Abstract
The phytochemical investigation of the ethyl acetate soluble fraction of the dried floral buds of Syringa patula palibiniana Nakai (Oleaceae) has led to the isolation of six compounds: β-amyrin acetate (1), β-sitosterol (2), nortropin (3), eugenol (4), syringaresinol (5), and oleoside 11-methyl ester (6). The structures were elucidated by spectroscopic and chemical means. Investigation of the biological activities indicated that 5 and 6 possessed a strong cytotoxic activity against Hep-G2 cells; 1 and 5 remarkably increased the macrophage proliferation index; 1 and 5 exhibited high anti-inflammatory activities as indicated by inhibited LPS-induced NO generation; and only 5 showed a strong scavenging activity against DPPH. Taken together, results indicate that 5 is a multipotent compound active as an anti-cancer, immunoproliferative, anti-inflammatory, and antioxidant agent.
Introduction
Lilac is the common name of the plants of the genus Syringa (Oleaceae). It is a popular ornamental bush, and is cultivated in the middle latitudes of Eurasia and North America (CitationLi et al., 2006). This genus includes 22–23 species according to different classisfications (CitationLi et al., 2006). The fragrance components of lilac are important constituents in perfumery. Many studies have been carried out on the plants of this genus in view of their interesting biological activities, including antibacterial, antivirus, and hypotensive (CitationLuo et al., 2006). Previous phytochemical investigations on the plants of this genus have led to the isolation of iridoids, secoiridoids, phenylethanoids, lignans, and their glycosides. Among them, secoiridoid glucosides have shown free radical scavenging activity and antihypertensive activity. Furthermore, some lignan glycosides have displayed hypotensive activity and cytotoxicity (CitationLuo et al., 2006).
Syringa patula palibiniana Nakai, formerly known Syringa velutina Kom., is a tall shrub with an upright habit and pale lilac flowers; this Korean native has foliage that is resistant to both mildew and leaf-roll necrosis. To date, only one phytochemical study has been carried out on this plant species (CitationPark et al., 1999). This previous study resulted in the occurrence of oleuropein, 3,4-dihydroxyphenylethyl alcohol-8-O-β-d-glucoside, coniferylaldehyde-4-O-glucoside, syringin, ligstroside, (+)-syringaresinol-4-O-glucoside, (+)-medioresinol-4-O-glucoside, and (–)-olivil-4-O-glucoside in the stem bark of S. patula. Here we report on the isolation and structural elucidation of six compounds isolated from the ethyl acetate-soluble fraction of the floral buds of S. patula: β-amyrin acetate (1), β-sitosterol (2), nortropin (3), eugenol (4), syringaresinol (5), and oleoside 11-methyl ester (6). Compounds 1, 5, and 6 were tested for their cytotoxic, immunoproliferative, anti- inflammatory, and antioxidant activities, and the results are reported herein.
Materials and methods
Plant material
Floral buds of S. patula were purchased from the Gyung Dong herbal drug market and identified by Prof. Young-Kyoon Kim, Department of Forest Products, Kookmin University. A voucher specimen (YKP04-626) has been kept in the herbarium of the Department of Forest Products, Kookmin University, Seoul, Korea.
Extraction and isolation
The dried powdered floral buds of Syringa patula (700 g) were extracted with hot MeOH (6 L). The combined extracts were concentrated under reduced pressure to yield a dark gum (37.8 g). The methanol extract was suspended in water and partitioned with hexane, ethyl acetate (EtOAc), and n-butanol (n-BuOH), respectively. The resulting fractions were concentrated in vacuo to give a hexane-soluble fraction (2.1 g), an EtOAc-soluble fraction (3.5 g), and a BuOH-soluble fraction (19.9 g).
The EtOAc-soluble fraction (3.5 g) was fractionated by vacuum liquid chromatography (silica gel 60, 0.063–0.200 mm; Merck) with n-hexane–EtOAc gradient to yield six fractions (E1–E6). Compounds 1 (84 mg) and 2 (37 mg) were crystallized, with hexane, from fractions E1 and E2, respectively. Compound 3 (43 mg) was obtained from fraction E3 by repeated silica gel column chromatography eluted with hexane–EtOAc (10:1). Fraction E4 was chromatographed on a silica gel column eluted with CHCl3–MeOH (40:1) to give 4 (68 mg). Fraction E5 was subjected to polyamide column chromatography, and eluted with EtOH–H2O (8:1) to give 5 (35 mg). Meanwhile, repeated chromatography of the last fraction E6 on a Sephadex LH-20 column (MeOH) yielded 6 (18 mg).
For column chromatography, silica gel 60, 0.040–0.063 mm (Merck), polyamide 6, 50–160 μm (Fluka), and Sephadex LH-20 (Pharmacia) were used. Thin layer chromatography (TLC) analysis was carried out using silica gel 60 F254 plates (Merck); chromatograms were visualized under ultraviolet (UV) light at 240 and 366 nm then sprayed with anisaldehyde reagent (Aldrich). UV spectra were recorded on an X-ma 2000 UV-visible spectrophotometer. Infrared (IR) spectra were obtained on a Bruker IFS 113v instrument. 1H and 13C nuclear magnetic resonance (NMR) spectra were obtained in CDCl3 using a Bruker Avance 300 DPX spectrometer. The chemical shifts are given in δ (ppm) relative to (Me)4Si. Electrospray ionization mass spectrometry (ESIMS) was performed on a Waters ZQ 2000 spectrometer.
Cell culture
Human hepatocellular carcinoma (Hep-G2) and murine macrophage (RAW 264.7) cell lines were purchased from ATCC, VA, USA. Hep-G2 cells were routinely cultured in DMEM (Dulbeco’s modified Eagle’s medium), while RAW 264.7 cells were grown in RPMI-1640. Media were supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, containing 100 units/mL penicillin G sodium, 100 units/mL streptomycin sulfate, and 250 ng/mL amphotericin B. Cells were maintained in humidified air containing 5% CO2 at 37°C. Monolayer cells were harvested using trypsin/EDTA (ethylenediaminetetraacetic acid), and were collected by scraping. Only compounds 1, 5, and 6 were tested for biological activities due to sufficient amounts remaining after structure analysis. Compounds were dissolved in dimethylsulfoxide (DMSO; 99.9%) and diluted 1000-fold in the assays. In all the cellular experiments, results were compared with DMSO-treated cells. All experiments were repeated four times, unless mentioned, and the data are represented as mean ± SD. All cell culture material was obtained from Cambrex Bio Science (Copenhagen, Denmark).
Cytotoxicity assay
The cytotoxic activity of the compounds against Hep-G2 was estimated by the 3-(4,5-dimethyl-2-thiazolyl)- 2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (CitationHansen et al., 1989), which is based on the cleavage of the tetrazolium salt by mitochondrial dehydrogenases in viable cells. Cells were seeded in a 96-well plate at a concentration of 5 × 104 cells/well and incubated overnight. After treatment with various concentrations of the compounds (0, 12.5, 25, 50, and 100 μg/mL, respectively), cells were incubated for an additional 24 h at 37°C in serum-free medium before being submitted to MTT assay. Serial concentrations of dexamethasone (0–100 μg/mL) were used as known growth inhibitor. The absorbance was measured with an enzyme-linked immunosorbent assay (ELISA) reader (BioRad, USA) at 570 nm. The relative cell viability was determined by the amount of MTT converted to the insoluble formazan salt. Data are expressed as the mean percentage of viable cells as compared to the respective control cultures treated with solvent.
Macrophage proliferation assay
The macrophage proliferation index was investigated using the MTT assay. RAW 264.7 cells were seeded in a 96-well plate at a concentration of 3 × 104 cells/well and incubated for 24 h with different concentrations of the compounds, at 37°C in serum-free medium. Then, cells were submitted to the MTT assay. The macrophage proliferation index was calculated in relation to control by the following formula: absorbance of treated cells/absorbance of untreated cells.
Nitrite assay
The accumulation of nitrite is an indicator of nitric oxide (NO) synthesis, which was measured in the culture medium using the Griess reaction (CitationGerhäuser et al., 2002). RAW 264.7 cells were grown in phenol red-free RPMI medium containing 10% FBS. Cells were incubated for 2 h with bacterial lipopolysaccharide (LPS; 1 μg/mL) before being treated with the compounds (20 μg/mL), or DMSO, and then being subjected to the Griess assay. A standard curve was plotted using serial concentrations of sodium nitrite. Data were normalized to the cellular protein content, which was measured by the bicinchoninic acid (BCA) assay (CitationSmith et al., 1985).
Antioxidant activity
The antioxidant capacity of the tested compounds was studied through their scavenging activity against 1,1-diphenyl-2-pycrylhydrazyl (DPPH) radical, using the method of CitationGerhäuser et al. (2003) as modified by CitationVan Amsterdam et al. (1992). The bleaching of DPPH was monitored at an absorbance of 515 nm. The percentage of DPPH bleaching was utilized to calculate the SC50 (half-maximal scavenging concentration), with 0% being the absorbance of DPPH with solvent (ethanol), and 100% being the absorbance of DPPH with an efficient scavenger (10 mM ascorbic acid; AA).
Results and discussion
Six compounds (), β-amyrin acetate (1) (CitationSeo et al., 1975; CitationAgeta & Arai, 1983; CitationChoi et al., 2004), β-sitosterol (2) (CitationKojima et al., 1990; CitationChen et al., 2008), nortropin (3) (CitationGiraudeau et al., 2007), eugenol (4) (CitationJakupovic et al., 1991), syringaresinol (5) (CitationShahat et al., 2004), and oleoside 11-methyl ester (6) (CitationShen et al., 1996) were isolated from an EtOAc-soluble extract of the floral buds of S. patula. The structures of the known compounds were identified by physical and spectroscopic data (1H-, 13C-NMR, and MS) measurement and by comparison with published values. Compounds (1) and (5) were found to be the major components of the EtOAc-soluble fraction of S. patula. To the best of our knowledge; there is only one prior report on the isolation and characterization of secondary metabolites from this plant species (CitationPark et al., 1999); accordingly, the metabolites (1–6) are reported here for the first time. Compounds 1, 5, and 6 were subjected to in vitro bioassays to evaluate their cytotoxic, anti-inflammatory, and antioxidant effects.
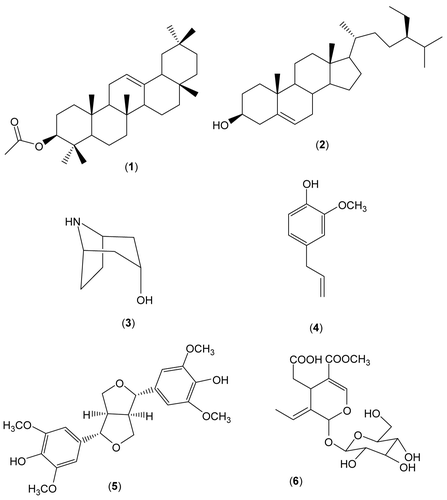
The cytotoxic effects of compounds 1, 5, and 6 were investigated against Hep-G2 cells. Using the MTT assay, a metabolic cytotoxicity assay, the results revealed that 5 possessed a strong dose-dependent cytotoxic effect against Hep-G2 cells with an IC50 value of 39.57 μg/mL and that 6 had a dose–response curve of low slope and a high IC50 value of 75.43 μg/mL (). Although 5 was cytotoxic starting from the lowest tested concentration (12.5 μg/mL), its IC50 value was high compared to that of dexamethasone (IC50 5.6 μg/mL) and known potent anti-cancer agents, e.g., paclitaxel (IC50 700 nM) (CitationGamal-Eldeen et al., 2006). On the other hand, 1 showed no cytotoxic effect against Hep-G2 cells ().
Figure 2. Cytotoxicity of tested compounds against Hep-G2 cells after treatment for 24 h with different concentrations of β-amyrin acetate (circles), syringaresinol (squares), and oleoside 11-methyl ester (triangles), as estimated by MTT assay. Results are presented as a percentage compared to control cells treated with solvent (mean of four readings ± SD).
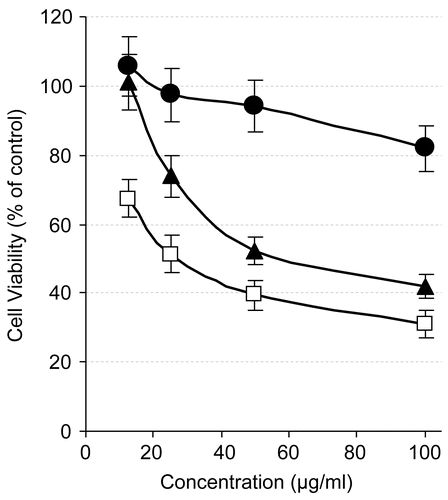
To determine whether the tested compounds had a growth stimulatory or inhibitory effect on immune cells, we monitored the alteration in macrophage proliferation index, using RAW 264.7 cells. Interestingly, treatment with 1 and 5 remarkably increased the macrophage proliferation index () to 1.6-fold and 1.95-fold that of control, respectively, at the highest tested concentration (100 μg/mL). Compound 6 was cytotoxic against RAW 264.7 cells, but in an insignificant manner (). The induction of macrophage proliferation may be a result of either an induced expression of specific growth factors such as interleukin-12 or another mechanism that needs to be explored.
Figure 3. Proliferation index of RAW 264.7 cells after treatment with different concentrations of β-amyrin acetate (circles), syringaresinol (squares), and oleoside 11-methyl ester (triangles), as estimated by MTT assay. As described in “Materials and methods”, the proliferation index was calculated relative to control.
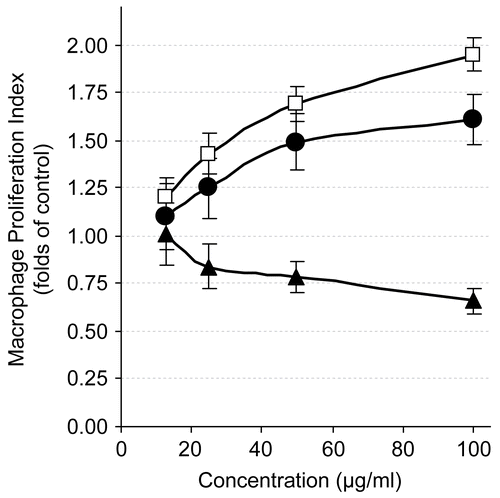
Figure 4. Evaluation of nitrite production (nmol nitrite/mg cellular protein, mean ± SD) by RAW 264.7 cells stimulated for 20 h by LPS (1 μg/mL) alone or in combination with 20 μg/mL of the different compounds.
The macrophage is an important antigen-presenting and effector cell in anti-tumor immunity. After activation, macrophages acquire a broad range of functions, including the enhancement of phagocytic activity, production of free radicals, release of proinflammatory cytokines, and secretion of enzymes. With the acquisition of these properties, macrophages can play an important role in antigen presentation to activate the immune response (CitationMacMicking et al., 1997).
Oxidative stress, chronic inflammation, and infections stimulate the inducible form of nitric oxide (NO) synthase (iNOS), which consequently enhances the generation of NO (CitationKovacic & Jacintho, 2001). Generally, NO is an important signaling molecule that is involved in the immune defense against pathogens and certain tumor cells. Long-term elevated levels of NO, however, have been linked to early steps in carcinogenesis via nitrosative desamination of DNA bases and DNA adduct formation (CitationOhshima & Bartsch, 1994).
The finding that treatment with 1 and 5 remarkably increased the macrophage proliferation index raised the question of whether the elevated proliferation of these immune cells is concomitant with an activation of their functions. To find an answer we tested one of the macrophage functions. We investigated the influence of the tested compounds on NO generation from macrophages as a reflection of the enhancement of iNOS.
In this experiment, 6 unexpectedly showed no change in NO generation level (), which was measured as nitrite content by the Griess reaction using a mimic inflammation model of LPS-mediated (iNOS) induction in murine macrophages. Surprisingly, all treatments with 1 and 5 markedly inhibited LPS-induced NO generation, with inhibition percentages of 49.97% and 33.21% of the nitrite level of LPS-treated cells, respectively, at a concentration of 20 μg/mL (). Results were normalized to the protein content to avoid interference of the increased or inhibited macrophage number at that concentration.
This inhibition could be due to direct NO scavenging. Therefore, we investigated the antioxidant capacity of the tested compounds using the DPPH assay. DPPH is a stable non-physiological radical, which could provide a relative figure of the antioxidant activity of a tested probe. The DPPH assay showed that 1 and 6 possessed a weak scavenging activity with high SC50 values, > 100 μg/mL, compared to the scavenging activity of a well-known antioxidant (AA; SC50 8.2 μg/mL), while 5 showed a strong scavenging activity against DPPH, with an effective SC50 value as low as 12.5 μg/mL. This antioxidant activity of 5 may be one of the reasons for its strong inhibition of NO. The findings of the DPPH assay in the case of 1 did not explain its potent inhibition of NO; however, it appears that this may be due to inhibition of iNOS expression or other unknown mechanisms.
Previously, 5 was reported as a topoisomerase I inhibitor (CitationMa et al., 2005) and as a new type of DNA strand-scission agent (CitationDeng et al., 2000). Compound 5 was also reported as a strong inhibitor of both LPS-induced production of NO, prostaglandin E2 (PGE2), and tumor necrosis factor α (TNF-α) from macrophages and LPS-induced expression of iNOS and cyclooxygenase-2 (COX-2) enzyme (CitationJung et al., 2003). Our findings support these reports, and add more information on the stimulating effect of 5 on macrophage growth, its cytotoxic effect on hepatocellular carcinoma cells, and its antioxidant activity, which may be due to the high number of hydroxyl groups.
There is a lack of studies on the biological activities of 6, and it is only reported as an effective antimicrobial agent against Lactobacillus pentosus (CitationMedina et al., 2007). However, our findings revealed the cytotoxic activity of 6 and its inactive anti-inflammatory and antioxidant effect.
Previous reports indicated that 1 exhibited significant antifungal activity against Candida (CitationJohann et al., 2007), pronounced antinociceptive properties in the writhing test and formalin test in mice (CitationKrogh et al., 1999), moderate inhibition of 5-HETE (5- hydroxyeicosatetraenoic acid) synthesis (CitationKweifio-Okai & Macrides, 1992), and weak cytotoxicity against the A2780 human ovarian cancer cell line (CitationChaturvedula et al., 2002). In this study, we have additionally reported that 1 exhibited a strong anti-inflammatory activity, a strong proliferative effect on macrophages, and a weak cytotoxicity against Hep-G2 cells.
Collectively, our results indicated that 5 and 6 possessed a strong cytotoxic activity against Hep-G2 cells; 1 and 5 remarkably increased the macrophage proliferation index; 1 and 5 exhibited high anti-inflammatory activities as indicated by inhibition of LPS-induced NO generation; and only 5 showed a strong scavenging activity against DPPH. Taking these results together, 5 is a multipotent compound active as an anti-cancer, immunoproliferative, anti-inflammatory, and antioxidant agent.
Acknowledgements
The authors wish to thank the Department of Forest Products, College of Forest Science, Kookmin University, South Korea for providing and botanical identification of the plant, and Professor Young-Kyoon Kim, College of Forest Science, Kookmin University, South Korea for his cooperation and assistance.
Declaration of interest: This work was financially supported by the National Research Center, Cairo, Egypt.
References
- Ageta H, Arai Y (1983): Fern constituents: Pentacyclic triterpenoids isolated from Polypodium niponicum and P. formosanum. Phytochemistry 22: 1801–1806.
- Chaturvedula VS, Schilling JK, Miller JS, Andriantsiferana R, Rasamison VE, Kingston DG (2002): Two new triterpene esters from the twigs of Brachylaena ramiflora from the Madagascar rainforest. J Nat Prod 65: 1222–1224.
- Chen Z, Liu YM, Yang S, Song BA, Xu GF, Bhadury PS, Jin LH, Hu DY, Liu F, Xue W, Zhou X (2008): Studies on the chemical constituents and anticancer activity of Saxifraga stolonifera (L) Meeb. Bioorg Med Chem 16: 1337–1344.
- Choi SZ, Choi SU, Lee KR (2004): Phytochemical constituents of the aerial parts from Solidago virga-aurea var. gigantea. Arch Pharm Res 27: 164–168.
- Deng JZ, Newman DJ, Hecht SM (2000): Use of COMPARE analysis to discover functional analogues of bleomycin. J Nat Prod 63: 1269–1272.
- Gamal-Eldeen AM, Amer H, Helmy WA (2006). Cancer chemopreventive and anti-inflammatory activities of chemically modified guar gum. Chem Biol Interact 161: 229–240.
- Gerhäuser C, Alt A, Heiss E, Gamal-Eldeen A, Klimo K, Knauft J, Neumann I, Scherf H, Frank N, Bartsch H, Becker H (2002): Cancer chemopreventive activity of xanthohumol, a natural product derived from hop. Mol Cancer Ther 1: 959–969.
- Gerhäuser C, Klimo K, Heiss E, Neumann I, Gamal-Eldeen A, Knauft J, Liu JU, Sitthimonchai S, Frank N (2003): Mechanism-based in vitro screening of potential cancer chemopreventive agents. Mut Res 523–524: 163–172.
- Giraudeau P, Guignard N, Hillion E, Baguet E, Akoka S (2007): Optimization of homonuclear 2D NMR for fast quantitative analysis: Application to tropine-nortropine mixtures. J Pharm Biomed Anal 43: 1243–1248.
- Hansen MB, Nielsen SE, Berg K (1989): Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods 119: 203–210.
- Jakupovic J, Tan RX, Bohlmann F, Jia ZJ, Huneck S (1991): Acetylenes and other constituents from Artemisia dracunculus. Planta Med 57: 450–453.
- Johann S, Soldi C, Lyon JP, Pizzolatti MG, Resende MA (2007): Antifungal activity of the amyrin derivatives and in vitro inhibition of Candida albicans adhesion to human epithelial cells. Lett Appl Microbiol 45: 148–153.
- Jung HJ, Park HJ, Kim RG, Shin KM, Ha J, Choi JW, Kim HJ, Lee YS, Lee KT (2003): In vivo anti-inflammatory and antinociceptive effects of liriodendrin isolated from the stem bark of Acanthopanax senticosus. Planta Med 69: 610–616.
- Kojima H, Sato N, Hatano A, Ogura H (1990): Sterol glucosides from Prunella vulgaris. Phytochemistry 29: 2351–2355.
- Kovacic P, Jacintho JD (2001): Mechanisms of carcinogenesis: Focus on oxidative stress and electron transfer. Curr Med Chem 8: 773–796.
- Krogh R, Kroth R, Berti C, Madeira AO, Souza MM, Cechinel-Filho V, Delle-Monache F, Yunes RA (1999): Isolation and identification of compounds with antinociceptive action from Ipomoea pes-caprae (L.) R. Br. Pharmazie 54: 464–466
- Kweifio-Okai G, Macrides TA (1992): Antilipoxygenase activity of amyrin triterpenes. Res Commun Chem Pathol Pharmacol 78: 367–372.
- Li ZG, Lee MR, Shen DL (2006): Analysis of volatile compounds emitted from fresh Syringa oblata flowers in different fluorescence by headspace solid-phase microextraction-gas chromatography-mass spectrometry. Anal Chim Acta 576: 43–49.
- Luo Y, Liu Y, Qi H, Wu Z, Zhang G, Luo Y, Liu Y, Qi H, Wu Z, Zhang G (2006): Steryl esters and phenylethanol esters from Syringa komarowii. Steroids 71: 700–705.
- Ma J, Jones SH, Marshall R, Wu X, Hecht SM (2005): DNA topoisomerase I inhibitors from Rinorea anguifera. Bioorg Med Chem Lett 15: 813–816.
- MacMicking J, Xie QW, Nathan C (1997): Nitric oxide and macrophage function. Annu Rev Immunol 15: 323–350.
- Medina E, Brenes M, Romero C, García A, de Castro A (2007): In vivo anti-inflammatory and antinociceptive effects of liriodendrin isolated from the stem bark of Acanthopanax senticosus. J Agric Food Chem 55: 9817–9823.
- Ohshima H, Bartsch H (1994): Chronic infections and inflammatory processes as cancer risk factors: Possible role of nitric oxide in carcinogenesis. Mutat Res 305: 253–264.
- Park HJ, Lee MS, Lee KT, Sohn IC, Han YN, Miyamoto K (1999): Studies on constituents with cytotoxic activity from the stem bark of Syringa velutina. Chem Pharm Bull (Tokyo) 47: 1029–1031.
- Seo S, Tomita Y, Tori K (1975): Carbon-13 NMR spectra of urs-12-enes and application to structural assignment of components of Isodon japonicus Hara tissue cultures. Tetrahedron Lett 16: 7–11.
- Shahat AA, Abdel-Azim NS, Pieters L, Vlietinck AJ (2004): Isolation and NMR spectra of syringaresinol-beta-d-glucoside from Cressa cretica. Fitoterapia 75: 771–773.
- Shen YC, Lin SL, Chein CC, Jaspolyside (1996): A new secoiridoid glycoside from Jasminum Polyanthum. Phytochemistry 42: 1629–1633.
- Smith PK, Krohn R, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985): Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76–80
- Van Amsterdam FT, Roveri A, Maiorino M, Ratti E, Ursini F (1992) Lacidipine: A dihydropyridine calcium antagonist with antioxidant activity. Biol Med 12: 183–187.