Abstract
The methanol extract of Urvillea ulmaceae Kunth (Sapindaceae) aerial parts and the hexane, ethyl acetate, and hydromethanol fractions were evaluated for their free radical scavenging activity with the DPPH assay. Among all the tested fractions, the ethyl acetate fraction was the most active, exhibiting an IC50 of 16.33 μg/mL, comparable to that of the commercial antioxidant BHT. Fractionation of the ethyl acetate fraction through chromatographic methods afforded trans-N-methyl-5-hydroxypipecolic acid, epicatechin, and proanthocyanidin A2 as the main constituents. Epicatechin and proanthocyanidin A2 showed potent DPPH radical scavenging activity, with IC50 values of 18.34 and 11.45 μg/mL, respectively. A new compound, trans-N-methyl-5-hydroxypipecolic acid, did not show any antioxidant effect (IC50 > 500 μg/mL).
Introduction
Reactive oxygen species (ROS), including free radicals, are reported to cause tissue damage of biological systems, and to be involved in aging and in the pathogenesis of some diseases such as arthritis, atherosclerosis, diabetes, and cancer (CitationAmes, 1983; CitationFeher et al., 1987; CitationHalliwel & Gutteridge, 1989; CitationAruoma, 1998). Antioxidants are compounds that protect cells against free mediated processes. Several studies have demonstrated that plants are natural antioxidant sources due, mainly, to the presence of flavonoids, which act in reducing free radical formation and also in scavenging free radicals (CitationMiller, 1996; CitationPietta, 2000; CitationKnekt et al., 2002). In this context, our research group has continued studying plants that occur naturally in the Brazilian stretch of the Upper Paraná River, Porto Rico (PR), to investigate the chemical composition and antioxidant properties of the species present in that area (CitationNazari et al., 2006).
The genus Urvillea is a group of perennial climbers with 13 species distributed in tropical and subtropical areas of the American continent (CitationNogueira et al., 1995). Urvillea ulmaceae Kunth (Sapindaceae) is a naturally occurring species in the Brazilian stretch of the Upper Paraná River, PR, Brazil. A decoction from the crushed stems of this plant is used by the French Guianan Wayapi as an antidiarrheic (CitationGrenand et al., 1987). Reported phytochemical studies on the Urvillea genus are restricted to the U. uniloba Radlk., from which fatty acids and cyanolipids were isolated (CitationMikolajczak et al., 1970; CitationSpitzer, 1996). There are no chemical or biological studies reported for Urvillea ulmaceae.
In the present study, we evaluated the free radical scavenging activity towards 1,1-diphenyl-1-picrylhydrazyl (DPPH) of the methanol extract and its fractions and of an isolated compound from Urvillea ulmaceae aerial parts. Also, the chemical composition of the active fraction was investigated through chromatographic methods. The isolated compounds were characterized by 1D and 2D nuclear magnetic resonance (NMR) spectral data and comparison with those reported in literature.
Materials and methods
Plant material
Aerial parts of Urvillea ulmaceae were collected in May 2004 in a Brazilian stretch of the Upper Paraná River, area of Porto Rico, state of Paraná, Brazil, and identified by Dr. Maria Conceição de Souza. A voucher specimen has been deposited at the Herbarium of the Departamento de Biologia, Universidade Estadual de Maringá, Paraná, Brazil.
Extraction and isolation of the chemical constituents
Air-dried aerial parts of Urvillea ulmaceae (950 g) were exhaustively extracted by maceration with methanol at room temperature. Evaporation of the solvent afforded the methanol extract (27.5 g). Part of this extract (24.5 g) was dissolved in MeOH–H2O 1:1 and partitioned with n-hexane and ethyl acetate. The solvents were evaporated to give the hexane (HF, 5.3 g), ethyl acetate (EAF, 12.5 g), and hydromethanol (HMF, 1.60 g) fractions.
The ethyl acetate fraction (3.5 g) was fractionated on Sephadex LH-20 using H2O, H2O:MeOH 75:25, 50:50, 25:75, and MeOH as solvent, to give five sub-fractions (UM-1 to UM-5). Sub-fraction UM-1 (924. 0 mg) was purified on a chromatographic column of silica gel and eluted with a mixture of CHCl3:MeOH, in increasing polarity, to afford trans-N-methyl-5-hydroxypipecolic acid (27. 3 mg). Purification of sub-fraction UM-3 (606. 5 mg) on Sephadex LH-20 using H2O, H2O:MeOH 75:25, 50:50, 25:75, and MeOH as solvent furnished epicatechin (69. 7 mg). The same procedure was used for purification of the sub-fraction UM-4 (439. 4 mg), which resulted in isolation of proanthocyanidin A2 (60. 0 mg).
Identification of the isolated compounds
The isolated compounds were identified by analysis of their NMR and electron ionization-mass spectroscopy (EI-MS) data. NMR measurements were carried out on a Varian Mercury Plus BB spectrometer operating at 300 MHz for 1H and 75.5 for 13C, using D2O or CD3OD as solvent and tetramethylsilane (TMS) as internal standard. EI-MS spectra were recorded on a Thermoelectron Corporation Focus-DSQ II spectrometer.
The structures of epicatechin and proanthocyanidin A2 were elucidated by comparison of their NMR data with those reported in the literature (CitationFoo et al., 1997). Compound trans-N-methyl-5-hydroxypipecolic acid was identified on the basis of MS and NMR spectroscopic data. EI-MS at 70eV, m/z (relative abundance): 159 (M+, 2), 130 (5), 114 (100), 96 (35), 94 (13), 81 (7), 70 (10). 1H NMR (300 MHz, CD3OD), δ ppm, (mult., J in Hz): 3.50 (m, H-2); 1.75 (q, J = 13 Hz, H-3ax); 2.32 (dd, J = 13 and 1.4, H-3eq); 1.56 (q, J = 11 Hz, H-4ax); 2.15 (m, H-4eq); 3.95 (m, H-5); 2.87 (m, H-6ax); 3.55 (m, H-6eq); 2.93 (N-CH3). 13C NMR (75.5 MHz; CD3OD, δ ppm): 70.8 (C-2), 28.7 (C-3), 32.6 (C-4), 66.6 (C-5), 60.2 (C-6), 45.6 (N-CH3), 176.4 (COOH).
DPPH free radical scavenging assay
Free radical scavenging activities of the test samples and of the positive control butylhydroxytoluene (BHT) were determined using the DPPH free radical method. Various concentrations of the samples were added to 3 mL of daily-prepared methanol DPPH solution (0.1 mM). The mixture was shaken and left to stand at room temperature in the dark. After 30 min, absorbance was measured at 517 nm against a blank (containing all reagents except the test samples). Assays were carried out in triplicate. The concentrations of the samples for 50% inhibition of DPPH (IC50) were obtained from the graph of I% (inhibition percentage) versus concentration of the sample in μg/mL. The percentage inhibition of DPPH (I%) was calculated using the equation: ![]() where Ablank is the absorbance of the blank solution and Asample is the absorbance of the test sample.
where Ablank is the absorbance of the blank solution and Asample is the absorbance of the test sample.
Results and discussion
The free radical scavenging activity was initially evaluated for the crude methanol extract of Urvillea ulmaceae. The results () showed that the crude methanol extract presented significant free radical scavenging activity, with an IC50 of 26.98 μg/mL. Fractionation of this extract by solvent partition furnished the hexane (HF), ethyl acetate (EAF), and hydromethanol (HMF) fractions, which were tested for their antioxidant capacity toward the radical DPPH. Comparison of the obtained IC50 data () indicated a potent activity for the EAF fraction (IC50 = 16.33 μg/mL), which was comparable to that of the commercial antioxidant BHT, and a moderate free radical scavenging effect for HF (IC50 = 111.96 μg/mL) and HMF (IC50 = 74.16 μg/mL) fractions. The most active fraction (EAF) was purified by chromatography column on Sephadex LH-20 or silica gel to afford the compound trans-N-methyl-5-hydroxypipecolic acid (), epicatechin, and proanthocyanidin A2.
Table 1. DPPH free radical scavenging activity (IC50) for methanol extract, HF, EAF, and HMF fractions, and isolated compounds from Urvillea ulmaceae.
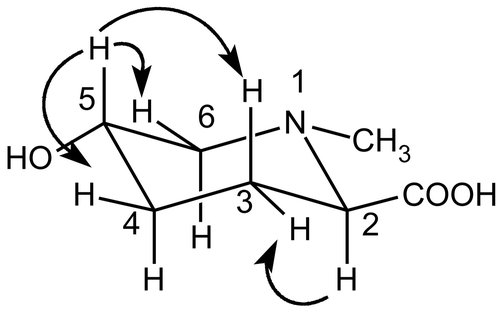
The compound trans-N-methyl-5-hydroxypipecolic acid () is being reported for the first time, according to our knowledge. Its structure determination was based on 1D and 2D NMR data (see “Materials and methods” section) and comparison with those of trans-5-hydroxypipecolic acid (CitationMester et al., 1979). Its 1H NMR spectrum was characteristic of a pipecolic acid derivative, showing signals for an oxymethine hydrogen at δ 3.95 (m, H-5), a carboxymethine hydrogen at δ 3.50 (m, H-2), and for three groups of methylene hydrogens at δ 1.75 (H-3ax) and 2.32 (H-3eq); δ 1.56 (H-4ax) and 2.15 (H-4eq); δ 2.87 (H-6ax) and 3.55 (H-6eq). The singlet at δ 2.93 (3H) was attributed to the methyl group on the nitrogen atom. The 13C NMR spectrum confirmed the proposed structure by the signals for a carboxyl group at δ 176.4, an N-CH3 at 45.6, two methine carbons at δ 70.8 (C-2) and 66.6 (C-5), and three methylene carbons at δ 28.7 (C-3), 32.6 (C-4), and 60.2 (C-6). Localization of the hydroxyl group at C-5 was confirmed from the J2 or J3 HMBCs (heteronuclear multiple bond correlations) between the signal at δ 66.6 (C-5) and those of H-3, H-4, and H-6. The NOESY (nuclear Overhauser enhancement spectroscopy) spectra permitted establishment of the relative stereochemistry of the isolated compound. The correlation between the signals for H-2 (δ 3.50) and H-3eq (δ 2.32) indicated that the group -COOH occupied an equatorial position. The equatorial position for the hydroxyl group was confirmed by the interactions observed between H-5 and H-3ax, H-4eq and H-6eq. EI-MS spectra showed the molecular peak at m/z 159, consistent with the proposed structure. From these data the isolated compound was identified as trans-N-methyl-5-hydroxylpipecolic acid. The new compound showed no free scavenging activity in the DPPH test (IC50 > 500 μg/mL; ).
Epicatechin and proanthocyanidin A2 are widespread in nature, occurring in various plants (CitationFoo et al., 1997; CitationOkawa et al., 2001; CitationPloss et al., 2001; CitationLin et al., 2002; CitationVerstraeten et al., 2005; CitationShahat, 2006; CitationLiu et al., 2007), including species belonging to the Sapindaceae family (CitationSarni-Manchado et al., 2000; CitationSakane et al., 2005). The isolated epicatechin and proanthocyanidin A2 demonstrated potent free radical scavenging activity in the DPPH assay, with IC50 values of 11.45 and 18.34 μg/mL, respectively. The antioxidant activities of these compounds are well described in the literature (CitationOkawa et al., 2001; CitationVerstraeten et al., 2005; CitationLiu et al., 2007).
In conclusion, our study demonstrated the potential antioxidant properties of the plant Urvillea ulmaceae as a scavenger of free radicals and showed that this effect can be attributed to the compounds epicatechin and proanthocyanidin A2.
Declaration of interest: We are grateful to CAPES and CNPq for fellowships (S.A.D., F.P.C.), and to Fundação Araucária (Paraná State, Brazil) for financial support.
References
- Ames BM (1983): Dietary carcinogens and anticarcinogens: oxygen radical and degenerative diseases. Science 221: 1256–1263.
- Aruoma OI (1998): Free radicals, oxidative stress and antioxidants in human health. J Am Oil Chem Soc 75: 199–212.
- Feher J, Csomos G, Vereckei A (1987): Free Radical Reactions in Medicine. Berlin-Heidelberg, Springer-Verlag, pp. 40–43.
- Foo LY, Lu Y, McNabb WC, Waghorn G, Ulyatt MJ (1997): Proanthocyanidins from Lotus peduncylatus. Phytochemistry 45: 1689–1696.
- Grenand P, Moretti C, Jacquemin H (1987): Pharmacopées Tradionnelles en Guyane: Créoles, Palikur, Wayapi. Paris, Editions de l´ORSTOM, pp. 569–570.
- Halliwel BG, Gutteridge JMC (1989): Free Radicals in Biology and Medicine. Oxford, Clarendon Press, pp. 416–494.
- Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliovaara M, Reunanen, A, Hakulinen T, Aromaa A (2002): Flavonoid intake and risk of chronic diseases. Am J Clin Nutr 76: 560–568.
- Lin LC, Kuo YC, Chou CJ (2002): Immunomodulatory proanthocyanidins from Ecdysanthera utilis. J Nat Prod 65: 505–508.
- Liu L, Xie BJ, Cao SQ, Yang EN, Xu Xy, Guo SS (2007): A-type procyanidins from Litchi chinensis pericarp with antioxidant activity. Food Chem 105: 1446–1451.
- Mester L, Szabados L, Mester M, Yadav N (1979): Identification by carbon-13 NMR spectroscopy of trans-5-hydroxypipecolic acid a new inhibitor of platelet aggregation induced by serotonin in the leaves of Xylia xylocarpa. Planta Med 35: 339–341.
- Mikolajczak KL, Smith CRJ, Tjarks LW (1970): Cyanolipids of Cardiospermun halicacabum and other sapindaceous seed oil. Lipids 5: 812–817.
- Miller AL (1996): Antioxidant flavonoids: Structure, function and clinical usage. Alt Med Rev 1: 103–111.
- Nazari AS, Dias SA, da Costa WF, Bersani-Amado CA, Vidotti GJ, de Souza MC, Sarragiotto MH (2006): Anti-inflammatory and antioxidant activities of Randia hebecarpa and major constituents. Pharm Biol 44: 7–9.
- Nogueira CZ, Ruas PM, Ruas CF, Ferrucci MS (1995): Karyotypic study of some species of Serjania and Urvillea (Sapindaceae, tribe Paullinieae). Am J Bot 82: 646–654.
- Okawa M, Kinjo J, Nohara T, Ono M (2001): DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity of flavonoids obtained from some medicinal plants. Biol Pharm Bull 24:1202–1205.
- Pietta P (2000): Flavonoids as antioxidants. J Nat Prod 63: 1035–1042.
- Ploss O, Petereit F, Nahrstedt A (2001): Procyanidins from the herb of Hypericum perforatum. Pharmazie 56: 509–511.
- Sakane W, Hara N, Fujimota Y, Takaish Y, Acuña R, Osorio C, Duque C (2005): Cupaniol, a new branched polyphenol, from Cupania latifolia. Chem Pharm Bull 53: 1037–1039.
- Sarni-Manchado P, Le Roux E, Le Guerneve C, Lozano Y, Cheynier V (2000): Phenolic composition of litchi fruit pericarp. J Agric Food Chem 48: 5995–6002.
- Shahat AA (2006): Procyanidins from Adansonia digitata. Pharm Biol 44: 445–450.
- Sptizer V (1996): Fatty acid composition of some seed oils of the Sapindaceae. Phytochemistry 42: 1357–1360.
- Verstraeten SV, Hammerstone JF, Keen CL, Fraga CG, Oteiza PI (2005): Antioxidant and membrane effects of procyanidin dimers and trimers isolated from peanut and cocoa. J Agric Food Chem 53: 5041–5048.