Abstract
Alzheimer’s disease (AD) is the most common form of dementia among the elderly, causing progressive cognitive dysfunction, particularly memory loss. Recently, modulation of Aβ toxicity, which is one of the major potential causes of AD, by plant extracts is emerging as a possible therapeutic approach to control the onset of AD. Therefore, in the present study, the methanol extracts of 400 traditional oriental herbal medicines were tested for their abilities to protect PC12 rat pheochromocytoma cells against Aβ-induced toxicity. Our results identified 29 plant extracts that protected PC12 cells against Aβ insult, with ED50 values of less than 100 μg/mL. Among these extracts, those from Chaenomeles sinensis Koehne (Rosaceae), Ephedra sinica Stapf. (Ephedraceae), and Drynaria fortunei Smith (Polypodiaceae) exhibited the most promising neuroprotective effects, with ED50 values of less than 10 μg/mL. Their neuroprotective effects against Aβ insult were not fully explained by their participation in antioxidant pathways, as demonstrated by a free radical scavenging assay with DPPH. Therefore, the mechanisms and the major constituents behind the neuroprotective effects of these plant extracts warrant further study.
Introduction
Alzheimer’s disease (AD), the most common form of dementia among the elderly, is characterized by progressive cognitive dysfunction, and it frequently presents with memory loss and depression (CitationGarber, 2001). AD affects approximately four million Americans and causes more than 100,000 deaths each year. One of the pathologic hallmarks of AD is senile plaques, which are extracellular deposits of insoluble β-amyloid (Aβ) protein (CitationHaass & Selkoe, 1994). The Aβ peptide is a product of the proteolytic cleavage of amyloid precursor protein (APP) by β- and γ-secretases. Since Aβ accumulation into senile plaques has been accepted as one of the major causes of AD pathology, modulation of Aβ toxicity has been speculated to be an important therapeutic approach to control the onset of AD.
Natural products have attracted relatively little attention in the area of AD drug discovery, but recently they have shown potential as a valuable resource for new therapeutic approaches to AD. Extensive investigations of Ginkgo biloba L. (Ginkgoaceae) extract EGb 761 (CitationOken et al., 1998) and Huperzia serrata (Thunb. ex Murray) Trevis. (Lycopodiaceae) (CitationBadia et al., 1998) have suggested their potential as natural therapeutic agents to treat AD patients. Several recent reports have demonstrated protection against Aβ-induced neurotoxicity by plant extracts, such as onji (Polygala tenuifolia Willd., Polygalaceae) (CitationIkeya et al., 2004), turmeric (Curcuma longa L., Zingiberaceae) (CitationPark & Kim, 2002), and Smilax china L. (Liliaceae) (CitationBan et al., 2006). Based on these promising reports, we conducted a large-scale screen of the myriad of plants used as traditional oriental herbal medicines, envisioning that one or more of them might protect neuronal cells from Aβ-induced toxicity. Specifically, we obtained methanol extracts from traditional oriental herbal medicines and tested their abilities to protect PC12 rat pheochromocytoma cells against Aβ-induced toxicity. Our results revealed that several of these herbal remedies show promise as potential therapeutic agents in the treatment of AD.
Materials and methods
Materials and reagents
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide], SDS (sodium dodecyl sulfate), DMSO (N,N-dimethyl sulfoxide), absolute ethanol, and DPPH (1,1-diphenyl-2-picrylhydrazyl) were purchased from Sigma (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and horse serum were obtained from Invitrogen (Carlsbad, CA, USA). Other general supplies needed for the bioassays were purchased from Fisher Scientific (Itasca, IL, USA). MTT was dissolved in phosphate buffered saline (PBS; 1 mg/mL), sterilized by filtration through a 0.2-μm filter, and stored at 4°C in an amber bottle until use. Lysis buffer was prepared by dissolving 10% (w/v) SDS in 0.01N HCl.
Culture of PC12 cells
PC12 rat pheochromocytoma cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in DMEM supplemented with 15% horse serum and 5% FBS, at 37°C under 5% CO2. Cells were utilized for experiments during the exponential growth phase.
Preparation of Aβ (25–35) aggregates
Aβ aggregates are most likely responsible for AD pathology, because Aβ oligomers (insoluble form) are more toxic to neurons than are monomers (soluble form) or fibrils (CitationKelly & Ferreira, 2006). Therefore, for this study, Aβ (25–35) was aggregated before use. Briefly, Aβ (25–35) ( 1 mg) was dissolved in 1 mL of DMEM and incubated in a 37°C water bath. After 3 days of aggregation, Aβ (25–35) was diluted to 100 μg/mL (100 μM) and stored at −20°C until use.
Preparation of plant extracts
The methanol extracts of Korean medicinal plants were obtained from the Plant Extract Bank, Plant Diversity Research Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB, Daejeon, Korea). All botanical samples were identified and authenticated at the Plant Extract Bank, where all of the voucher specimens were deposited (http://extract.pdrc.re.kr).
Assay for the ability to protect PC12 cells against Aβ insult
In order to evaluate the abilities of the plant extracts to protect or treat Aβ-induced neurotoxicity, we performed a bioassay according to a previously published method (CitationPark & Kim, 2002). A test compound was scored as being able to protect PC12 cells from Aβ (25–35) insult if it enhanced the cells’ conversion of MTT to MTT-formazan within the cells, which directly reflects cell viability. For the assay, we seeded 90 μL of exponentially growing PC12 cells (4 × 104 cells per well) in 96-well tissue culture plates for at least 2 h. Cells were then pretreated with various concentrations (100, 20, and 4 μg/mL) of the experimental methanol extracts, rosmarinic acid as a positive control, or DMSO as a negative (vehicle) control. One hour later, Aβ (25–35) aggregates (10 μM) were added to the pretreated cells and incubated for 24 h. Cells were then incubated with MTT solution (20 μL per well, 1 mg/mL stock solution) for 3 h at 37°C, followed by lysis overnight at 37°C in 100 μL lysis buffer. We determined the optical density of the resulting solutions colorimetrically at 590 nm using a microplate reader. We then prepared dose–response curves for each extraction, with results expressed as ED50 values in μg/mL. The ED50 values of samples were defined as the concentrations (μg/mL) required to achieve 50% cell viability under Aβ insult.
To investigate changes in cell morphology, PC12 cells plated on coverslips were treated with Aβ (25–35) aggregates for 24 h in the presence or absence of plant extract. Cells were then incubated with MTT for 1 h, and cell morphology was examined under a microscope.
Antioxidant activity
We determined the antioxidant activity of plant extracts based on their ability to scavenge a stable free radical, 1,1-diphenyl-2-picrylhydrazyl (DPPH), and convert it into 1,1-diphenyl-2-picrylhydrazine (CitationSmith & Reeves, 1987); this change was measured colorimetrically. Briefly, 99 μL DPPH (0.316 mM in ethanol) and 1 μL plant extract were mixed and incubated at 37°C for 30 min. The resulting optical density was measured at 517 nm using a microplate reader. Various concentrations of each plant extract were tested, as indicated, and BHA (butylated hydroxyanisole) and α-tocopherol were used as positive controls.
Statistical analysis
All data in the text and figures are expressed as the mean ± SEM. Two-group comparisons were evaluated by paired t-tests. Differences were considered statistically significant at p < 0.05.
Results and discussion
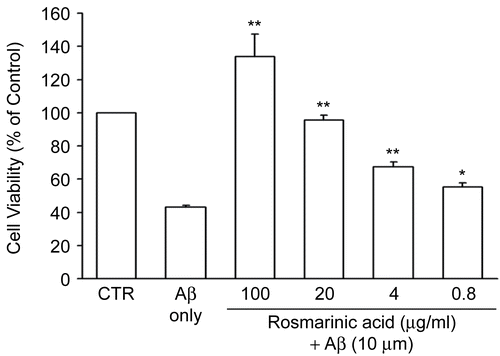
We designed this study to evaluate the potential neuroprotective effects of natural products against Aβ-mediated toxicity. First, we selected 10 μM as the Aβ concentration for all subsequent assays, since about 50% of cells were considered dead at this concentration. Second, to confirm the effectiveness of our toxicity bioassay (see “Materials and methods”), we tested rosmarinic acid, a compound previously demonstrated to mediate anti-Aβ effects (CitationIuvone et al., 2006), as a positive control. In agreement with previous results, our bioassay demonstrated that rosmarinic acid protected PC12 cells against Aβ insult with an ED50 value of 6.6 μg/mL. At pretreatment concentrations greater than 20 μg/mL, rosmarinic acid completely protected PC12 cells against Aβ insult (). Pretreatment with 4 and 0.8 μg/mL rosmarinic acid also significantly improved cell viability compared to treatment with Aβ alone.
Figure 1. Rosmarinic acid inhibited Aβ (25–35)-induced cell death in PC12 cells. Different concentrations of rosmarinic acid were applied to PC12 cells for 1 h prior to treatment with 10 μM Aβ (25–35). The cell viability was measured by MTT assay. The absorbance of 0.1% DMSO-treated cells (controls) was set at 100%. The results are expressed as mean ± SEM from three independent experiments, each performed in triplicate. * p < 0.05, ** p < 0.01 compared to 10 μM Aβ (25–35).
To investigate the potential for traditional oriental herbal remedies to ameliorate Aβ-induced toxicity in PC12 cells, a total of 400 methanol extracts were tested for neuroprotective effects against Aβ. Three independent assays were performed in order to calculate ED50 values. Our assay revealed that 29 plant extracts protected PC12 cells against Aβ insult, all of them with ED50 values of less than 100 μg/mL (). Among these species, Chaenomeles sinensis Koehne (Rosaceae), Ephedra sinica Stapf. (Ephedraceae), and Drynaria fortunei Smith (Polypodiaceae) showed the most promising neuroprotective effects, with ED50 values of less than 10 μg/mL (). In addition, Acorus gramineus Solander (Araceae), Anethum graveolens L. (Apiaceae), Cimicifuga heracleifolia Komarov (Ranunculaceae), Cornus officinalis S. et Z. (Cornaceae), Lycium chinense Miller (Solanaceae), Terminalia chebula Retzius (Combretaceae), Fraxinus rhynchophylla Hance (Oleaceae), Scutellaria baicalensis Georgi (Lamiaceae), Polygonum multiflorum Thunberg (Polygonaceae), and Amomum xanthioides Wallich (Zingiberaceae) demonstrated moderate neuroprotective effects with ED50 values between 11 and 30 μg/mL.
Table 1. Traditional oriental herbal medicines that demonstrated a neuroprotective effect against Aβ insult.
Figure 2. The methanol extracts of C. sinensis, E. sinica, and D. fortunei protected PC12 cells against Aβ in a dose-dependent manner. (A) Different concentrations of methanol extracts from C. sinensis, E. sinica, and D. fortunei were incubated with PC12 cells for 1 h, followed by 24 h incubation with 10 μM Aβ (25–35). Resulting cell viabilities were measured using MTT assay. The absorbance of 0.1% DMSO-treated cells (controls) was set to 100%. The results are expressed as mean ± SEM from three independent experiments, each performed in triplicate. (B–D) The methanol extract from D. fortunei (10 μg/mL) was incubated with PC12 cells for 1 h, followed by 24 h incubation with 10 μM Aβ (25–35). The morphological changes of PC12 cells induced by Aβ were examined under a microscope after incubation with MTT for 1 h (×200). Note that DMSO-treated negative controls (B) and cells treated with the extract from D. fortunei (D) and Aβ generated MTT-formazan granules, whereas Aβ-treated cells (C) formed MTT-formazan spikes. Bar = 10 μm.
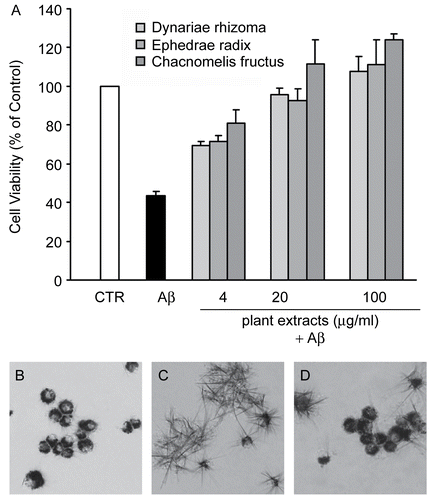
In order to confirm the neuroprotective effect of plant extracts, we investigated the effect of the methanol extract of D. fortunei on the Aβ-induced changes in cell morphology. As shown in , negative controls (DMSO-treated) formed MTT-formazan granules in the cytoplasm, whereas Aβ-treated positive controls showed the formation of MTT-formazan spikes (). On the other hand, cells treated with Aβ in the presence of the methanol extract of D. fortunei () formed MTT-formazan granules, suggesting that the D. fortunei plant extract protected cells from Aβ-induced toxicity.
Despite previous studies on the ethnomedical uses or pharmacological activities of the plants examined herein, in most cases, there were no previous reports examining the effects of these plants on memory improvement, dementia, or amnesia, nor had these plants been used traditionally to treat any of these ailments. However, some of these plants, namely D. fortunei, A. gramineus, S. baicalensis, and C. officinalis, have been reported as having beneficial neurological effects when used in traditional settings or when analyzed pharmacologically.
In our study, D. fortunei showed one of the most potent neuroprotective effects against Aβ insult, with an ED50 value of 5.7 μg/mL. Recently, Dr. Yang reported that D. fortunei boosted cognitive abilities, suggesting that this plant could be developed as a treatment for senile dementia (CitationYang, 2005), supportive of the neuroprotective effect of D. fortunei demonstrated herein. Taken together, these results warrant future studies of D. fortunei to identify its active compounds and their mechanisms of action.
A. gramineus has been reported to improve learning and memory, even following a stroke, in combination with other herbal medicines (CitationYun et al., 2007; CitationZhang et al., 2007). The neuroprotective activity of A. gramineus in the central nervous system (CNS) is mediated by signaling through NMDA (N-methyl-d-aspartate) receptors (CitationCho et al., 2001). This observation sheds light on the antitoxicity of this plant against Aβ, since Aβ induces neurotoxicity by disturbing NMDA-mediated calcium homeostasis (CitationShankar et al., 2007). CitationIrie and Keung (2003) reported previously that A. gramineus essential oil protected PC12 cells against Aβ, and they eliminated both eugenol and β-asarone as possible active compounds within this essential oil. In the study herein, a methanol extract of A. gramineus protected neuronal cells against Aβ with an ED50 value of 11.6 μg/mL. Subsequent high-performance liquid chromatography (HPLC) measurements revealed that the extract contained 15.5% β-asarone and negligible eugenol, and that the isolated compounds had no protective effect in PC12 cells against Aβ (data not shown). Therefore, the neuroprotective constituent(s) within the A. gramineus extract still need to be identified and validated in vivo for anti-AD effects.
C. officinalis is used traditionally as a therapy for diabetes, dizziness, and high blood pressure. In our experimental system, C. officinalis extract protected PC12 cells against Aβ insult with an ED50 value of less than 25 μg/mL. In previous animal studies, C. officinalis extract administered in a rat model of cerebral infarction decreased the area of infarction and the levels of proinflammatory molecules such as nitric oxide (NO), iNOS, and NF-κB (CitationLi et al., 2005). Since both NO and NF-κB are mediators of Aβ-induced neurotoxicity (CitationValerio et al., 2006), these results suggest that our observed neuroprotective effect could result from a C. officinalis-mediated decrease of these molecules.
S. baicalensis is a traditional Asian herbal anti-inflammatory and antioxidant agent. In previous reports, baicalin and baicalein, which are the major constituents of S. baicalensis, not only protected neuronal cells against Aβ-induced neurotoxicity, but also alleviated AD-associated cognitive deficits, such as cerebral ischemia-induced neuronal damage (CitationHeo et al., 2004). In our study, S. baicalensis protected PC12 cells against Aβ insult with an ED50 value of 11.1 μg/mL. Subsequent HPLC analysis revealed that the levels of baicalin and baicalein in methanolic S. baicalensis extract were 30.4% and 1.9%, respectively. Interestingly, when tested separately, baicalein and baicalin protected PC12 cells with ED50 values of 25.7 and 119.2 μg/mL, respectively, indicating that these two compounds alone could not account for the neuroprotective effects of S. baicalensis extract. We propose that additional active constituent(s) might be present in the extract, and that our preliminary results warrant their isolation and testing.
The oxidative damage induced by Aβ is widely accepted as one of the major pathologic contributors to AD (Onyango & Khan, Citation2006), and known antioxidants are currently being studied for their effect in slowing AD progression. Several plants and plant compounds with antioxidant activity have shown favorable effects in the CNS. In an effort to identify the relevance of antioxidant properties in relation to neuroprotective effects against Aβ, we tested the same 400 plant extracts for antioxidant properties using a DPPH-based scavenging assay (). Two well-documented antioxidants, BHA (butylated hydroxyanisole) and β-tocopherol, were used as positive controls. The DPPH radical scavenging efficiencies of BHA or β-tocopherol were 91 and 90%, respectively, both at concentrations of 100 μg/mL. Among the traditional herbal medicines that exhibited neuroprotective effects against Aβ insult, D. fortunei, E. sinica Stapf. (Ephedraceae), Euonymus alatus Sieb. (Celastraceae), P. multiflorum, S. baicalensis, and Terminalia chebula Retzius (Combretaceae) showed only weak DPPH scavenging efficiencies at 100 μg/mL. It is worth noting, however, that most of the neuroprotective plants tested in this study had no scavenging activity at 100 μg/mL. The results of the scavenging assay, therefore, suggest that activation of the antioxidant pathway could not sufficiently account for the neuroprotective effects of these plants against Aβ insult.
In this study, 400 methanolic plant extracts were tested for protective effects against Aβ-induced neurotoxicity. To the best of our knowledge, this is the first large-scale, systematic screening of oriental medicinal plants for anti-Aβ activity. Our results identified 29 extracts that protected PC12 cells against Aβ insult with ED50 values of less than 100 μg/mL. Among them, C. sinensis, E. sinica, and D. fortunei showed the most promising neuroprotective effects, with ED50 values of less than 10 μg/mL. With notable exceptions, most of the plants that were effective at some level against Aβ insult have not been used traditionally to treat neuronal defects, nor have they been reported to have neuroprotective pharmacological activities. Our analyses of the free radical scavenging efficiencies of the plant extracts demonstrate that the neuroprotective effects of most of the plants in this study were not directly related to antioxidant properties. Therefore, the major bioactive components within these plants and their mechanisms of neuroprotection remain a mystery and warrant further investigation. Nonetheless, this study has identified over two-dozen biologically active plant extracts that show potential as therapies against AD, and we are confident that our results will serve as a starting point for the future development of new neuroprotective pharmacological agents.
Acknowledgements
The authors are grateful to Dr. Hyeong-Kyu Lee of the Plant Extract Bank, Plant Diversity Research Center, KRIBB, for the plant extracts used in this study.
Declaration of interest: This work was supported by the Medical Research Center for Environmental Toxico-Genomics and Proteomics of Korea University.
References
- Badia A, Banos JE, Camps P, Contreras J, Gorbig DM, Munoz-Torrero D, Simon M, Vivas NM (1998): Synthesis and evaluation of tacrine-huperzine A hybrids as acetylcholinesterase inhibitors of potential interest for the treatment of Alzheimer’s disease. Bioorg Med Chem 6: 427–440.
- Ban JY, Cho SO, Koh SB, Song K-S, Bae KW, Seong YH (2006): Protection of amyloid beta protein (25-35)-induced neurotoxicity by methanol extract of Smilacis chinae rhizome in cultured rat cortical neurons. J Ethnopharmacol 106: 230–237.
- Cho J, Kong JY, Jeong DY, Lee KD, Lee DU, Kang BS (2001): NMDA receptor-mediated neuroprotection by essential oils from the rhizomes of Acorus gramineus. Life Sci 68: 1567–1573.
- Garber K (2001): Isolation of key genes at the heart of Alzheimer’s disease has set off a frenzied race to find a drug to stop this cruel affliction in its tracks. Technol Rev 104: 70–77.
- Haass C, Selkoe D (1994): Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell 7: 1039–1042.
- Heo HJ, Kim DO, Choi SJ, Shin DH, Lee CY (2004): Potent inhibitory effect of flavonoids in Scutellaria baicalensis on amyloid beta protein-induced neurotoxicity. J Agric Food Chem 52: 4128–4132.
- Ikeya Y, Takeda S, Tunakawa M, Karakida H, Toda K, Yamaguchi T, Aburada M (2004): Cognitive improving and cerebral protective effects of acylated oligosaccharides in Polygala tenuifolia. Biol Pharm Bull 27: 1081–1085.
- Irie Y, Keung WM (2003): Rhizoma acori graminei and its active principles protect PC-12 cells from the toxic effect of amyloid-beta peptide. Brain Res 963: 282–289.
- Iuvone T, De Filippis D, Esposito G, D’Amico A, Izzo AA (2006): The spice sage and its active ingredient rosmarinic acid protect PC12 cells from amyloid-beta peptide-induced neurotoxicity. J Pharmacol Exp Ther 317: 1143–1149.
- Kelly BL, Ferreira A (2006): beta-Amyloid-induced dynamin 1 degradation is mediated by N-methyl-D-aspartate receptors in hippocampal neurons. J Biol Chem 281: 28079–28089.
- Li CY, Li L, Li YH, Ai HX, Zhang L (2005): Effects of extract from Cornus officinalis on nitric oxide and NF-kappaB in cortex of cerebral infarction rat model. Zhongguo Zhong Yao Za Zhi 30: 1667–1670.
- Oken BS, Storzbach DM, Kaye JA (1998): The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol 55: 1409–1415.
- Onyango IG, Khan SM (2006): Oxidative stress, mitochondrial dysfunction, and stress signaling in Alzheimer’s disease. Curr Alzheimer Res 3: 339–349.
- Park SY, Kim DSHL (2002): Discovery of natural products from Curcuma longa that protect cells from beta-amyloid insult: A drug discovery effort against Alzheimer’s disease. J Nat Prod 65: 1227–1231.
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL (2007): Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci 27: 2866–2875.
- Smith RC, Reeves JC (1987): Antioxidant properties of 2-imidazolones and 2-imidazolthiones. Biochem Pharmacol 36: 1457–1460.
- Valerio A, Boroni F, Benarese M, Sarnico I, Ghisi V, Bresciani LG, Ferrario M, Borsani G, Spano P, Pizzi M (2006): NF-kappaB pathway: a target for preventing beta-amyloid (Abeta)-induced neuronal damage and Abeta42 production. Eur J Neurosci 23: 1711–1720.
- Yang D (2005): Gu Sui Bu (Rhizoma Drynariae) – a good drug for senile dementia. J Tradit Chin Med 25: 290–291.
- Yun YJ, Lee B, Hahm DH, Kang SK, Han SM, Lee HJ, Pyun KH, Shim I (2007): Neuroprotective effect of palmul-chongmyeong-tang on ischemia-induced learning and memory deficits in the rat. Biol Pharm Bull 30: 337–342.
- Zhang H, Han T, Yu CH, Rahman K, Qin LP, Peng C (2007): Ameliorating effects of essential oil from Acori graminei rhizoma on learning and memory in aged rats and mice. J Pharm Pharmacol 59: 301–309.