Abstract
In this study we report the screening of the in vitro trypanocidal activity of 20 extracts obtained from 10 different plant species growing in the Brazilian Cerrado: Aspidosperma macrocarpum Mart. (Apocynaceae), Aegiphila sellowiana Cham. (Verbenaceae), Byrsonima intermedia Juss. (Malpighiaceae), Cyperus rotundus L. (Cyperaceae), Leandra lacunosa Cogn. (Melastomataceae), Miconia ligustroides (DC.) Naudin. (Melastomataceae), Miconia sellowiana Naudin. (Melastomataceae), Myrcia variabilis Mart. ex DC. (Myrtaceae), Solanum lycocarpum St. Hil. (Solanaceae), and Tibouchina stenocarpa Cogn. (Melastomataceae). The most active extracts were submitted to phytochemical analyses. High-resolution gas chromatography analysis of the n-hexane extract of T. stenocarpa (IC50 = 23.6 μg/mL), the most active extract amongst all the tested samples, allowed the identification of β-amyrin, α-amyrin, lupeol, friedelin, β-friedelanol, campesterol, stigmasterol, and β-sitosterol. Oleanolic and ursolic acids were isolated from the methylene chloride extract of T. stenocarpa (IC50 = 51.5 μg/mL), while ursolic acid was isolated from the methylene chloride extract of M. variabilis (IC50 = 38.4 μg/mL). Solasonine and solamargine were identified as major compounds by mass spectrometry analysis in the hydroalcoholic extract of the fruits of S. lycocarpum (IC50 = 57.1 μg/mL). The results showed that the trypanocidal activity may be related to the major compounds identified in the crude active extracts.
Introduction
Herbs and other plants have been used as medicinal agents since ancient times. At first, this happened on a folkloric basis only, but later developed on a scientific basis into single agent drugs (CitationLee, 2004). Nowadays, despite the competition from other drug discovery methods, research into natural products (NPs) still provides its fair share of new clinical drug candidates (CitationButler, 2004; CitationNewman et al., 2003).
In recent years, there has been growing interest from the pharmaceutical industry in “hotspot” areas of biodiversity, such as the Brazilian Cerrado (CitationCordell, 2000; CitationMyers et al., 2000). In this context, plants from the Brazilian Cerrado have attracted much interest from many researchers and pharmaceutical companies for two main reasons: (1) great devastation is occurring in the area due to the spread of both cattle raising and soybean culture, which may result in the extinction of a number of endemic species in the Cerrado; (2) many plant species from the Cerrado that are currently being used in Brazilian folk medicine may contain biologically active secondary metabolites.
Chagas’ disease is endemic in Latin America: it affects 16–18 million people, while another 100 million are exposed to the risk of infection (CitationWorld Health Organization, 2001). Trypanosoma cruzi, the etiological agent of the disease, causes a pathology whose features depend on both the inherent characteristics of the host and the virulence of the parasite (CitationBrener & Andrade, 1997). Infection most commonly occurs via the feces of the triamine bug. However, blood transfusion has been recognized as having an important role in the transmission of the disease (CitationWorld Health Organization, 2000). Gentian violet is the only effective compound available for elimination of the parasites from the blood prior to its transfusion. Despite the effectiveness of this compound, there are several restrictions to its use (CitationNussenzweig & Sonntaeg, 1953). Therefore, in recent years there has been an intense search for trypanocidal compounds from natural sources, which has led to the identification of several classes of active plant metabolites (CitationBoza & Cassels, 1996; CitationDa Silva Filho et al., 2004; CitationGrael et al., 2000; CitationMolinar-Toribio et al., 2006).
As part of our ongoing research on trypanocidal constituents from plants (CitationCunha et al., 2003, Citation2006), we report here the evaluation of the in vitro trypanocidal activity of 10 Brazilian Cerrado species. Additionally, we have carried out phytochemical investigation of Tibouchina stenocarpa Cogn. (Melastomataceae) on the basis of its trypanocidal results, since, to the best of our knowledge, this has not been described in the literature to date.
Materials and methods
Plant material
All the screened plants were collected from the Brazilian Cerrado area located in the northeast of the São Paulo state (20°309 S, 47°209 W G, altitude 810–870 m, area 19,660 m2), where more than 70 different plant species from 30 different families may be found. The predominant families were Myrtaceae and Melastomataceae, the latter having the highest density value (CitationAraújo et al., 1999). The species evaluated in this study were collected by Dr. Wilson R. Cunha in September, 2004 and identified by Dr. Angela Borges Martins, Instituto de Biologia, UNICAMP, Brazil. Voucher specimens were deposited in the Herbarium of this same Institute.
Extraction procedures
The collected plants were dried in a stove with circulating air (40°C) and powdered using a blender. The extracts of Aspidosperma macrocarpum Mart. (Apocynaceae), Cyperus rotundus L. (Cyperaceae), and Solanum lycocarpum St. Hil. (Solanaceae) were respectively obtained from the leaves, rhizomes, and fruits, while all the other evaluated plant extracts were obtained from aerial parts. The furnished powders were exhaustively extracted with different solvents by maceration at room temperature (25°C), followed by filtration. Ethanol and ethanol/H2O (8:2 v/v) were the solvents of choice for the extraction, although n-hexane and methylene chloride were also used. The filtered extracts were concentrated under reduced pressure, affording the crude extracts of all the plant species.
Gas chromatography analysis
A portion (50 mg) of the Tibouchina stenocarpa n-hexane extract was cleaned up and submitted to high-resolution gas chromatography (HRGC) analysis, according to a previously reported methodology (CitationCrevelin et al., 2006). HRGC analysis was performed on a Hewlett-Packard model 5980 series II gas chromatograph, equipped with a split injector (split ratio 1:60) operating at 260°C and a flame ionization detector operating at 330°C. The injected volume was 2 μL. HP-50 (cross-linked 50% phenyl-methyl-silicone, 30 m × 0.25 m × 0.25 μm) and HP-1 (cross-linked methyl-silicone, 30 m × 0.25 m × 0.25 μm) capillary columns were employed. Hydrogen was used as the carrier gas at an average linear velocity of 44 cm s−1. Data were processed on a Hewlett-Packard model 3395 integrator.
Mass spectrometry analysis
The mass spectra of the hydroalcoholic extract of the fruits of S. lycocarpum (SL) and the standards solasonine and solamargine were acquired on an ultrOTOFq mass spectrometer (Bruker Daltonics, Billerica, MA, USA), previously calibrated with sodium trifluoroacetate (Na-TFA; 10 mg mL−1). The samples were dissolved in methanol and infused directly into the electrospray ion (ESI) source at a flow rate of 300 μL h−1. The product ion spectra of the protonated solasonine and solamargine (m/z 868 and 884, respectively) were obtained using collision-induced dissociation (CID) with N2 as gas collision at 55 eV. The mass spectrum of SL was obtained at the same conditions as for the standards with no previous treatment. The ions of m/z corresponding to the protonated standards (m/z 868 and 884) were selected in the first-stage mass analysis and activated to fragmentation by CID under the same collision energy. Comparison between the product ion spectra obtained and the spectra of the authentic standards was used to confirm the presence of solamargine and solasonine in that extract.
Fractionation
Based on its trypanocidal activity, the methylene chloride extract of T. stenocarpa (12.0 g) was chromatographed over 300 g silica gel 60 (0.063–0. 200 mm; Merck, Darmstadt, Germany) by vacuum liquid chromatography (CitationColl & Bowden, 1986), furnishing five fractions of 1000 mL each (F1: hexane; F2: methylene chloride; F3: methylene chloride/EtOAc 1:1; F4: EtOAc; F5: EtOH). Fraction F3 (4.33 g) exhibited a significant trypanocidal effect and was filtered over a mixture of celite:norit (3:1; 60 g); elution with methylene chloride afforded 350 mg of a white amorphous solid. Nuclear magnetic resonance (NMR)-1H and -13C data analysis allowed the identification of both oleanolic acid (2) and ursolic acid (4) in this sample as a mixture (CitationMahato & Kundu, 1994). The methylene chloride extract (15.0 g) of M. variabilis was submitted to the same fractionation procedure as described for T. stenocarpa. Fraction F3 (5.22 g), which showed significant trypanocidal activity, was chromatographed over 200 g of silica gel 60 by chromatography column. Elution with hexane/ethyl acetate (3:2 v/v) afforded 650 mg of ursolic acid (1).
In vitro trypanocidal assay
The bioassays of all extracts were carried out using blood collected by cardiac puncture of Swiss albino mice in the parasitemic peak (7th day) after infection with the Y strain of T. cruzi. The blood was diluted with normal murine blood to give a concentration of ca. 2 × 106 trypomastigote forms/mL. Stock solutions of the tested extracts were prepared by dissolving the extracts in pure dimethylsulfoxide (DMSO), to obtain a final concentration of 20.0 mg/mL for each extract. The bioassays were performed in triplicate on microtiter plates (96 wells, which contained 400 μL of mixture/well). Aliquots of the stock solution were added to the diluted blood in such quantities as to give final concentrations of 8.0, 32.0, and 128.0 μg/mL of each extract. The plates were incubated at 4°C during 24 h under stirring, and the lysis percentages of the trypomastigote forms were determined according to a previous methodology (CitationBrener, 1962). The negative control group (infected blood and the solvent) and the positive control group (infected blood and gentian violet) were all run in parallel.
Results are expressed as mean ± standard deviation (SD) of the lysis percentages of trypomastigote forms induced by addition of the extracts to the infected blood. The sigmoidal dose–response curve was used as the statistical method for IC50 determination. Extracts that showed IC50 lower than 100 μg/mL (about three times higher than IC50 of the positive control) were considered active.
Results and discussion
In this study we obtained 20 crude extracts from 10 different Brazilian Cerrado plants (). In we may observe that, among all the assayed extracts, seven are active, including the extracts from Aspidospema macrocarpum (AM-M), Myrcia variabilis (MV-M, MV-E), Solanum lycocarpum (SL), and Tibouchina stenocarpa (TS-E, TS-H, TS-M), which displayed IC50 values lower than 100 μg/mL. We can also observe that all the assayed extracts from T. stenocarpa are active, including the methylene chloride extract (TS-M; IC50 value of 51.5 μg/mL) and the n-hexane extract (TS-H), which exhibits the highest trypanocidal activity among all the tested samples, with an IC50 value of 23.6 μg/mL. Gentian violet, used as positive control, showed an IC50 value of 31 μg/mL (76 μM).
Table 1. Plant extracts from the Brazilian Cerrado assayed for their in vitro trypanocidal activity.
Table 2. In vitro trypanocidal activity of plant extracts from the Brazilian Cerrado against the Y strain of Trypanosoma cruzi.
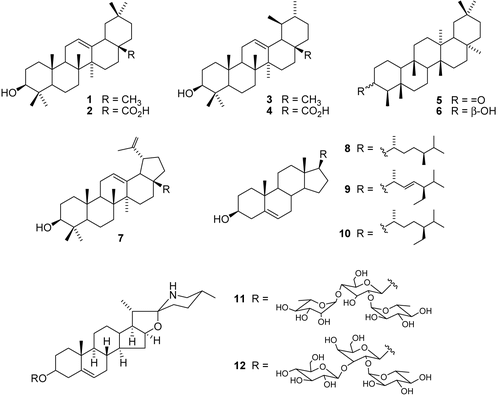
On the basis of their trypanocidal activity, TS-H, TS-M, MV-M, and SL were submitted to phytochemical investigation. TS-H was analyzed by HRGC in order to determine its chemical composition. HRGC analysis allowed the identification of the triterpenesβ-amyrin (1), α-amyrin (3), friedelin (5), β-friedelanol (6), and lupeol (7), as well as the sterols campesterol (8), stigmasterol (9), and β-sitosterol (10) in TS-H (). The phytochemical investigation of TS-M led to the isolation of oleanolic acid (2) and ursolic acid (4) () from T. stenocarpa for the first time. Also, the phytochemical investigation of MV-M revealed the presence of ursolic acid (4) as a major constituent. On the other hand, mass spectrometry analysis of SL allowed the identification of the alkaloids solasonine (11) and solamargine (12) () as major compounds present in SL.
Regarding the trypanocidal activity, previous studies had shown that the pure compounds 1, 3, and 5-10 are inactive (CitationDa Silva Filho et al., 2004; CitationFournet et al., 1992). However, according to literature data (CitationCrevelin et. al., 2006; CitationGaertner et al., 1999; CitationOtuki et al., 2005), some triterpene mixtures may present better biological activities than their isolated compounds. Therefore, since TS-H is active in comparison with its major identified compounds (1, 3, 5-10), it is suggested that the trypanocidal activity of TS-H may be due to the presence of all the compounds present in this crude extract.
Oleanolic acid (2) and ursolic acid (4) are pentacyclic acid triterpenes previously isolated from other Melastomataceae species (CitationCunha et al., 2003, Citation2006, Citation2008; CitationSpessoto et al., 2003), and they display several biological activities such as anti-inflammatory (CitationVasconcelos et al., 2006), analgesic (CitationVasconcelos et al., 2006), antimicrobial (CitationDa Silva Filho et al., 2008), and antimutagenic (CitationResende et al., 2006). In vitro and in vivo trypanocidal assays undertaken in our laboratory demonstrated that these triterpene acids exhibited trypanocidal effects (CitationCunha et al., 2006). For this reason, we suggest that the trypanocidal activity of TS-M may be related to the presence of compounds 2 and 4, which displayed IC50 values of 12.8 μM and 17.1 μM, respectively (CitationCunha et al., 2006). Also, the trypanocidal activity of MV-M may be related to the presence of compound 4.
Moreover, several natural alkaloids have demonstrated trypanocidal activity (CitationBoza & Cassels, 1996), such as the glycoalkaloids solasonine (11) and solamargine (12) (CitationChataing et al., 1998), which are present in the fruits of S. lycocarpum (CitationSchwartz et al., 2007). Since 11 and 12 are the major compounds in the fruits of S. lycocarpum, they may be related to the trypanocidal activity of SL.
In summary, we carried out the screening of several plant extracts from the Brazilian Cerrado for their in vitro trypanocidal activity, which led to the identification of seven active extracts. The phytochemical analyses of the active extracts showed that their trypanocidal activities may be related to their major identified compounds.
Acknowledgements
We wish to thank Alba Regina Barbosa and Maria Inês Junqueira Garcia Teixeira for helping with plant collection and Dr. Angela Borges Martins for plant identification.
Declaration of interest: The authors alone are responsible for the content and writing of the paper. The authors are grateful to FAPESP for financial support.
References
- Araújo ARB, Teixeira MIJG, Rodrigues RR (1999): Florística e fitossociologia de um trecho de cerrado no município de Franca. Naturalia 24: 153–170.
- Boza SS, Cassels BK (1996): Plant metabolites active against Trypanosoma cruzi. Planta Med 62: 98–105.
- Brener Z (1962): Therapeutic activity and criterion of cure on mice experimentally infected with Trypanossoma cruzi. Rev Inst Med Trop São Paulo 4: 389–396.
- Brener Z, Andrade Z (1997): Trypanosoma cruzi e a doença de Chagas. Rio de Janeiro, Guanabara-Koogan, pp. 1–41.
- Butler MS (2004): The role of natural product chemistry in drug discovery. J Nat Prod 67: 2141–2153.
- Chataing B, Concepción JL, Lobatón R, Usubillaga A (1998): Inhibition of Trypanosoma cruzi growth in vitro by Solanum alkaloids: A comparison with ketoconazole. Planta Med 64: 31–36.
- Coll JC, Bowden BF (1986): The application of vacuum liquid chromatography to the separation of terpene mixtures. J Nat Prod 49: 934–936.
- Cordell GA (2000): Biodiversity and drug discovery – a symbiotic relationship. Phytochemistry 55: 463–480.
- Crevelin EJ, Turatti ICC, Crotti AEM, Veneziani, RCS, Lopes JLC, Lopes NP, Cunha WR (2006): Identification of biologically activity triterpenes and sterols present in hexane extracts from Miconia species using high-resolution gas chromatography. Biomed Chromatogr 20: 827–830.
- Cunha WR, Crevelin E J, Arantes GM, Crotti AEM, Silva MLA, Furtado NAJC, Albuquerque S, Ferreira DSA (2006): A study of the trypanocidal activity of triterpene acids isolated from Miconia species. Phytother Res 20: 474–478.
- Cunha WR, Martins C, Ferreira DS, Crotti AEM, Lopes NP, Albuquerque S (2003): In vitro trypanocidal activity of triterpenes from Miconia species. Planta Med 69: 470–472.
- Cunha WR, Silva MLA, Dos Santos FM, Montenegro IM, Oliveira ARA, Tavares HR, Leme dos Santos HS, Da Silva Bizário JC (2008): In vitro inhibition of tumor cell growth by Miconia fallax. Pharm Biol 46: 292–294.
- Da Silva Filho AA, Albuquerque S, Silva MLA, Tomazela DM, Eberlin MN, Bastos JK (2004): Tetrahydrofuran lignans from Nectandra megapotamica with trypanocidal activity. J Nat Prod 67: 42–45.
- Da Silva Filho AA, Sousa JPB, Soares S, Furtado NAJC, Silva MLA, Cunha WR, Gregório LE, Nanayakkara NPD, Bastos JK (2008): Antimicrobial activity of the extract and isolated compounds from Bacharis dracunculifolia DC (Asteraceae). Z Naturforsch C 63: 40–46.
- Fournet A, Angelo A, Munoz V, Roblot F, Hocquemiller R, Cave A (1992): Biological and chemical studies of Pera benensis, a Bolivian plant used in folk medicine as a treatment of cutaneous leishmaniasis. J Ethnopharmacol 37: 159–164.
- Gaertner M, Muller L, Roos JF, Cani G, Santos AR, Niero R, Calixto JB, Yunes RA, Delle Monache F, Cechinel-Filho V (1999): Analgesic triterpenes from Sebastiana schottiana roots. Phytomedicine 6: 41–44.
- Grael CFF, Vichnewski W, De Souza GEP, Lopes JLC, Albuquerque S, Cunha WR (2000): A study of the trypanocidal and analgesic properties from Lychnophora granmogolense (Duarte) Semir & Leitão Filho. Phytother Res 14: 203–206.
- Lee KH (2004): Current developments in the discovery and design of new drugs candidates from plant natural product leads. J Nat Prod 67: 273–283.
- Mahato SB, Kundu AP (1994): 13CNMR spectra of pentaciclic triterpenoids – a compilation and some salient features. Phytochemistry 37: 1517–1575.
- Molinar-Toribio E, González J, Ortega-Barria E, Capson TL, Coley PD, Kursar TA, McPhail K, Cubilla-Rios L (2006): Antiprotozoal activity against Plasmodium falciparum and Trypanosoma cruzi of xanthones isolated from Chrysochlamys tenuis. Pharm Biol 44: 550–553.
- Myers N, Mittermeier RA, Fonseca GAB, Kent J (2000): Biodiversity hotspots for conservation priorities. Nature 403: 853–858.
- Newman DJ, Cragg GM, Snader KM (2003): Natural products as sources of new drugs over the period 1981-2002. J Nat Prod 66: 1022–1037.
- Nussenzweig V, Sonntaeg R (1953): Ação da violeta genciana sobre o T. cruzi in vitro: Sua importância na esterilização do sangue destinado a transfusão. Rev Paul Med 42: 85–86.
- Otuki MF, Ferreira J, Lima FV, Meyre-Silva C, Malheiros A, Muller LA, Cani GS, Sabtos ARS, Yunes RA, Calixto JB (2005): Antinociceptive properties of mixture of α-amyrin and β-amyrin trterpenes: Evidence for participation of protein kinase C and protein kinase A pathways. J Pharmacol Exp Ther 313: 310–318.
- Resende FA, Barcala CAMA, Faria MCS, Kato FH, Cunha WR, Tavares DC (2006): Antimutagenicity of ursolic and oleanoic acid against doxorubicin-induced clastogenesis in Balb/c mice. Life Sci 79: 1268–1273.
- Schwarz A, Pinto E, Haraguchi M, De Oliveira CA, Bernardi MM, Spinosa HS (2007): Phytochemical study of Solanum lycocarpum (St. Hil) unripe fruit and its effects on rat gestation. Phytother Res 21: 1025–1028.
- Spessoto MA, Ferreira DS, Crotti AEM, Silva MLA, Cunha WR (2003): Evaluation of the analgesic activity of extracts of Miconia rubiginosa (Melastomataceae) Phytomedicine 10: 606–609.
- Vasconcelos MAL, Royo VA, Ferreira DS, Crotti AEM, Silva MLA, Carvalho JCT, Bastos JK, Cunha WR (2006): In vivo analgesic and anti-inflammatory activities of ursolic acid and oleanoic acid from Miconia albicans (Melastomataceae). Z Naturforsch 61c: 477–486.
- World Health Organization (2000): Special Programme for Research and Training in Tropical Disease (TDR). Natural products for parasitic diseases. TDR News 62: 4.
- World Health Organization (2001): Chagas’ disease intervention development and implemention research: transfer to the WHO regional office for the Americas. TDR News 66: 17.