mucosa is necessary for the BTB to confer gastroprotection. In the ethanol model, pretreatment with indomethacin, a well known inhibitor of cyclooxygenase, did not suppress the gastroprotective effect of BTB. This suggests that BTB is unlikely to act as a mild irritant and relies on endogenous gastric mucosal prostaglandins to exert the gastroprotective effect: mild irritants are gastroprotective, via endogenous mucosal prostaglandins (CitationRobert, 1979; CitationRobert et al., 1983). Collectively, these observations indicate that BTB induces gastroprotection via prostaglandin-independent protective mechanisms, possibly by stimulating the release of other mediators. BTB contains caffeine (CitationBalentine et al., 1997; CitationModder & Amarakoon, 2002), which is known to induce gastroprotection in an ethanol model (CitationKoyama et al., 1999). In this study, decaffeination of BTB impaired its gastroprotective potential considerably, indicating that caffeine plays a major role in eliciting the gastroprotection of BTB.
Viscous paste-like adhesive agents offer gastroprotection by forming a physical barrier on the gastric mucosa (CitationSzabo, 1989). Viscosity measurements and the appearance of BTB showed that it is neither viscous nor paste-like. Further, at autopsy, a paste-like adherent layer was not evident on the gastric mucosa. Hence, BTB is unlikely to have mediated gastroprotection via viscosity. Agents having osmotic pressure greater than 1000 milliosmoles are known to offer gastroprotection (CitationDanon & Assouline, 1979). However, BTB-induced gastroprotection cannot be attributed to this mechanism as it had a low osmolarity. An increase in gastric secretory volume has been implicated with gastroprotection (CitationLacy & Ito, 1982). Such a mode of action is unlikely with BTB as it caused a marked reduction in gastric content in pyloric ligated rats. It is claimed that an increase in protein level in gastric contents may induce gastroprotection (CitationAnandan et al., 1988), but such a mode of action is unlikely with BTB as there was no increase in protein level in gastric fluid in pyloric ligated rats. A reduction in peptic activity has been implicated with gastroprotection (CitationGoel & Bhattacharya, 1991): pepsin leads to autodigestion of the gastric mucosa (CitationRang et al., 1995). However, this mode of action is also unlikely to operate in this study since BTB failed to reduce pepsin levels.
The conductivity of BTB was three-fold higher than that of distilled water, indicating the existence of charged phytoconstituents in the BTB. Charged molecules are claimed to contribute to gastroprotection of drugs by adhering to the gastric mucosal layer and forming a protective barrier (CitationSzabo, 1989). A possibility exists that this mode of action may operate with BTB. This action could be mediated via tannins present in the BTB (CitationBalentine et al., 1997; CitationModder & Amarakoon, 2002), which are known to “tan/strengthen” the outermost layer of the gastric mucosa rendering it less permeable and more resistant to gastric injury (CitationBorrelli & Izzo, 2000).
It is well known that impairment of gastric acidity provides gastroprotection (CitationRang et al., 1995). BTB caused an increase in gastric pH and a simultaneous drastic reduction in acid output. Further, gastroprotection of BTB in the indomethacin-induced gastric lesion model also reinforces the fact that it inhibits the gastric acid output (CitationHemamalini & Varma, 2006). Obviously, this mechanism is likely to play a key role in triggering gastroprotection by BTB in this study. Since BTB has antihistamine activity (in terms of the wheal test) it is possible that impairment of acid secretion may be mediated via gastric histamine receptors, as evident with cimetidine (CitationRang et al., 1995). BTB is a rich source of flavonoids (CitationBalentine et al., 1997; CitationModder & Amarakoon, 2002), which impair histamine secretion (CitationDiCario et al., 1999; CitationBorrelli & Izzo, 2000) and gastric acid release (CitationDiCario et al., 1999; CitationBorrelli & Izzo, 2000). Inhibition of the gastric proton pump can impair acid output (CitationRang et al., 1995). Such an action may be possible with BTB as it contains catechins, quercetin, and rutin, which are potent inhibitors of this pump (CitationMurakami et al., 1992; CitationDiCario et al., 1999; CitationBorrelli & Izzo, 2000). Impairment of acid output can also be mediated via cholinoreceptor antagonism (CitationRang et al., 1995). However, BTB is devoid of such activity (CitationRatnasooriya, 2008), and therefore this mode of action is unlikely to be operative here.
The gastric mucus coat is claimed to play an important role in the defensive mechanism against gastric ulceration by acting as a physical barrier (CitationWallace & Whittle 1986). Allopathic drugs such as sucralfate (CitationSzabo, 1989), carbenoxolone (CitationFrancp et al., 1993), ranitidene (CitationRang et al., 1995), and several herbal decoctions (CitationRatnasooriya et al., 1995, Citation2005; CitationBorrelli & Izzo, 2000; CitationMunasinghe et al., 2002) offer gastroprotection by stimulating mucus production and secretion. BTB induced a marked increase in gastric mucus content (as evident with the Alcian blue technique) and thickness of the gastric mucus layer (as judged from hi stopathology). Obviously, this mode of action is likely to contribute substantially to the gastroprotective action of BTB. Anthocyanosides and quercetin, which are present in BTB (CitationBalentine et al., 1997; CitationModder & Amarakoon, 2002), are shown to be capable of increasing the gastric mucus secretion (CitationBorrelli & Izzo, 2000). An increase in carbohydrate content of the gastric mucus layer can assist in the induction of gastroprotection (CitationMunasinghe et al., 2002), but this mechanism is unlikely to be operative with BTB as it did not change the carbohydrate content.
Active oxygen species are linked with the pathogenesis of gastric mucosal injury (CitationItoh & Guth, 1985), and antioxidants are known to offer gastroprotection (CitationDiCario et al., 1999; CitationBorrelli & Izzo, 2000). BTB is known to possess antioxidant activity (CitationModder & Amarakoon, 2002; CitationAbeywickrama et al., 2005) and the ability to inhibit lipid peroxidation (CitationAbeywickrama et al., 2005). In this study too, antioxidant activity of BTB was also demonstrated. Thus, it is reasonable to attribute the gastroprotective activity of BTB also to its antioxidant activity, which could be mediated via its flavonoids (CitationDiCario et al., 1999; CitationBorrelli & Izzo, 2000). Helicobacter pylori are now linked with the induction of gastric ulcers (CitationDeCross & Marshall, 1993; CitationO’Connor, 1994). Recently, BTB has been shown to possess strong bactericidal activity against H. pylori (CitationO’Mahony et al., 2005), and this mechanism is also likely to play a role in inducing gastroprotection in this study.
An increase in gastric mucosal blood flow is claimed to offer gastroprotection (CitationHemamalini & Varma, 2006). In this study, BTB impaired serotonin-induced gastric lesions, indicating that it enhances gastric mucosal blood flow (CitationHemamalini & Varma, 2006). This action of BTB is likely to contribute to its gastroprotective activity. A reduction in gastric motility is also considered as a mechanism to offer gastroprotection (CitationRang et al., 1995). It is possible that this mechanism may have operated in offering gastroprotection by BTB since it decreased the gut transit time (by charcoal plug test).
In conclusion, this study scientifically demonstrates, for the first time, the acute oral gastroprotective activity of the black tea brew of C. sinensis (mediated via multiple mechanisms), justifying the claim made by Sri Lankan traditional practitioners.
Declaration of interest: This investigation received financial support from the National Science Foundation of Sri Lanka (NSF) under grant number NSF/Fellow/2005/01.
References
- Abeywickrama KRW, Amarakoon AMT, Ratnasooriya WD (2005): In-vitro and In-vivo antioxidant activity of high grown Sri Lankan Black tea (Camellia sinensis L.) Sri Lanka J Tea Sci 70: 57–68.
- Al-Qarawi AA, Abdel-Rahman H, Ali BH, Mousa HM, El-Mougy SA (2005): The ameliorative effect of dates (Phoenix dactylifera L.) on ethanol-induced gastric ulcer in rats. J Ethnopharmacol 98: 313–317.
- Anandan R, Reckha RD, Saravanan N, Devaki T (1988): Protective effects of Picrorrhiza lauroa against HCl/ethanol-induced ulceration in rats. Fitoterapia 70: 498–501.
- Balentine DA, Wiseman SA, Bouwers LCM (1997): The chemistry of tea flavonoids. Crit Rev Food Sci Nutri 37: 693–704.
- Borrelli F, Izzo AA (2000): The plant kingdom as a source of anti-ulcer remedies. Phytotherapy Res 14: 581–591.
- Corn SJ, Morrissey SM, Woods RJ (1974): A method for quantitative estimation of gastric barrier mucus. J Physiol 242: 116–117.
- Danon A, Assouline G (1979): Antiulcer activity of hypertonic solutions in the rat: Role of endogenous prostaglandins. Eur J Pharmacol 58: 425–431.
- Dassanayake D, Forsberg FR (1981): A Revised Hand Book to the Flora of Ceylon. New Delhi, Amreind, pp. 394–396.
- DeCross AJ, Marshall BJ (1993): The role of Helicobacter pylori in acid-peptic disease. Am J Med Sci 306: 381–392.
- DiCario G, Mascolo N, Izzo AA, Capasso F (1999): Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci 64: 337–353.
- Fernandopulle BMR, Ratnasooriya WD, Karunanayake EH (1996): Evaluation of two cucurbits (Genus: Mormodica) for gastroprotection and ulcer healing activity in rats. Med Sci Res 24: 85–88.
- Francp L, Mahara P, Erbetti I, Velo GP (1993): Anti-ulcer activity of carbenoxolone and ISF 3401 on PGE2 release in gastric mucosa. Pharmacol Res 27: 141–150.
- Goel RK, Bhattacharya SK (1991): Gastroduodenal mucosal defense and mucosal protective agents. Ind J Expt Biol 29: 701–714.
- Hemamalini L, Varma VK (2006): Antiulcer activity of Indigofera aspalathoids on chemically induced ulcer models in rats and guinea pigs. Adv Pharmacol Toxicol 7: 25–29.
- International Organization for Standardization (1980): Tea-preparation of liquor for use in sensory tests: ISO 3103: 1532. Geneva, ISO, pp. 1–4.
- Itoh M, Guth PH (1985): Role of oxygen-derived free radicals in hemorrhagic shock-induced gastric lesions in the rat. Gastroenterology 88: 1162–1167.
- Jayakody JRAC, Ratnasooriya WD (2008): Blood glucose level lowering activity of black tea brew Camellia sinensis in rats. Pharmacog Mag 4: 341–349.
- Jayasinghe DM (1976): Ayurveda Pharmacopia. Colombo, Department of Ayurveda, pp. 1–334.
- Koyama R, Kataoka H, Tanaka Y, Nakatsugi S, Furukawa M (1999): Effect of caffeine on ibuprofen-induced gastric mucosal damage in rats. J Pharm Pharmacol 51: 817–824.
- Lacy ER, Ito S (1982): Microscopic analysis of ethanol damage to rat gastric mucosa after treatment with a prostaglandin. Gastroenterology 83: 619–625.
- Modder WWD, Amarakoon AMT (2002): Tea and Health. Talawakelle, Sri Lanka, Tea Research Institute, pp. 1–179.
- Morikawa T, Li N, Nagatoma A, Matsuda H, Li X, Yoshikawa M (2006): Triterpene saponins with gastroprotective effects from tea seed (the seeds of Camellia sinensis). J Nat Prod 69: 185–190.
- Munasinghe SL, Vithanage DK, Ratnasooriya WD (2002): Gastroprotective activity of patoladi decoction in rats. Vidyodaya J Sci 11: 47–55.
- Murakami S, Muramatsu M, Otomo S (1992): Gastric H+, K+-ATPase inhibition by catechins. J Pharm Pharmacol 44: 926–928.
- O’Connor HJ (1994): The role of Helicobacter pylori in peptic ulcer disease. Scand J Gastroenterol 29: 11–15.
- O’Mahony R, Al-Khtheeri H, Weerasekara D, Fernando N, Vaira D, Holton J, Basset C (2005): Bactericidal and anti-alhesive properties of culinary and medicinal plants against Helicobacter pylori. World J Gastroenterol 11: 7499–7507.
- Pavia DL, Lampman GM, Kriz GS (1976): Introduction to Organic Laboratory Techniques. Philadelphia, Saunders, pp. 58–62.
- Rang HP, Dale MM, Ritter JM (1995): Pharmacology. London, Churchill-Livingstone, pp. 385–402.
- Ratnasooriya WD (2008): An Assessment on Potential Health Benefits of Sri Lankan Black Tea by Studying its Bioactivities, Final Report (NSF/Fellow/2005/01). Colombo, National Science Foundation of Sri Lanka, pp. 85–105.
- Ratnasooriya WD, Hewageegama HGSP, Jayakody JRAC, Ariyawansa HAS, Kulatunga RDH (2005): Gastroprotective activity of Evolvulus alsinoides L. powder. Aust J Med Herbalism 17: 55–59.
- Ratnasooriya WD, Premakumara GAS, Ananda UVDS (1995): Protection by Murraya koenigii leaf extract against ethanol induced gastric lesions in rats. Med Sci Res 23: 11–13.
- Robert A (1979): Cytoprotection by prostaglandins. Gastroenterology 77: 761–767.
- Robert A, Nazamis JE, Lancaster C, Davis JP, Field SO, Hanchar AJ (1983): Mild irritants prevent gastric necrosis through “adaptive cytoprotection” mediated by prostaglandins. Gastroenterology 91: 660–666.
- Spector WG (1956): The mediation of altered capillary permeability in acute inflammation. J Pathol Bacteriol 72: 367–373.
- Szabo S (1989): Pathways of gastrointestinal protection and repair: Mechanisms of action of sucralfate. Am J Med 86: 23–31.
- Varley H (1962): Practical Clinical Biochemistry. New York, William Heinemann Medical Books Ltd., pp. 252–263.
- Wallace JL, Whittle BJR (1986): Role of mucus in the repair of gastric epithelial damage in the rat: Inhibition of epithelial recovery by mucolytic agents. Gastroenterology 91: 601–603.