Abstract
The effects of water extract of Cajanus cajan (L.) Millsp. leaves (WECML) on differentiation and function of primary osteoblasts and osteoclasts were studied. The results indicated that WECML inhibited the proliferation of osteoblasts at higher concentration of 100 μg/mL and the inhibition rate was 12.12%. When its concentration descended to 0.01~10 μg/mL, it turned to stimulate the proliferation of the osteoblasts. The effects of WECML on differentiation of osteoblasts depended on concentrations and time. It promoted the mineralization function of osteoblasts at concentrations of 0.01, 0.1, and 1 μg/mL, but inhibited the mineralization function at concentrations of 10 and 100 μg/mL. It inhibited bone resorption activity as indicated by the dose-dependent reduction in the pit numbers and areas (P < 0.05). This indicates that the WECML may be a potential source of natural anti-osteoporosis products.
Introduction
As defined by the World Health Organization, osteoporosis is a systemic skeletal disease characterized by low bone mass and bone matrix deterioration, leading to bone fragility and an increased risk of fracture. As the general population is ageing, osteoporosis is becoming more prevalent, not just in China, but worldwide. In the western world, the prevalence of osteoporosis and osteopenia in those aged 55 to 64 years is 20% and 37%, respectively. After the age of 80 years, the prevalence of osteoporosis reaches almost 70%. Therefore, the projected incidence of hip fractures is expected to increase to three-fold the current value by 2050 (CitationMargaret et al., 2008).
The rationale for prevention and treatment of osteoporosis is directed along two basic approaches, namely, agents preventing bone resorption (estrogen, calcitonin, bisphosphonates, calcium, vitamin D, raloxifene) and those stimulating bone formation (fluoride, anabolic steroids). Estrogen replacement therapy (ERT) was a popular regime in prevention and treatment of postmenopausal osteoporosis. However, recent evidence suggests that ERT is associated with increased risk of breast, ovarian and endometrial cancer development (CitationDavison & Davis, 2003). In addition, the most frequently used anti-osteoporosis drugs are developed in affluent countries and the costs are too high to benefit a large population in developing or even developed countries for prevention and treatment of osteoporosis. Thus, alternative treatment or prevention regimes for osteoporosis are urgently needed (CitationSakamoto et al., 2000).
Traditional Chinese medicine has been widely used in orthopedic clinical practice for thousands of years for the treatment of fractures and joint diseases. Their therapeutic actions are believed to be mediated through multiple signaling pathways and cellular targets that in turn restore the pathogenic status of multiple physiological systems in the body. Many herbs that are known to possess “kidney-tonifying” activities or “bone and gonad nourishing” effects have been demonstrated to be effective in reducing bone loss and promoting fracture healing (CitationWu et al., 2003). Cajanus cajan (L.) Millsp. is an important drug in traditional Chinese medicine for reducing swellings, alleviating pain, invigorating kidney and strengthening bones, treating a wide range of diseases including osteoporosis (CitationSun et al., 2001; CitationDornstauder et al., 2001). A drug made of WECML has been approved for clinical use by the State Food and Drug Administration (SFDA) in China, and has a remarkable preventive effect on glucocorticoid-induced avascular necrosis of the femoral head. Some experimental results indicate that this drug can increase the number of osteoblasts and decrease the number of osteoclasts by the animal model experiment (CitationYuan et al., 2005). Accordingly, WECML may prevent osteoporosis, but WECML’s effects on osteoblasts and osteoclasts were not reported in vitro so far. In order to further elucidate the mechanism of the action of WECML on osteoblasts and osteoclasts, in this paper the effects of WECML on differentiation and function of primary osteoblasts and osteoclasts were studied in vitro.
Materials and methods
Materials
Newborn Japanese white rabbits and Kun Ming (KM) mice were purchased from Guangming Weiwu Biological Product Factory (Shenzhen, China). Bovine femur was stored at −70°C. Minimum essential medium alpha (α-MEM), collagenase A, trypsin and fetal calf serum (FCS) were purchased from Gibco (Carlsbad, CA). Benzylpenicillin, streptomycin, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT), β-glycerophosphate, dexamethasone and ascorbic acid were obtained from Sigma Chemical Co (St Louis, MO). Demethyl sulfoxide (DMSO) was purchased from Sangon (Shanghai, China). An alkaline phosphatase (ALP) activity kit was obtained from Nanjing Jiancheng Biological Engineering Institute (Nanjing, China), micro-protein assay kit was purchased from Beyotime Biotechnology (Haimen, China). Alendronate was purchased from Shijiazhuang Pharma (Shijiazhuang, China). The leaves of Cajanus cajan were purchased in Hainan province in China in August 2005 and were identified by Zhu Guo-Yuan of the Shenzhen Research Institute of the City University of Hong Kong. A voucher specimen was deposited in Shenzhen Research Institute of the City University of Hong Kong, Shenzhen, China.
Preparation of WECML
Leaves of Cajanus cajan (5 kg) were extracted with water (50 L) three times, 1.5 h each time. The extraction was filtered. The filtered extraction was concentrated to get a relative density of 1.05~1.10 (60°C), and then subjected to spray drying. The total flavonoids was measured according to the method previously reported (CitationHuang et al., 2006). The dried substance (50 mg) was dissolved in 10 mL water and subjected to column chromatography over Amerlite XAD 7HP and eluted with distilled water and ethanol respectively. The eluant was collected and evaporated to dryness, the residue was dissolved in ethanol, and AlCl3 was added. Vitexin in solution was measured by spectrophotometry at a wavelength of 274 nm. The vitexin was calculated according to standard curve. Polysaccharide was measured according to the method previously reported (CitationZhao & Cheng, 2006).The dried substance (50 mg) was dissolved in water (5 mL) and ethanol (15 mL) was added. The solution was centrifuged 4,000 rpm/min) and the supernatant was removed. The sediment was dissolved in water (5 mL) and ethanol (20 mL) was added again. The solution was centrifuged (4,000 rpm/min) and the supernatant was removed. The sediment was dissolved in water again and 0.6% phenol solution was added. Glucose in solution was measured by spectrophotometry at a wavelength of 490 nm. Glucose was calculated according to standard curve. The total flavonoids in the WECML (0.5 g) is no less than 50 mg calculated as vitexin, vitexin in the WECML (0.5g) is no less than 0.8 mg, polysaccharide in the WECML (0.5 g) is no less than 50 mg calculated as glucose.
Preparation of devitalized bone slices
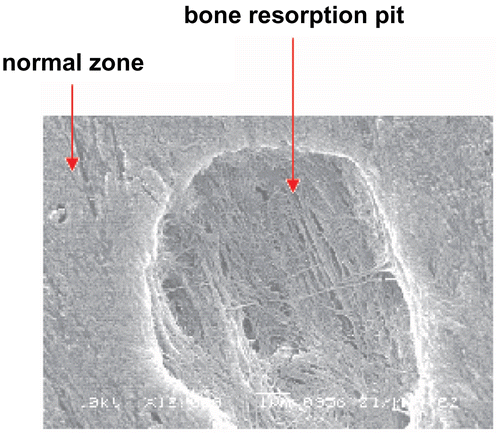
Bone slices (6 × 6×0.05 mm) were prepared according to the method of Arnett and Dempster (CitationArett & Dempster, 1986). Transverse slices of dense cortical bone were cut from the diaphysis of adult bovine femurs using a low-speed diamond saw. Slices were cleaned by ultrasonication three times for 10 min each time in distilled water and further sterilized. Scanning electron microscopy (SEM, S250 Mk3, Link AN 10000) was performed on a number of uncultured bone slices to ensure that their surface was smooth and that no cavities were present ().
Isolation and culture of primary osteoblasts
Osteoblasts were isolated enzymatically from newborn mouse skull as described previously (CitationLi et al., 2005). Briefly, the skull (frontal and parietal bones) was dissected from KM mice, the endosteum and periosteum were stripped off, and the bone was cut into approximately 1~2 mm2 pieces and digested with trypsin (2.5 g/L) for 30 min, and the digestion was discarded. The bone was then digested twice with collagenase A (2 g/L) for 1 h each time. The cells were collected and cultured in a culture flask. After being placed overnight in a 5% CO2 humidified incubator at 37ºC, the α-MEM was removed. The medium was changed every 3 days in all experiments.
Isolation and culture of rabbit osteoclasts
The isolation of rabbit osteoclasts was performed according to the procedures previously described (CitationZhang et al., 2003). Briefly, the femur, tibia and humerus were removed from neonatal Japanese white rabbits, dissected free of adherent tissue, placed in α-MEM and cut into small fragments. The bone fragments were pipetted vigorously to release the osteoclasts, and then allowed to sediment for 30 s for experiment.
Osteoblast proliferation
The protocol described by CitationMosmann (1983) was followed with some modifications. Briefly, osteoblasts were plated in 96-well culture plates (104 cells per well) and grown overnight at 37°C in a 5% CO2 incubator. WECML and NaF were then added to the wells to achieve final concentrations. Control wells were prepared by addition of α-MEM. Wells containing α-MEM without cells were used as blanks. The plates were incubated at 37°C in a 5% CO2 incubator for 44 h. Upon completion of the incubation, stock MTT dye solution (20 μL, 5 mg/mL) was added to each well. After 4 h incubation, 2-propanol (100 μL) was added to solubilize the MTT formazan. The optical density (OD) was then measured on a microplate spectrophotometer (Bio-Rad Model 3550) at a wavelength of 570 nm. The proliferation rate was calculated according to the formula (1–ODtreated/ODcontrol) ×100%.
Measurement of ALP activity
The protocol described by CitationGray (1987) was followed. Briefly, osteoblasts were plated in 48-well culture plates (104 cells per well) and grown overnight at 37°C in a 5% CO2 incubator. WECML and NaF were then added to the wells to achieve final concentrations. Control wells were prepared by addition of α-MEM. The plates were incubated at 37°C in a 5% CO2 incubator for 2 days and 12 days, respectively. The medium was then removed and the cells were washed with 10 mmol/L Tris-HCl (pH 7.2), and sonicated in 1 mL of 50 mmol/L Tris-HCl (pH 7.2), that contained 0.1% Triton X-100 and 2 mmol/L MgCl2 for 15 s with a sonicator (Ultrasonic Disruptor UD-201, Japan). The ALP activity and concentration of protein were measured by alkaline phosphatase activity kit and micro-protein kit, respectively.
Quantitation of Ca2+
Osteoblasts were subcultured in α-MEM containing 15% fetal bovine serum, 10 mmol/L β-glycerophosphate, 50 μg/mL ascorbic acid. WECML and NaF were then added to the wells to achieve final concentrations. The amount of Ca2+ in the cell layer was measured as follows. The layers of cells in 24-well plates were washed with phosphate-buffered saline (pH 7.4, 20 mmol/L sodium phosphate and 130 mmol/L NaCl) and incubated with 1 mL of 2 mol/L HCl overnight with gentle shaking. The [Ca2+] in the samples was measured with an atomic absorption spectrometer (2100, PE company).
Measurement of bone resorption pit numbers and areas
The cell suspension of osteoclasts was transferred into 24-well tissue culture plates containing the bovine bone slices prewetted with 1 mL α-MEM. After incubation in α-MEM containing 15% fetal calf serum at 37ºC for 16 h, the osteoclasts stuck to the bone slices, then the bone slices were vigorously washed twice to remove non-adherent cells. WECML and alendronate were added to the wells to achieve final concentrations. The bone slices were then incubated in α-MEM containing 15% fetal calf serum for 7 days at 37ºC in a humidified atmosphere with 5% CO2. The resorption pits were captured using a microscope equipped with a digital camera. The pit numbers on each slice were counted. The total resorption pit areas were measured by image analyzing software (LEICA Q550IW, Germany) (CitationYuhara et al., 1999; CitationMoonga & Dempster, 1995).
Calcium ion concentration measurement
The supernatant was collected at the end of the experiment, and the total concentration of calcium in the supernatant was measured with an atomic absorption spectrometer (2100, PE company) (CitationSugawara et al., 1998).
Statistical analysis
Data were collected from at least three separate experiments. The results were expressed as mean ± standard deviation. The statistical differences were analyzed using SPSS t-test; p values less than 0.05 were considered to indicate statistical differences.
Results
Effects of WECML on the proliferation of osteoblasts
The exposure of osteoblasts to WECML (0.01~10 μg/mL) promoted the proliferation of the osteoblasts, moreover the proliferation rate reached maximal value at concentration of 1 μg/mL. WECML inhibited the proliferation of osteoblasts at the higher concentration of 100 μg/mL. The inhibition rate was 12.12% ().
Table 1. Effects of WECML on the proliferation of osteoblasts for 48 h (n = 6).
Effects of WECML on the differentiation of osteoblasts
The alkaline phosphatase activity of osteoblasts that had been exposed to WECML (0.01, 0.1, 10, and 100 μg/mL) for 2 days was significantly higher than that in control cells (P < 0.05), but had no significant effect on alkaline phosphatase activity at concentration of 1 μg/mL (P > 0.05). On day 12, the alkaline phosphatase activity of osteoblasts that had been exposed to WECML (0.01, 0.1 and 1 μg/mL) was significantly higher than that in control cells (P <0.05), but lower than that in control at concentrations of 10 and 100 μg/mL (P <0.05) ().
Table 2. Effects of WECML on the differentiation of osteoblasts.
Effects of WECML on the mineralization function of osteoblasts
WECML promoted the deposition of Ca2+ by osteoblasts at concentrations of 0.01, 0.1, and 1 μg/mL, but decreased the deposition of Ca2+ at concentrations of 10 and 100 μg/mL ().
Table 3. Effects of WECML on the mineralization of osteoblasts for 14 days.
Effects of WECML on bone resorption activity of osteoclasts
WECML (0.01~100 μg/mL) inhibited bone resorption activity as indicated by the dose-dependent reduction in the pit numbers and areas on the surface of bone slices ( and ).
Table 4. Effects of WECML on the bone resorption pit number of osteoclasts for 7 days.
Table 5. Effects of WECML on areas of bone resorption pits of osteoclasts for 7 days.
Effects of WECML on the calcium release
After treatment of osteoclasts with WECML for 7 days, WECML (0.01~100 μg/mL) reduced the calcium release from bone slices in a dose-dependent manner ().
Table 6. Effects of WECML on the calcium release from bone slices induced by osteoclasts for 7 days.
Discussion
The process of bone remodeling is controlled by a balance of bone formation and bone resorption. The formation and resorption of bone are maintained by the interactions of osteoblasts with osteoclasts. Excessive bone resorption that overcomes bone formation results in osteoporosis.
Osteoblasts are bone-forming cells. The formation of bone involves a complex series of events that include the proliferation and differentiation of osteoprogenitor cells and result eventually in the formation of a mineralized extracellular matrix (CitationYuhara et al., 1999). The sequential expression of type I collagen, alkaline phosphatase and osteocalcin, and the deposition of Ca2+ are known as markers of osteoblastic differentiation and function. Osteoclasts are multinucleated cells that are responsible for resorption of bone. The osteoclastic bone resorption consists of several processes (CitationSuda et al., 1997): the development of osteoclasts from hematopoietic progenitor cells, the fusion of osteoclasts, the attachment of osteoclasts to the surface of the bone and the secretion of acids and lysosomal enzymes into the space beneath the osteoclast. So osteoclasts and osteoblasts are important cells in the process of pathology of osteoporosis.
In the present work, osteoblast and osteoclast models were used to evaluate the anti-osteoporosis activity of WECML. Our results indicated that WECML inhibited the proliferation of osteoblasts at the higher concentration of 100 μg/mL; when its concentration reduced to 0.01~10 μg/mL, it turned to stimulate the proliferation of the osteoblasts. The effects of WECML on differentiation of osteoblasts depend on concentrations and time. On day 2, it had no effect on differentiation of osteoblasts at a concentration of 1 μg/mL, but promoted differentiation of osteoblasts at other concentrations. On day 12, WECML promoted differentiation of osteoblasts at concentrations of 0.01, 0.1, and 1 μg/mL, but inhibited differentiation of osteoblasts at concentrations of 10 and 100 μg/mL. In addition, WECML promoted mineralization of osteoblasts at concentrations of 0.01, 1, and 10 μg/mL, but inhibited mineralization of osteoblasts at concentrations of 10 and 100 μg/mL. The effects of WECML on rabbit osteoclast bone-resorbing activity exhibited a dose-dependent reduction in the pit numbers and areas, and was further confirmed by calcium release from bone slices. These results show that WECML has a positive effect on osteoporosis in vitro.
Cajanus cajan leaves has been reported to contain vitexin, isovitexin, apigenin, luteolin, naringenin-dimethylether, longistyline A, longistyline C, pinostorbin and salicylic acid, etc. (CitationBhanumati et al., 1979; CitationCooksey et al., 1982; CitationDuker-Eshun et al., 2004). Several in vitro studies indicated the osteoblastic proliferation stimulating activity of the total flavonoids and main flavonoid constituents from Herba Epimedii toward primary osteoblasts and osteoblast-like UMR106 cells (CitationZhang et al., 2008). So we deduce the major effective components in WECML may be flavonoids. The defined active ingredients in WECML and their mechanisms of action remain to be further studied.
Declaration of interest: This work was supported by the Foundation for Key Program of the Ministry of Education of China (No. 208018) and the Natural Science Foundation of Hebei University. Returned Scholars of Hebei Province (No.207041). The authors alone are responsible for the content and writing of the paper.
References
- Arett TR, Dempster DW (1986): The effect of pH on bone resorption by rat osteoclasts in vitro. Endocrinology 119: 119–124.
- Bhanumati S, Chhabra SC, Gupta SR (1979): A new isoflavone glucoside from Cajanus cajan. Phytochemistry 18: 365–366.
- Cooksey CJ, Dahiya JS, Garratt PJ (1982): Two novel stilbene 2-carboxylic acid phytoalexins from Cajanus cajan. Phytochemistry 21: 2935–2938.
- Davison S, Davis SR (2003): Hormone replacement therapy: Current controversies. Clin. Endocrin 58: 249–261.
- Dornstauder E, Jisa E, Unterrieder I (2001): Estrogenic activity of two standardized red clover extracts (menoflavon) intended for large scale use in hormone replacement therapy. Steroid Biochem Mol Biol 78: 67–75.
- Duker-Eshun G, Jaroszewski JW, Asomaning WA (2004): Antiplasmodial constituents of Cajanus cajan. Phytotherapy Res 18: 128–130.
- Gray TK (1987): 17β-Estradiol acts directly on the clonal osteoblastic cell line UMR106. Proc Natl Acad Sci USA 84: 6267–6271.
- Huang YM, Zhao L, Wang ZZ, Pan LH, Li CE (2006): Determination of flavonoids in Danhong injection by spectrophtometric method. Chin Hosp Pharm J 26: 845–846.
- Li XH, Zhang JC, Sui SF, Yang MS (2005): Effect of daidzin, genistin and glycitin on the osteogenic and adipogenic differentiation of bone marrow stromal cells and the adipocytic trans- differentiation of osteoblasts. Acta Pharmacol Sin 26: 1081–1086.
- Margaret WMF, Leung HB, Lee WM (2008): Osteoporosis: Public awareness, commitment, and perspectives. Hong Kong Med J 14: 1–6.
- Moonga B, Dempster DW (1995): Zinc is a potent inhibitor of osteoclast bone resorption in vitro. J Bone Miner Res 10: 453–457.
- Mosmann T (1983): Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63.
- Sakamoto S, Sassa S, Kudo H, Suzuki S, Mitamura T, Shinoda H (2000): Preventive effects of a herbal medicine on bone loss in rats treated with a GnRH agonist. Eur J Endocrinol 143: 139–142.
- Suda T, Nakamura I, Jimi E, Takahashi N (1997): Regulation of osteoclast function. J Bone Miner Res 12: 869–879.
- Sugawara K, Hamada M, Hosoi S, Tamaoki T (1998): A useful method to evaluate bone resorption inhibitors, using osteoclast-like multinucleated cells. Anal Biochem 255: 204–210.
- Sun L, Yang J, Liu JS (2001): Effects of lower dose of estrogen on trabecular structure of femur in ovariectomized-induced osteoporosis rats. Acta Acad Med Sin 23: 224–227.
- Wu H, Lien EJ, Lien LL (2003): Chemical and pharmacological investigations of Epimedium species: A survey. Prog Drug Res 60: 51–57.
- Yuan J, Lin J, Xu CY, Ye QX, Xiong YH, Huang L, Yuan H (2005): Experimental research on prevention of glucocorticoid-induced avascular necrosis of the femoral head with Tongluo Shenggu Capsule. Zhongguo Xinyao Yu Linchuang Yaoli. 16: 185–188.
- Yuhara S, Kasagi S, Inoue A, Otsuka E, Hirose S, Hagiwara H (1999): Effects of nicotine on cultured cells suggest that it can influence the formation and resorption of bone. Eur J Pharmacol 383: 387–393.
- Zhang DW, Chen Y, Zhang JC, Wang XL, Wang NL, Yang MS, Yao XS (2008): Synergistic effect of trace elements and flavonoids from Epimedium koreanum Nakai on primary osteoblasts. Chinese Sci Bull 53: 347–356.
- Zhang JC, Xu SJ, Wang K, Yu SF (2003): Effects of the rare earth ions on bone resorbing function of rabbit mature osteoclasts in vitro. Chinese Sci Bull 48: 2170–2175.
- Zhao WB, Cheng YH (2006): Extraction and content determination of polysaccharide from Rheum wittrochii L.by ultrasonic technology. Lishizhen Med Mat Med Res 17: 223–224.