Abstract
This study evaluated the antimicrobial activity of three different ethanol extracts of Agaricus brasiliensis S. Wasser et al. (Agaricaceae) on the growth and cell adherence of mutans streptococci. Crude ethanol extracts (100% EtOH, 75% EtOH, and 50% EtOH, v/v) obtained from dried basidiomes of A. brasiliensis were assessed against Streptococcus mutans UA159 and Streptococcus sobrinus 6715 by the agar diffusion method, determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC), and inhibition of cell adherence to a glass surface. In the agar diffusion method, only the 100% ethanol extract revealed inhibition of bacterial growth. Yet, in MIC and MBC tests, the three extracts presented antimicrobial properties and MIC and MBC values between 87.4 and 444.5 μg mL−1. The MBC was predominantly similar to MIC against S. mutans, but only the 75% ethanol extract was bactericidal against S. sobrinus. Among the ethanol extracts tested, the 100% ethanol extract was the most active (MIC 87.4 μg mL−1). The cellular adherence also was inhibited at concentrations from 100 to 400 μg mL−1 of 50% and 75% ethanol extracts. These results provide promising baseline formation for the potential use of A. brasiliensis against mutans streptococci. These findings warrant more in-depth studies of the active principles of this mushroom ethanol extract.
Introduction
Several natural products have been used and studied as potential agents to prevent oral diseases, especially plaque-related diseases such as dental caries. The development of dental caries involves a series of events in the biofilm on the tooth surface, where bacterial interactions with a person’s diet occur (CitationGibbons & von Houte, 1975; CitationLoesche, 1986). There is a general consensus that the frequent consumption of carbohydrates, mainly sucrose, can result in the appearance of cariogenic microorganisms, such as mutans streptococci (CitationAires et al., 2006; CitationDuarte et al., 2008). Streptococcus mutans is generally accepted to be the most important cause of dental caries (CitationHamada et al., 1984; CitationNapimoga et al., 2005). Therefore, special attention should be paid to mutans streptococci in the development of any therapeutic agent which aims at preventing dental biofilm-related diseases, such as dental caries.
Recent studies (CitationRosa et al., 2003; CitationLindequist et al., 2005; CitationTalcott et al., 2007) showed that mushrooms are a significant source of new potential therapeutic agents, as they make use of antibacterial and antifungal compounds to survive in their natural environment. Therefore, it is not surprising that antimicrobial compounds have been isolated from many mushrooms and that they may be used to benefit humans. Nevertheless, in Kingdom Fungi, only compounds from microscopic fungi such as penicillin (derived from Penicillium notatum) and cephalosporin (derived from Cephalosporium salmosynnematum) can be found as antibiotics in the current market.
Agaricus brasiliensis S. CitationWasser et al. (2002) (syn., Agaricus blazei Murrill ss. Heinem.) (Agaricaceae family), known in Brazil as “Cogumelo-do-sol” and worldwide as “sun mushroom” or “Himematsutake”, has been largely cultivated in Brazil because of its medicinal properties (CitationMachado et al., 2005; CitationFaccin et al., 2007). Strains of this species were exported to Japan from Brazil in 1965, and their fruiting bodies have been used as medicines in Japan and many other countries (CitationMizuno et al., 1999; CitationTalcott et al., 2007).
Preparations of “Cogumelo-do-sol” have therapeutic effects, such as immunomodulatory activity and antineoplasic activities (CitationKawagishi et al., 1989; CitationMizuno et al., 1999; CitationOhno et al., 2001; CitationDong et al., 2002; CitationKobayashi et al., 2005; CitationChan et al., 2007; CitationTalcott et al., 2007).
The relevance of this study is supported by the use of ethanol (EtOH) extracts in phytotherapy, and by the fact that A. brasiliensis is found throughout the world and is used for medicinal purposes (CitationKoo et al., 2000a, Citation2000b; CitationDuarte et al., 2003; CitationLindequist et al., 2005; CitationFaccin et al., 2007).
Few investigations into the antimicrobial activity of medicinal mushrooms have been carried out, and even fewer regarding oral microorganisms (CitationOsaki et al., 1994; CitationBerg et al., 1999; CitationPaccola et al., 2001; CitationRosa et al., 2003; CitationGrinde et al., 2006; CitationFaccin et al., 2007). Hence, the aim of this article is to report the antimicrobial activity of three ethanol extracts of A. brasiliensis against mutans streptococci.
Materials and methods
Preparation of ethanol extracts
Dried basidiomes of A. brasiliensis – strain ABL99/30 – were obtained from the Faculdade de Ciências Agronômicas, UNESP, in Botucatu, São Paulo, Brazil. A voucher specimen is kept in the Mycoteca of the Experimental Laboratory of Mycology, Federal University of Pelotas (Pelotas, RS, Brazil). The mushroom was identified by Dr. José Soares do Nascimento (taxonomist).
The mushrooms were harvested with the pileus closed, washed, and dried at 50°C for 72 h. After that, they were ground into powder.
Dried and crushed fruiting bodies of the mushroom (30 g) were divided into three different portions. Ten grams of dried powder were submitted to maceration with 50 mL of 100% ethanol, v/v (ABL 100% EtOH extract), 10 g with 50 mL of 75% ethanol (ABL 75% EtOH extract), and another 10 g with 50 mL of 50% ethanol (ABL 50% EtOH extract), at 4°C, for 216 h.
During this time, at every 72 h, the extracts were filtered, and another 50 mL of the corresponding ethanol solutions was added to the residues. Then, the supernatants were concentrated under reduced pressure in a Rotavapor and lyophilized. The lyophilized compounds were resuspended in their corresponding ethanol solutions (100, 75, or 50%, v/v) at concentrations of 15.74% (w/v) for ABL 100% EtOH, and 20% (w/v) for ABL 75% EtOH and ABL 50% EtOH, just prior to performance of the assays.
Strains
The in vitro antimicrobial activity of A. brasiliensis ethanol extracts was tested against the oral streptococci: Streptococcus mutans UA159 and Streptococcus sobrinus 6715. These Gram-positive bacteria were obtained from the Department of Pharmacology, Anesthesiology and Therapeutics, Faculty of Dentistry, University of Campinas (UNICAMP) (Piracicaba, São Paulo, Brazil).
Antimicrobial activity assays
Agar diffusion assay
The disk diffusion method was performed to evaluate the antimicrobial activity of the A. brasiliensis ethanol extracts (CitationKoo et al., 2000a). The microorganism suspension (0.1 mL of 108 cells/mL) was spread onto solid media plates. The microorganisms were seeded by “pour plate” in brain–heart infusion (BHI) agar and incubated for 18–24 h. The oral streptococci grown on BHI agar were suspended in sterile BHI broth. The suspension was adjusted spectrophotometrically to match the turbidity of a McFarland 0.5 scale (1.5 × 108 CFU mL−1). A 400 μL portion of each tested suspension was mixed with 40 mL BHI agar at 45°C, and poured onto a previously set layer of Mueller Hinton agar. The nutritive media were prepared according to the manufacturer’s instructions. All agar plates were prepared in 90 mm Petri dishes with 22 mL of agar, giving a final depth of 4 mm. The inoculum procedure was appropriate to provide a semiconfluent growth of the microorganisms tested. Six sterilized stainless-steel cylinders of 8.0 × 10.0 mm (inside diameter: 6 mm) were placed on each inoculated agar plate. Both the tested extracts and the controls (40 μL) were placed inside the cylinders. The plates were kept for 2 h at room temperature to allow diffusion of the agents through the agar. Afterwards, the plates were incubated at 37°C under microaerophilic conditions (5–10% CO2) for a period of 24–48 h. Cylinders with 40 μL of 100% EtOH, 50% EtOH, or 75% EtOH were used as negative controls.
The zones of inhibition of microbial growth around the cylinder containing the extracts were measured after the incubation time using a ruler, and the results expressed in millimeters. The plates used for each treatment were chosen randomly and each extract was processed in triplicate. Three replicates were made for each of the tested microorganisms. The experiment described here was repeated twice.
Broth dilution assay (MIC and MBC)
The antimicrobial activity of ethanol extracts of A. brasiliensis was examined by determining the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) in accordance with CitationKoo et al. (2000b) and CitationDuarte et al. (2003). For MIC determination, the starting inoculum was 5 × 105 CFU mL−1. Two-fold dilution series of extracts (concentrations ranging from 1777.8 to 27.778 μg mL−1 two-fold) were tested. The control vehicles were 100%, 75%, and 50% ethanol (final ethanol concentrations in the culture medium of: 0.900%, 0.675%, and 0.450%, v/v, respectively). The MIC was defined as the lowest concentration of extract that restricted the growth to a level lower than an optical density (OD) of 0.05 at 660 nm (no visible growth). For determination of the MBC, aliquots (50 μL) from all incubated test tubes with concentrations higher than the MIC were sub-cultured on Columbia agar plates supplemented with 5% of defibrinated sheep blood. The MBC was defined as the lowest concentration that enabled no growth on the blood agar (99.9% killed). Three replicates were made for each concentration of the tested extracts for both assays (MIC and MBC), in each experiment. The experiment was repeated three times.
Inhibition of growing cell adherence to glass surface
To assess the bacterial adherence to a glass surface, microorganisms were grown in BHI broth plus 1% sucrose (w/v) at 37°C, 10% CO2, at an angle of 30° for 18 h in glass test-tubes, containing sub-MIC levels of the A. brasiliensis ethanol extracts (or vehicle controls) as detailed in CitationHamada and Torii (1978) and CitationKoo et al. (2000a). Isolated colonies, after culture on BHI agar plates during 18–24 h, were suspended in 4.5 mL of sterile BHI broth, and the suspension adjusted to 0.5 on the McFarland scale (1.5 × 108 CFU mL−1). A portion of the suspension was mixed with BHI broth (1:1000 dilution, v/v) containing 1% sucrose, and 2.48 mL was transferred to a test tube. Subsequently, 20 μL of a two-fold dilution series of the test extracts (ABL 50% and ABL 75%) (concentrations ranging from 19.7 to 1600 μg mL−1 reaction) and their controls (75% or 50% ethanol, v/v) were inoculated, stirred, and then incubated. The final concentrations of 75% and 50% ethanol within the culture medium were 0.6% and 0.4% (v/v), respectively. After incubation, the adhered cells were washed and re-suspended in an ultrasonic bath. The amount of adhered cells was measured spectrophotometrically at 550 nm (CitationHamada & Torii, 1978; CitationDuarte et al., 2003).
The inhibition of adherence was defined as the lowest concentration that allowed no visible cell adherence on the glass surface. Three replicates were made for each concentration of the tested extracts in each experiment. The experiment was repeated three times.
Results and discussion
The diffusion method is the most employed method in this kind of research despite some limitations. It is a model with low credibility for samples that are difficult to diffuse in the media because there is no relationship between their solubility in water, diffusion power, and antimicrobial study. In some cases, diffusion techniques can be used for antimicrobial screening, but they may not be used as a definitive method because there is no relationship between MIC values and inhibition diameters (CitationRios et al., 1988). Among the tested ethanol extracts of A. brasiliensis (ABL EtOH), only treatment with the ABL 100% ethanol extract presented a growth inhibition zone, and this was larger against S. sobrinus. However, the agar diffusion assay is a qualitative non-standardized method that is useful only for the detection but not for the comparison of antimicrobial properties of different samples. Comparison of the size of inhibition halos of different extracts cannot be used for determination of the relative antimicrobial potencies, since a more diffusible but less active extract could give a larger diameter than a non-diffusible but more active extract.
The MIC values from the different A. brasiliensis ethanol extracts ranged from 87.4 to 444.5 μg mL−1 (). The MBC values of the ethanol extracts were similar to the MIC values against Streptococcus sobrinus, whereas only ABL 75% showed a bactericidal effect against Streptococcus mutans in the concentrations tested. In general, ABL 100% EtOH extract was most active against the cell viability of S. mutans and S. sobrinus. The broth dilution tests showed that all ethanol extracts of Agaricus brasiliensis had an effect against the in vitro microbial growth when compared to their controls (100% EtOH, 75% EtOH, or 50% EtOH, v/v).
Table 1. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values (n = 9) of ethanol extracts of A. brasiliensis against S. mutans UA159 and S. sobrinus 6715.
When the MIC is similar to the MBC, it must be considered that this concentration is simply bactericidal. There is no need to give definitions of both MIC and MBC for the same concentration, because there is no bacteriostatic effect when more than 99.9% of the inoculum is killed after determining the MIC. However, in this study, we assigned the same concentration to two classifications (MIC and MBC) because the two different assays were carried out separately (first, MIC, and later, MBC).
The antimicrobial activity increased when the concentration of EtOH in the extract increased, indicating, possibly, that the compounds responsible for the biological activity are present in a larger amount in the ethanol extract with higher concentrations. It is almost certain for all biological assays that this phenomenon will always be true. Some ethanol extracts presented an MIC value but not an MBC value, being considered just bacteriostatic agents. Others showed MIC values similar to MBC values, showing bactericidal behavior of the extracts. Among the ethanol extracts, ABL 100% EtOH exhibited the lowest values of MIC and MBC (87.4 μg mL−1) against mutans streptococci, being considered a bacteriostatic agent against S. mutans and bactericidal against S. sobrinus, because the MIC value was equal to the MBC. ABL 75% EtOH showed intermediate MIC and MBC values. It showed a lower MIC against S. mutans (111.1 μg mL−1) than against S. sobrinus (222.2 μg mL−1), but the MIC value was similar to the MBC, being simply considered bactericidal against S. sobrinus. ABL 50% EtOH showed higher MIC and MBC values (444.5 μg mL−1) against mutans streptococci.
Usually, the controls used to evaluate the efficacy of plant compounds are those used to evaluate standard antibiotics, which are used in treatment against different microorganisms. However, there is still no agreement regarding the use of this technique when natural products are being tested (CitationDuarte et al., 2005). CitationAligiannis et al. (2001) proposed a classification for plant materials based on MIC results as follows: strong inhibitors – MIC up to 0.5 mg mL−1; moderate inhibitors – MIC between 0.6 and 1.5 mg mL−1; and weak inhibitors – MIC above 1.6 mg mL−1. CitationDuarte et al. (2005) have established the concentration of 2 mg mL−1 as the highest concentration acceptable to consider a vegetable extract as having potentially antimicrobial activity. According to the MIC values found in this study and the statements of CitationAligiannis et al. (2001) and CitationDuarte et al. (2005), it was observed that the hydroalcoholic extracts from Agaricus brasiliensis assessed were potentially antimicrobial, but their antibacterial behavior ranged from highest to lowest when assessed against mutans.
Furthermore, this study investigated whether the ethanol extracts of A. brasiliensis would affect the sucrose-dependent adherence of the growing cells of mutans streptococci to a glass surface. The adherence assays were conducted using sub-MIC levels, as false positive results could be generated due to the antimicrobial effects of the extract. The results obtained in this study were very close to the MIC and MBC values, which suggests a bacteriostatic/bactericidal effect instead of the inhibition of a virulence factor of the oral streptococci, which is the bacterial adherence.
The in vitro inhibition of adherence of these microorganisms was evident () when they were grown in broth, which contained sucrose and different concentrations of ABL 75% EtOH and ABL 50% EtOH. The rate of inhibition was 100% against S. mutans at concentrations of 200 μg mL−1 (ABL 75% EtOH) and 100 μg mL−1 (ABL 50% EtOH) and against S. sobrinus at concentrations of 200 μg mL−1 (ABL 75% EtOH) and 100 μg mL−1 (ABL 50% EtOH).
Figure 1. The minimum concentration of ABL 75% EtOH and ABL 50% EtOH capable of totally inhibiting the adherence of growing S. mutans UA159 and S. sobrinus 6715 cells.
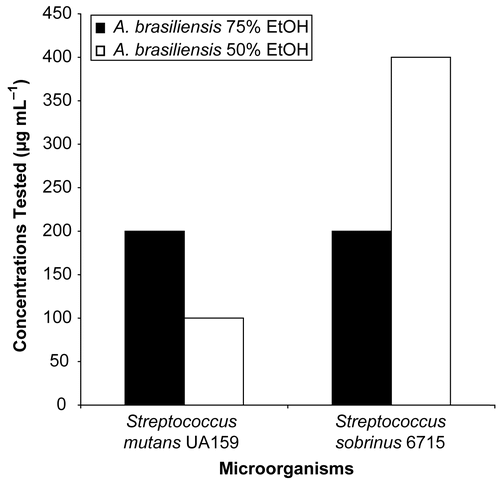
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) always had higher values than those of total inhibition of cell adherence against mutans streptococci. Sub-MIC levels of crude ethanol extracts inhibited bacteria cell adherence to a glass surface. The ethanol extracts from A. brasiliensis were able to inhibit the adherence at sub-MIC levels, as shown in , because in this test we assessed the action of the extracts against a virulence factor, mainly involved in adhesion, without bacteria killing. In determination of the MIC, the lowest concentration of the extract that restricted the growth to a level lower than an OD of 0.05 at 660 nm (no visible growth) was assessed, and in MBC determination, the concentrations were usually higher than the MIC, defined as the lowest concentration that allowed no growth on the blood agar (99.9% killed). Thus, it is normal that the concentrations required for the MIC and MBC will be higher than those for inhibiton of bacteria cell adherence.
The present data suggest that A. brasiliensis has bioactive compounds that possess antimicrobial activity in vitro, and provide an important basis for the use of ethanol extracts from basidiomes of Agaricus brasiliensis for the treatment of infections associated with oral microorganisms, such as mutans streptococci. The remarkable inhibitory effects of ethanol extracts of this mushroom could be a useful source for the development of new and promising antimicrobial agents against oral pathogens, such as mutans streptococci. However, further pharmacological and toxicity studies will be necessary to confirm this hypothesis. These findings also suggest that further studies should be conducted regarding the active principles of the ethanol extracts of this mushroom.
Declaration of interest: The authors would like to thank CAPES and CNPq (Brazilian agencies) for financial support.
References
- Aligiannis N, Kalpoutzakis E, Mitaku S, Chinou IB (2001): Composition and antimicrobial activity of the essential oils of two Origanum species. J Agric Food Chem 49: 4168–4170.
- Aires CP, Tabchoury CP, Del Bel Cury AA, Koo H, Cury JA (2006): Effect of sucrose concentration on dental biofilm formed in situ and on enamel demineralisation. Caries Res 40: 28–32.
- Berg A, Zipper H, Dörfelt H, Walter G, Udo G (1999): Agaridiol, a new 1-arylpropan-1,2-diol produced by Agaricus sp. J Basic Microbiol 39: 213–215.
- Chan Y, Chang T, Chan CH, Yeh YC, Chen CW, Shieh B, Li C (2007): Immunomodulatory effects of Agaricus blazei Murrill in Balb/cByJ mice. J Microbiol Immunol Infect 40: 201–208.
- Dong Q, Yao J, Yang XT, Fang JN (2002): Structural characterization of a water-soluble β-d-glucan from fruiting bodies of Agaricus blazei Murr. Carbohydr Res 337: 1417–1421.
- Duarte S, Koo H, Bowen WH, Hayacibara MF, Cury JA, Ikegaki M, Rosalen PL (2003): Effect of a novel type of propolis and its chemical fractions on glucosyltransferases and on growth and adherence of mutans streptococci. Biol Pharm Bull 26: 527–531.
- Duarte MC, Figueira GM, Sartoratto A, Rehder VL, Delarmelina C (2005): Anti-Candida activity of Brazilian medicinal plants. J Ethnopharmacol 97: 305–311.
- Duarte S, Klein MI, Aires CP, Cury JA, Bowen WH, Koo H (2008): Influences of starch and sucrose on Streptococcus mutans biofilms. Oral Microbiol Imunol 23: 206–212.
- Faccin LC, Benati F, Rincão VP, Mantovani MS, Soares SA, Gonzaga ML, Nozawa C, Carvalho Linhares RE (2007): Antiviral activity of aqueous and ethanol extracts and of an isolated polysaccharide form Agaricus brasiliensis against poliovirus type 1. Lett Appl Microbiol 45(1): 24–28.
- Gibbons RJ, von Houte J (1975): Bacterial adherence in oral microbial ecology. Annu Rev Microbiol 29: 19–44.
- Grinde B, Hetland G, Johnson E (2006): Effects on gene expression and viral load of a medicinal extract from Agaricus blazei in patients with chronic hepatitis C infection. Int Immunopharmacol 6: 1311–1314.
- Hamada S, Torii M (1978): Effect of sucrose in culture media on the location of glucosyltransferase of Streptococcus mutans and cell adherence to glass surfaces. Infect Immun 20: 592–599.
- Hamada S, Koga T, Oshima T (1984): Virulence factors of Streptococcus mutans and dental caries prevention. J Dent Res 63: 407–411.
- Kawagishi H, Inagaki R, Kanao T, Mizuno T, Shimura K, Ito H, Hagiwara T, Nakamura T (1989): Fractionation and antitumor activity of the water-in-soluble residue of Agaricus blazei fruiting bodies. Carbohydr Res 186: 267–273.
- Kobayashi H, Yoshida R, Kanada Y, Fukuda Y, Yagyu T, Inagaki K, Kondo T, Kurita N, Suzuki M, Kanayama N, Terao T (2005): Suppressing effects of daily oral supplementation of β-glucan extracted from Agaricus blazei Murill on spontaneous and peritoneal disseminated metastasis in mouse model. J Cancer Res Clin Oncol 131: 527–538.
- Koo H, Gomes BPFA, Rosalen PL, Ambrosano GMB, Park YK, Cury JA (2000a): In vitro antimicrobial activity of propolis and Arnica montana against oral pathogens. Arch Oral Biol 45: 141–148.
- Koo H, Rosalen PL, Cury JA, Ambrosano GMB, Murata R, Yatsuda R, Ikegaki M, Alencar SM, Park YK (2000b): Effect of a new variety of Apis mellifera propolis on mutans streptococci. Curr Microbiol 41: 192–196.
- Lindequist U, Niedermeyer THJ, Jülich W-D (2005): The pharmacological potential of mushrooms. Evid Based Complement Alternat Med 2: 285–299.
- Loesche WJ (1986): Role of Streptococcus mutans in human dental decay. Microbiol Rev 50: 353–380.
- Machado MP, Filho ER, Terezan AP, Ribeiro LR, Mantovani MS (2005): Cytotoxicity, genotoxicity and antimutagenicity of hexane extracts of Agaricus blazei determined in vitro by the comet assay and CHO/HGPRT gene mutation assay. Toxicol In Vitro 19: 533–539.
- Mizuno M, Minato K, Ito H, Kawade M, Terai H, Tsuchida H (1999): Anti-tumor polysaccharide from the mycelium of liquid-cultured Agaricus blazei Mill. Biochem Mol Biol Int 47: 707–714.
- Napimoga MH, Hofling JF, Klein MI, Kamiya RU, Gonçalves RB (2005): Transmission, diversity and virulence factors of Streptococcus mutans genoptypes. J Oral Sci 47: 59–64.
- Ohno N, Furukawa M, Miura NN, Adachi Y, Motoi M, Yadomae T (2001): Antitumor β–glucan from the cultured fruit body of Agaricus blazei. Biol Pharm Bull 24: 820–828.
- Osaki Y, Kato T, Yamamoto K, Okubo J, Miyazaki T (1994): Antimutagenic and bactericidal substances in the fruit body of a basidiomycete Agaricus blazei. Yakugaku Zasshi 114: 342–350.
- Paccola EAS, Maki CS, de Nobrega GMA, Paccola-Meirelles LD (2001): Antagonistic effect of edible mushroom extract on Candida albicans growth. Braz J Microbiol 32: 176–178.
- Rios JL, Recio MC, Villar A (1988): Screening methods for natural products with antimicrobial activity: a review of the literature. J Ethnopharmacol 23: 127–149.
- Rosa LH, Machado KN, Jacob CC, Capelari M, Rosa CA, Zani CL (2003): Screening of Brazilian basidiomycetes for antimicrobial activity. Mem Inst Oswaldo Cruz 98: 967–974.
- Talcott JA, Clark JA, Lee IP (2007): Measuring perceived effects of drinking an extract of basidiomycetes Agaricus blazei Murrill: a survey of Japanese consumers with cancer. BMC Complement Altern Med 7: 32.
- Wasser SP, Didukh MY, Amazonas MALA, Nevo E, Stamets P, Eira AF (2002): Is a widely cultivated culinary-medicinal royal sun Agaricus (the Himematsutake mushroom) indeed Agaricus blazei Murrill? Int J Med Mushrooms 4: 267–290.