Abstract
Fungal infections represent a significant cause of morbidity and mortality especially in immunocompromised patients in the world today. Dichloromethane (DM) and aqueous (W) extracts of nine plants used traditionally for the treatment of fungal infections in Bukoba rural district in Tanzania were screened for antifungal activity against Candida albicans, Cryptococcus neoformans, and Aspergillus niger using agar well and disk diffusion methods. Dichloromethane extracts of Capparis erythrocarpos [CE] Isert (Capparaceae), Cussonia arborea [CA] Hochst. Ex A. Rich (Araliaceae), Dracaena steudneri [DS] Engl. (Dracaenaceae), Lannea schimperi [LS] (A. Rich) Engl. (Anacardiaceae), Rauvolfia vomitoria [RV] Afz (Apocynaceae), and Sapium ellipticum [SE] (Krauss) Pax (Euphorbiaceae) showed activity against all three fungi. Extracts of Rumex usambarensis [RU] (Dammer) Dammer (Polygonaceae) and Zehneria scabra [ZS] (L.f.) Sond. (Cucurbitaceae) had an activity limited to only one or two of the test organisms. Rhoicissus tridentata [RT] (L.f.) Wild & Drum (Vitaceae) was the only plant without activity. Fractions of the active extracts CE, CA, DS, LS, and SE exhibited higher antifungal activity against one or more of the three fungi. Four compounds isolated from S. ellipticum also exhibited antifungal activity against one or more of the three fungi. The minimum inhibitory concentrations (MICs) and minimum fungicidal concentrations (MFCs), determined using the microplate assay method, ranged between 0.4 and 50.0 μg/mL for crude extracts, 1.6 and 50.0 μg/mL for semi-purified fractions, and 0.12 and 1.0 μg/mL for pure compounds, as compared to 0.016–1.5 μg/mL for fluconazole. We confirm the potential of traditionally used plants as a source of new drugs for treatment of fungal infections.
Introduction
For centuries, medicinal plants have been used throughout the world for the treatment and prevention of various ailments, particularly in developing countries where infectious diseases are endemic and modern health facilities and services are inadequate (CitationSamie et al., 2005). In Africa, the use of medicinal plants in traditional medicine has been practiced since antiquity, and more than 70% of the population depend on it for their primary health care (CitationKamanzy et al., 2002). In Tanzania, traditional medicine is well recognized, and more than 60% of the population use medicinal plants to treat various diseases (CitationMinistry of Health and Social Welfare, 2002).
In the last two decades, invasive fungal infections have been recognized as a major cause of morbidity and mortality in the world (CitationHossain & Ghannoum, 2000). In sub-Saharan Africa, fungal infections, especially Candida infections, are very common and cause significant morbidity among patients (CitationFeldmesser, 2003; CitationMoshi et al., 2007). Fungal infections tend to be chronic, often require prolonged chemotherapy, and present particular risks for immunocompromised individuals (CitationMartin & Ernst, 2004). Most fungal infections are caused by Candida albicans, Aspergillus spp., and Cryptococcus neoformans, but the emergence of other fungal infections is changing the spectrum of the disease (CitationMaertens et al., 2001; CitationLemos et al., 2005). Most common fungal infections are frequently difficult to eradicate with topical preparations and may require long-term use of systemic drugs. Virtually all synthetic antifungals are associated with serious adverse effects; for some fungal infections, there is still no effective cure (CitationErnst, 2001).
Effective management of fungal infections has thus been impaired by the emergence of resistant fungal strains and side effects associated with most conventional antifungal drugs such as polyenes (amphotericin B) and the azoles (itraconazole and fluconazole) (CitationHossain & Ghannoum, 2000; CitationLemos et al., 2005). In some cases, especially for Candida, there is a tendency of pathogen shift from C. albicans to less sensitive species such as C. glabrata and C. krusei, thus reducing the number of available, effective antifungal agents (CitationBastert et al., 2001). The high cost of antifungal agents also renders them unaffordable by the majority of the population in developing countries including Tanzania, where resources are limited (CitationMoshi et al., 2007).
The difficulties associated with the management of fungal infections necessitate the development of new antifungal remedies. For the majority of the rural population in Tanzania, the only option for them is to utilize locally available plant remedies to manage the infections. Plants that are widely used in Tanzanian traditional medicine could offer a potentially useful resource for new and safe drugs for the treatment of fungal infections. The aim of the present study was therefore to determine the antifungal activity of some plants used in traditional medicine to manage fungal infections in Tanzania. Very little or no work has so far been done on the antifungal activities of some of the plant species analyzed in this study, particularly for Capparis erythrocarpos Isert (Capparaceae), Cussonia arborea Hochst. Ex A. Rich (Araliaceae), Dracaena steudneri Engl. (Dracaenaceae), Lannea schimperi (A. Rich) Engl. (Anacardiaceae), and Sapium ellipticum (Krauss) Pax (Euphorbiaceae). There are currently no chemical data known for S. ellipticum, and there is therefore the need to investigate the antifungal activity of possible bioactive compounds from the plant. The study has confirmed the potential of traditionally used plants as a source of new drugs for the treatment of fungal infections.
Materials and methods
Ethnobotanical survey
Ethnobotanical surveys were carried out between September and October 2006 in Bukoba rural district, Kagera region in Northern Tanzania. Having obtained a prior informed consent (PIC), information on plants used in traditional medicine to treat fungal infections was obtained from 30 authentic herbal practitioners (21 women and nine men). With the assistance of local administrative officers, the herbal practitioners were identified through a Participatory Rural Appraisal (PRA) approach according to CitationMartin (1995). They were then interviewed using semi-structured open-ended questionnaires. Interviews were conducted in the local Kihaya language except for a few cases where the respondents could understand Kiswahili. The respondents were requested to give the local names of the plants, parts used, preparation, administration, and the fungal conditions treated with the plants. Since most of the respondents were not aware of the clinical manifestations of various fungal infections, the symptoms of the infections were described to the healers to enable them to give the appropriate plant species they usually use to manage the infections.
Collection and identification of plant materials
Within the same period of ethnobotanical surveys, the practitioners were used as guides in field excursions to collect plant material. Plant materials were collected on the basis of ethnopharmacological surveys. Voucher specimens were identified by Mr. Suleiman Haji and Mr. Frank Mbago of the Department of Botany, University of Dar es Salaam. The voucher specimens were then assigned collection numbers and deposited at the department’s herbarium. The plant species collected for analysis were Capparis erythrocarpos Isert (Capparaceae) [roots] (Collection No. DK028/06), Cussonia arborea Hochst. Ex A. Rich (Araliaceae) [bark] (Collection No. DK022/06), Dracaena steudneri Engl. (Dracaenaceae) [bark] (Collection No. DK014/06), Lannea schimperi (A. Rich) Engl. (Anacardiaceae) [bark] (Collection No. DK047/06), Rauvolfia vomitoria Afz. (Apocynaceae) [bark] (Collection No. DK030/06), Rhoicissus tridentata (L.f.) Wild & Drum. (Vitaceae) [roots] (Collection No. DK072/06), Rumex usambarensis (Dammer) Dammer (Polygonaceae) [leaves] (Collection No. DK060/06), Sapium ellipticum (Hochst.) Pax (Euphorbiaceae) [bark] (Collection No. DK019/06), and Zehneria scabra (L.f.) Sond. (Cucurbitaceae) [leaves] (Collection No. DK017/06).
Preparation and extraction of the plant material
Plant materials were cut into small pieces and then air dried under shade for a period of 10 days at ambient temperature. The dried plant materials were then pulverized using a grinder. About 600 g of each of the pulverized materials was partitioned into two. One part of the material (about 300 g) was extracted by maceration in dichloromethane while the other part was extracted using 100% water. The organic crude extracts were concentrated in vacuo using a rotary evaporator at a temperature not exceeding 40°C. The aqueous extracts were lyophilized using a freeze dryer according to CitationMonks et al. (2002) and CitationBanjaw and Schmidt (2004). Both the organic and the aqueous extracts were dissolved in dimethylsulfoxide (DMSO) and kept in a refrigerator before use. The yield of extraction for organic crude extracts ranged between 7.8 and 16.51 g, while that of aqueous extracts ranged between 2.6 and 9.3 g.
Fractionation of crude extracts and isolation of pure compounds
Fractionation of crude extracts of five plant species, C. erythrocarpos (CE), C. arborea (CA), D. steudneri (DS), L. schimperi (LS), and S. ellipticum (SE), which have scanty literature information, was done using vacuum liquid chromatography (VLC). Column chromatography (CC) was used to isolate compounds from the SE 3 fraction (5.59 g), one of the six fractions obtained from S. ellipticum (SE) crude extracts. The isolated compounds were identified using standard spectroscopic methods 1H nuclear magnetic resonance (NMR), 13C NMR, distortionless enhancement by polarization transfer (DEPT), H,H-correlation spectroscopy (COSY), heteronuclear multiple quantum coherence (HMQC), heteronuclear multiple bond coherence (HMBC), electron ionization, and high resolution mass spectrometry) and by comparison of their data to those reported in the literature.
Test microorganisms and preparation of the inoculum
Three fungal species, Candida albicans (ATCC 90028), Cryptococcus neoformans (ATCC 90112), and Aspergillus niger (AZN 8240), were used for the in vitro antifungal assays. The microbes were obtained from the Department of Molecular Biology and Biotechnology (MBB), University of Dar es Salaam and the Department of Microbiology and Immunology, Muhimbili University of Health and Allied Sciences (MUHAS). Malt extract broth (MEB) was prepared according to the manufacturer’s instructions and dispensed (10 mL) in capped test tubes, autoclaved, and left overnight. Pure isolates of subcultured fungal colonies were then asceptically transferred into the broth and adjusted to a turbidity equivalent to 0.5 McFarland standard with approximate cell concentration of 1 × 106 CFU/mL. The inoculated broth was transferred to a shaking incubator at 150 rpm orbital for an overnight period.
Media preparation and antifungal activity tests
Kirby–Bauer disk and agar well diffusion methods were used to determine the in vitro antifungal activity of crude extracts according to CitationBauer et al. (1966) and CitationPerez et al. (1990), respectively. Semi-purified fractions and pure compounds were tested using the agar well diffusion method only. Malt extract agar was prepared according to the manufacturer’s instructions. The molten medium was inoculated with 0.2 mL of the broth culture, which was spread evenly on the agar surface using a sterile Drigalski spatula. For the agar well diffusion method, equidistant wells were prepared in the plates with the help of a sterile cork-borer. About 75 μL of the plant extracts, semi-purified fractions, or pure compounds and their controls were introduced into the wells (CitationRojas et al., 2006; CitationParekh & Chanda, 2007). For the disk diffusion method, sterile 5 mm Whatman No. 1 filter paper disks were saturated with 75 μL of the test crude extracts and controls, allowed to dry, and then introduced on the upper layer of the seeded agar plates. In both cases, the treated plates were pre-incubated in a refrigerator at 4°C for 4–6 h to allow diffusion of the extracts and controls into the agar while arresting the growth of the test microbes. They were then transferred to an incubator for 24 h at 30°C. The tests were carried out in duplicate.
Determination of minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC)
A broth microdilution method (CitationMurray & Hospenthal, 2004; CitationClinical and Laboratory Standards Institute, 2006) was used to determine the minimum inhibitory concentration (MIC) of crude extracts, semi-purified fractions, and pure compounds. The test was performed in Sero-Wel® sterile flat-bottomed 96-well microplates. Solutions of extracts and compounds were made in DMSO in the concentration range of 50–0.4 μg/mL for crude extracts and semi-purified fractions, and 0.5–0.0078 μg/mL for pure compounds and the positive control fluconazole. Serial dilutions of 100 μL of the test extracts and compounds were dispensed in duplicate into columns 1–10, while fluconazole was dispensed into columns 11 and 12. The serial dilutions were made vertically from rows A to G. The negative controls of inoculated and uninoculated broth were made in row H. An inoculum of approximately106 CFU/mL (100 μL) was added into the wells to make a volume of 200 μL for each well with the test drugs, followed by the addition of 40 μL of 0.2% iodonitrotetrazolium chloride (Sigma-Aldrich, St. Louis, MO, USA) and incubation of the plates at 30 ± 2°C for 24 h. Fungal growth in the plates was observed when the indicator turned from colorless to purple, thus indicating the absence of extract or compound activity. The MIC end-point was recorded when the color of the indicator remained clear, indicating activity of the extracts or compounds. The contents of the microplate wells that showed no visible fungal growth were cultured into malt extract agar plates to determine the fungicidal effects of the extracts. The plates were incubated for a further 24–48 h. The minimum fungicidal concentration was recorded as the lowest concentration of the extract that did not show any fungal growth on the solid agar medium (CitationMann et al., 2008).
Results
During the ethnobotanical surveys, the nine plant species analyzed in this study were found to treat one or more of the following fungal infections: skin rashes, oral candidiasis, ringworm, aspergillosis, cryptococcal meningitis, general skin eruptions, and skin lesions. Extracts of eight out of nine plants showed antifungal activity against one or more of the three fungal species tested (). Generally, activity was detected in the dichloromethane extracts except for C. erythrocarpos, C. arborea, D. steudneri, R. vomitoria, and Z. scabra, whose activity was also in the aqueous extract. Dichloromethane extracts of C. erythrocarpos, C. arborea, D. steudneri, L. schimperi, R. vomitoria, and S. ellipticum showed activity against all three fungi, while extracts of R. tridentata were inactive against all three fungal species. Out of a total of 33 active crude extracts in both agar well and disk diffusion assays, only six of them were aqueous.
Table 1. Antifungal activity of crude extracts observed in the agar well and disk diffusion methods.
Fractionation of dichloromethane extracts yielded 23 fractions with relatively higher antifungal activity than their initial crude extracts, as indicated by bigger growth inhibition zones (). Only dichloromethane crude extracts were fractionated, as they exhibited better activities than water extracts during the preliminary screening. Three fractions, CE 1, CE 2, and CE 3, were obtained from C. erythrocarpos, where CE 3 was the most active against all three fungi. Five fractions CA 1–5 were obtained from C. arborea extract, of which CA 5 was the most active, inhibiting the growth of the three fungal species. The D. steudneri dichloromethane extract yielded five fractions, DS 1–5, and DS 3 was the most active against the three fungi, with excellent activity against A. niger. LS 1–4 fractions of L. schimperi also showed higher activity than the crude extract, but none of them was active against all three fungi. The S. ellipticum dichloromethane extract produced six fractions, SE 1–6, all with higher activity than the original extract, with SE 3 being the most active against all three fungal species. Only one fraction, CA 3 from the dichloromethane extract of C. arborea, was inactive. Overall, the fractions that were active against all three fungal species were CE 3, CA 5, DS 3, and SE 3.
Table 2. Growth inhibition zone means ± SD (mm) of semi-purified fractions observed in agar well diffusion assay.
Column chromatography of extracts of the SE 3 fraction of S. ellipticum yielded four compounds identified as lup-20(29)-en-3-one (1), a triterpene; β-sitosterol (2), a steroid; α-amyrin (3), a triterpene; and acetyl aleuritolic acid (4), a triterpene (). Lup-20(29)-en-3-one (1) was active against C. albicans and A. niger, β-sitosterol (2) and α-amyrin (3) were active against A. niger only, while acetyl aleuritolic acid (4) was active against C. albicans. None of the compounds was active against C. neoformans ().
Figure 1. Chemical structures of compounds isolated from SE 3 fraction of S. ellipticum stem bark extract.
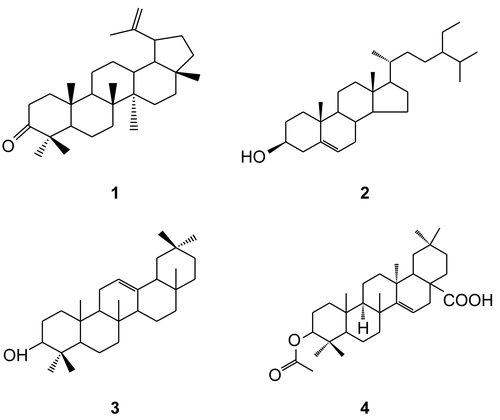
Table 3. Antifungal activity of pure compounds by agar well diffusion method.
The MIC values ranged between 12.5 and 0.4 μg/mL for crude extracts, 25 and 1.6 μg/mL for fractions, 0.5 and 0.12 μg/mL for pure compounds, and 0.5 and 0.016 μg/mL for fluconazole ( and ). For the crude extracts, the lowest MIC value (0.4 μg/mL) was recorded for R. vomitoria against A. niger. Fraction DS 3 had the lowest MIC value of 1.6 μg/mL against C. albicans, while compound α-amyrin (3) had the lowest MIC value of 0.12 μg/mL against A. niger. Crude extract of R. vomitoria, fraction DS 3, and compound α-amyrin (3) were also the most fungicidal, with MFCs of 1.2, 3.2, and 0.36 μg/mL, respectively ( and ).
Table 4. Minimum inhibitory concentrations (MICs) and minimum fungicidal concentrations (MFCs) of dichloromethane crude extracts and semi-purified fractions.
Table 5. Minimum inhibitory concentrations (MICs) and minimum fungicidal concentrations (MFCs) of pure compounds.
Discussion
The present study indicates that extracts of the plants studied have potential for treating fungal infections. This is confirmed by the fact that the majority of the extracts were active against one or more of the three fungal species tested. There was basically no activity for most water extracts, probably because the active constituents are soluble in organic solvents and not in water, as similarly observed by CitationNair and Chanda (2006). Semi-purified fractions and pure compounds were tested using the agar well diffusion method only, as it was superior to the disk assay method in the screening tests.
Although it is not recommended to compare the biological activity of standard drugs such as fluconazole with the activity of a complex mixture of substances (CitationSuffredini et al., 2006), the zones of inhibition of crude extracts for C. erythrocarpos and S. ellipticum and fractions DS 3 and DS 5 against A. niger were relatively comparable to that of fluconazole. The MIC and MFC values for these extracts were, however, not comparable to the standard except for fraction DS 3, which was relatively comparable against C. albicans. The MIC and MFC values of active compounds were also quite favorably comparable with those of fluconazole. Compounds lup-20(29)-en-3-one (1) and acetyl aleuritolic acid (4) were more active than fluconazole against C. albicans, while compound α-amyrin (3) was also favorably comparable against A. niger (), indicating a strong potency of these natural compounds. Since no synergistic action can be associated with pure compounds, it can be argued that the isolated compounds were relatively very active against the respective fungal species. Therefore, probably the compounds were responsible for the strong activity observed in the dichloromethane crude extract of S. ellipticum, especially against A. niger.
In closely related studies, CitationSama and Ajaiyeoba (2006) have reported inhibitory activities of the leaf and stem extracts of Capparis thonningii and C. tomentosa against C. albicans and A. flavus, while CitationMahasneh (2002) reported antifungal activity of aerial parts of C. spinosa against the same fungal strains. These findings augment the antifungal activity of C. erythrocarpos observed in the present study. CitationOkunji et al. (1996) reported fungistatic and fungicidal activity of spiroconazole A, a saponin compound from Dracaena mannii and D. arborea. In a related study by CitationDiallo et al. (2001), extracts from Cussonia barteri and Lannea velutina gave a positive response against C. albicans, thus corroborating the anticandida activity of C. arborea and L. shimperi observed in the present study. R. vomitoria has also been reported to possess antifungal activities (CitationENVIS (Environmental Information System) Center, 2007). This work also confirms the report by CitationDesta (1993) on the anticandida activity of the leaf extract of Z. scabra, though with very mild sensitivity. Nevertheless, methanolic leaf extracts of this plant were non-inhibitory against C. albicans, A. niger, and Trichophyton mentagrophytes, as reported in a different study by CitationMessele et al. (2004). A probable reason for this discrepancy could be that the anticandida compounds were less polar and therefore extractable by dichloromethane used in the present study rather than by methanol used in the previous study.
The present study confirms reports by CitationGhosal et al. (1978) and CitationTangmouo et al. (2006) on the anticandida and antiaspergillus activity of compound lup-20(29)-en-3-one (1). There is, however, no information on the anticryptococcal activity of this compound. In a different study, the antifungal activity of compound β-sitosterol (2) showed a percentage inhibition of spore germination and germ-tube elongation of A. niger (CitationAderiye et al., 1989). An anticandida activity of compound α-amyrin (3) and its derivatives has been reported by CitationJohann et al. (2007). However, information on its antifungal potential against C. neoformans and A. niger is not yet known. So far, there is no report on the antifungal activity of compound acetyl aleuritolic acid (4) against the three fungal species tested.
The antifungal activities of the extracts tested confirm many of their traditional applications in the treatment of fungal infections caused by the studied pathogens. For example, C. erythrocarpos, D. steudneri, R. vomitoria, R. usambarensis, S. ellipticum, and Z. scabra were all used traditionally in the treatment of oral candidiasis. The present in vitro anticandida activity for these extracts confirmed these claims, except for R. usambarensis extract, which did not inhibit the growth of C. albicans. The antifungal assays also confirm the use of C. erythrocarpos, D. steudneri, R. vomitoria, S. ellipticum, Z. scabra, and C. arborea in the treatment of cryptococcal meningitis by traditional healers. An exception is for R. tridentata, whose claim is not confirmed. Only C. erythrocarpos and S. ellipticum were associated with the treatment of aspergillosis, and their antiaspergillus activity is confirmed in this study.
Conclusion
This study authenticates that the information obtained from traditional healers could lead to the discovery of therapeutically useful agents. The detection of antifungal activity in nearly all the studied plant species gives a significant credibility to the claims by traditional healers in their treatment of fungal infections. Crude extracts of C. erythrocarpos, C. arborea, D. steudneri, L. schimperi, and S. ellipticum have been screened for the first time in this study against one or more of the fungal species studied, where they have portrayed various significant activities. The study supports the importance of ethnopharmacological leads in identifying bioactive molecules in drug discovery. Based on the results, extracts of the studied plants could effectively be used to manage the fungal infections associated with the test organisms. There would be a need to scale up the antifungal investigations of the bioactive compounds isolated to in vivo levels as an onset for preclinical trials. Alternatively, herbal formulations of the active crude extracts could be developed based on standardized dosages, for cheaper accessibility by the majority of the population in Tanzania who cannot afford the usually expensive conventional medicines.
Acknowledgements
We are grateful to the respondents and the general community in Bukoba Rural District for their cooperation during the period of collecting field data. Messrs F. M. Mbago and S. Haji of the Herbarium, Botany Department of the University of Dar es Salaam, are thanked for identifying plant voucher specimens.
Declaration of interest: We acknowledge financial support from DAAD/NAPRECA, The International Foundation for Science (IFS) in association with the Organization for the Prohibition of Chemical Weapons (OPCW), and The Inter-University Council of East Africa Research Initiative (VicRes).
References
- Aderiye BI, Ogundana SK, Adesanya SA, Roberts MF (1989): The effect of β-sitosterol on spore elongation of Aspergillus niger and Botryodiplodia theobromae. Int J Food Microbiol 8: 73–78.
- Banjaw MY, Schmidt WJ (2004): Lyophilization and freeze-precipitation as a method for crude extraction of cathinone from Catha edulis leaves with minimum thermal injury. Chem Nat Comp 40: 502–503.
- Bastert J, Schaller M, Korting HC, Evans EG (2001): Current and future approaches to antimycotic treatment in the era of resistant fungi and immunocompromised hosts. Int J Antimicrob Agents 17: 81–91.
- Bauer AW, Kirby WMM, Sherris JC, Turck M (1966): Antibiotic susceptibility testing by a single disc method. Am J Clin Pathol 45: 493–496.
- Clinical and Laboratory Standards Institute (2006): Performance Standards for Antimicrobial Susceptibility Testing; Sixteenth Informational Supplement. M100-S16. Wayne, PA, CLSI, pp. 1–37.
- Desta B (1993): Ethiopian traditional herbal drugs, Part II: Antimicrobial activity of 63 medicinal plants. J Ethnopharmacol 39: 129–139.
- Diallo D, Marston A, Terreaux C, Toure Y, Paulsen S, Hostettmann K (2001): Screening of Malian medicinal plants for antifungal, larvicidal, molluscicidal, antioxidant and radical scavenging activities. Phytother Res 15: 401–406.
- ENVIS Center (2007): Medicinal plants with antifungal activities. http://parisaramahiti.kar.nic.in/med_plant_Antifungal_1.html.
- Ernst E (2001): The desktop guide to complementary and alternative medicine: An evidence-based approach. J R Soc Med 94: 650–651.
- Feldmesser M (2003): New and emerging antifungal agents: Impact on respiratory infections. Am J Respir Med 2: 371–383.
- Ghosal S, Biswas K, Chattopadhyay BK (1978): Differences in the chemical constituents of Mangifera indica, infected with Aspergillus niger and Fusarium moniliformae. Phytochemistry 17: 689–694.
- Hossain MA, Ghannoum MA (2000): New investigational antifungal agents for treating invasive fungal infections. Expert Opin Investig Drugs 9: 1797–1813.
- Johann S, Soldi C, Lyon JP, Pizzolatti MG, Resende MA (2007): Antifungal activity of the amyrin derivatives and in vitro inhibition of Candida albicans adhesion to human epithelial cells. Lett Applied Microbiol 45: 148–153.
- Kamanzy AK, Kone M, Terreaux C, Traore D, Hostettmann K, Dosso M (2002): Evaluation of the antimicrobial potential of medicinal plants from the Ivory Coast. Phytother Res 16: 497–502.
- Lemos JA, Passos XS, Fátima O, Fernandes L, Paula JR, Ferri PH, Souza LKH, Aline de Aquino Lemos AA, Silva MRR (2005): Antifungal activity from Ocimum gratissimum L. towards Cryptococcus neoformans. Mem Inst Oswaldo Cruz 100: 55–58.
- Maertens J, Vrebos M, Boogaerts M (2001): Assessing risk factors for systemic fungal infections. Eur J Cancer Care 10: 56–62.
- Mahasneh AM (2002): Screening of some indigenous Qatari medicinal plants for antimicrobial activity. Phytother Res 16: 751–753.
- Mann A, Banso A, Clifford LC (2008): An antifungal property of crude plant extracts from Anogeissus leiocarpus and Terminalia avicennioides. Tanzania J Health Res 10: 34–38.
- Martin GJ (1995): Ethnobotany: A People and Plants Conservation Manual. London, Chapman & Hall; pp. 112–113.
- Martin KW, Ernst E (2004): Herbal medicines for treatment of fungal infections: A systematic review of controlled clinical trials. Mycoses 47: 87–92.
- Messele B, Lemma H, Abdel-Mohsen MG, Gebre-Mariam T (2004): In vitro evaluation of the antimicrobial activities of selected medicinal plants. Ethiopia Pharmaceut J 22: 1–14.
- Ministry of Health and Social Welfare (2002): Annual Health Statistical Abstract. Dar es Salaam, The United Republic of Tanzania, MoH, pp, 1–10.
- Monks NR, Ferraz A, Bordignon S, Machado KR, Lima M FS, Rocha AB, Schwartsmann G (2002). In vitro cytotoxicity of extracts from Brazilian Asteraceae. Pharm Biol 40: 494–500.
- Moshi MJ, Beukel CJP, Hamza OJM, Mbwambo Z.H, Nondo ROS, Masimba PJ, Matee MIN, Kapingu MC, Mikx F, Verweij PE, Ven AJM (2007): Brine shrimp toxicity evaluation of some Tanzanian plants used traditionally for the treatment of fungal infections. Afr J Trad CAM 4: 19–225.
- Murray CK, Hospenthal DR (2004): Broth microdilution susceptibility testing for Leptospira spp. Antimicrob Agent Chemother 48: 1548–1552.
- Nair R, Chanda S (2006): Activity of some medicinal plants against certain pathogenic bacterial strains. Indian J Pharmacol 38: 142–144.
- Okunji CO, Iwu M M, Jackson JE, Tally JD (1996): Biological activity of saponins from two Dracaena species. Adv Exp Med Biol 404: 415–428.
- Parekh J, Chanda SV (2007): In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turk J Biol 31: 53–58.
- Perez C, Paul M, Bazerque P (1990): An antibiotic assay by the agar well diffusion method. Acta Bio Med Exp 15: 113–115.
- Rojas JJ, Ochoa VJ, Ocampo SA, Munoz JF (2006): Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: A possible alternative in the treatment of non-nosocomial infections. BMC Complement Altern Med 6: 2–8.
- Sama W, Ajaiyeoba EO (2006): Phytochemical and antimicrobial studies of Capparis thonningii and Capparis tomemtosa. Pharmacog Mag 2: 119–122.
- Samie A, Obi CL, Bessong PO, Namrita L (2005): Activity profile of fourteen selected medicinal plants from Venda communities in South Africa against fifteen clinical bacteria species. Afr J Biotechnol 4: 1443–1453.
- Suffredini IB, Paciencia MLB, Varella AD, Younes RN (2006): Antibacterial activity of Brazilian Amazon plant extracts. Braz J Infect Dis 10: 400–402.
- Tangmouo JG, Meli AL, Komguem J, Kuete V, Ngounou FN, Lontsi D, Beng VP, Choudhary MI, Sondengam BL (2006): Crassiflorone, a new naphthoquinone from Diospyros crassiflor (Hien). Tetra Lett 47: 3067–3070.
