Abstract
The approach to new drugs through natural products has proved to be the single most successful strategy for the discovery of new drugs, but in recent years its use has been deemphasized by many pharmaceutical companies in favor of approaches based on combinatorial chemistry and genomics, among others. Drug discovery from natural sources requires continued access to plant, marine, and microbial biomass, and so the preservation of tropical rainforests is an important part of our drug discovery program. Sadly, many of the tropical forests of the world are under severe environmental pressure, and deforestation is a serious problem in most tropical countries. One way to combat this loss is to demonstrate their value as potential sources of new pharmaceutical or agrochemical products. As part of an effort to integrate biodiversity conservation and drug discovery with economic development, we initiated an International Cooperative Biodiversity Group (ICBG) to discover potential pharmaceuticals from the plant biodiversity of Suriname and Madagascar. The Group, established with funding from agencies of the United States government, involved participants from the USA, Suriname, and Madagascar. The basic approach was to search for bioactive plants in the Suriname and Malagasy flora, and to isolate their bioactive constituents by the best available methods, but the work included capacity building as well as research. Progress on this project is reported, drawing on results obtained from the isolation of bioactive natural products from Suriname and Madagascar. The benefits of this general approach to biodiversity and drug discovery are also discussed.
Introduction
Humans have depended on nature for their basic needs, including medicines, since the beginning of civilization. Today, natural products are responsible for over one-third of the approved drugs on the market, with 39% of the total drugs approved between 1983 and 1994 in 33 different disease areas being natural products or compounds derived from natural products (CitationCragg et al., 1997). Of the 1184 new chemical entities for all diseases worldwide approved between January 1981 and June 2006, 28% were natural products or derived from natural products, while 24% were synthesized using natural product scaffolds (CitationNewman & Cragg, 2007). The use of natural products and natural product scaffolds to produce compounds with therapeutic potential has been described recently (CitationNewman, 2008).
Plants have formed the basis of traditional medicine systems that have been in existence for thousands of years (CitationCragg & Newman, 2001). The plant-based systems continue to play an essential role in health care, and it has been estimated by the World Health Organization that approximately 80% of the world’s inhabitants rely predominantly on traditional medicines for their primary health care. Plant products also play an important role in the health care system of the remaining 20% of the population, who mainly reside in developed countries (CitationCragg & Newman, 2005a). Of 119 drugs derived from 90 plants, 74% were discovered as a result of chemical studies directed at isolation of the active substances from plants used in traditional medicine (CitationCragg & Newman, 2005b).
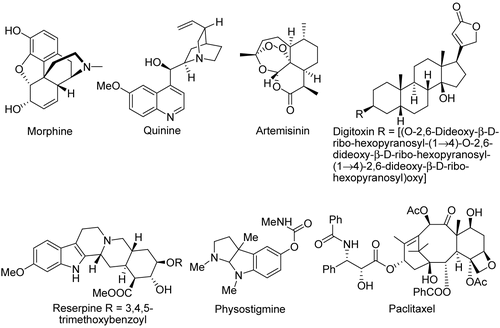
In 1805, morphine was crystallized from opium (Papaver somniferum L., Papaveraceae). Since then, many drugs have been developed from compounds obtained from plants (). For example, the anti-malarial drugs quinine and artemisinin were isolated from the bark of the South American cinchona tree Cinchona officinalis L. (Rubiaceae) and the Chinese herb Artemisia annua L. (Asteraceae), respectively. The cardenolide digitoxin was obtained from the leaves of foxglove, Digitalis purpurea L. (Scrophulariaceae), a biennial herb that is widely distributed throughout Europe and common in England. The antihypertensive indole alkaloid drug reserpine was isolated from the dried root of Indian snakeroot, Rauwolfia serpentina (L.) Benth. ex Kurz (Apocynaceae). Physostigmine, which is used for memory enhancement, was obtained from the west African plant Physostigma venenosum Balf. f. (Fabaceae). The Pacific Yew Taxus brevifolia Nutt. (Taxaceae), native to the Pacific Northwest of North America, and the Canada Yew Taxus canadensis Marshall, are the sources of paclitaxel, a chemotherapeutic drug used in breast and lung cancer treatment. Despite its potent bioactivity, natural products such as paclitaxel could never be prepared by standard “medicinal chemistry” approaches to drug discovery, even including the newer methods of combinatorial chemistry (CitationKingston et al., 2000).
Well over 50% of the estimated 250,000 plant species found on earth come from tropical forests, which cover less than 7% of the earth’s land surface. The majority of these tropical plants have not been studied for their chemical and biological properties. Unfortunately, tropical forests have been disappearing rapidly, and they have been reduced from about 16% of the earth’s land surface in 1950 to a mere 7% today (CitationKingston, 2004). The loss of tropical rainforests results in a catastrophic reduction of biodiversity.
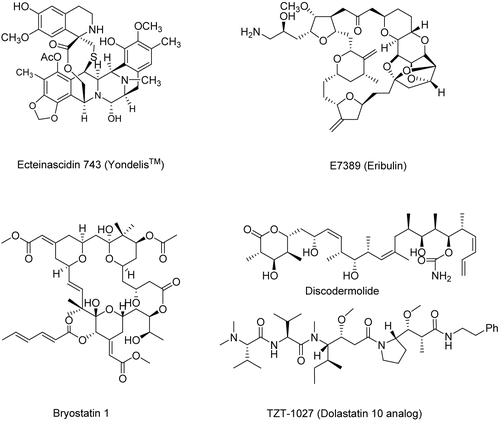
Marine natural products have also attracted the attention of biologists and chemists the world over for the past five decades. This interest has led to the discovery of almost 8500 marine natural products to date, and many of the compounds have shown very promising biological activity (CitationBhakuni & Rawat, 2005). The first drug directly derived from a marine source, namely ecteinascidin 743 (Yondelis®; ), has been granted Orphan Drug designation in Europe and the USA, and was recommended for approval by the European Medicines Evaluation Agency (EMEA) in late July, 2007, for the treatment of soft tissue sarcomas (STS). There are also significant numbers of very interesting molecules that have come from marine sources, or have been synthesized as a result of knowledge gained from a prototypical compound, that are either in or approaching Phase II/III clinical trials in cancer, analgesia, allergy, and cognitive diseases (CitationNewman & Cragg, 2004).
E7389 (Eribulin) is a simplified analog of halichondrin B which has been prepared by total synthesis; it is an inhibitor of tubulin assembly and is in Phase I and II clinical trials (CitationDabydeen et al., 2006, CitationNewman, 2007). Dolastatin 10, a linear peptide, from Dolabella auricularia Lightfoot (syn.: Symploca sp.) (Aplysiidae) (mollusc/cyanobacterium), strongly affected microtubule assembly and tubulin-dependent guanosine triphosphate hydrolysis. TZT-1027, which is now in clinical trial, was designed with the goal of maintaining potent antitumor activity while reducing toxicity of the parent compound, dolastatin 10 (CitationSimmons et al., 2005). Bryostatin 1, a macrocyclic lactone, from Bugula neritina L. (Bugulidae) (bryozoan), is a potent activator of protein kinase C (PKC) (CitationSimmons et al., 2005). Discodermolide, isolated in 1990 from the Caribbean marine sponge Discodermia dissoluta Schmidt (Discodermiidae), has been shown to inhibit the proliferation of human cells by arresting the cell cycle in G2- and M-phase. It hyper-stabilizes microtubules, especially prevalent during cell division, and competes with paclitaxel for microtubule binding, but with higher affinity (CitationKlein et al., 2005). The ocean is thus also an excellent source of potential drugs.
Plant collection and biodiversity conservation in Suriname and Madagascar
Background
The preceding discussion has highlighted the importance of natural products in drug discovery and development. It goes without saying that continuing natural products-based drug discovery requires access to a high degree of biodiversity, including tropical forests and reefs. Sadly, these parts of our world are under threat from deforestation, pollution, and a host of other pressures, many of them related to poverty. Drug discovery thus needs to be coupled with conservation and economic development to ensure continuing access to the necessary biodiversity for future generations. As part of an effort to integrate biodiversity conservation and drug discovery with economic development, we initiated an International Cooperative Biodiversity Group (ICBG) to discover potential pharmaceuticals from the plant biodiversity of Suriname and Madagascar. Early results from the work in Suriname have been summarized previously (CitationKingston et al. 1999), and this report summarizes work since then.
Why Suriname and Madagascar?
Suriname is located in the shoulder of South America, sharing borders with the Atlantic Ocean to the north, Guyana to the west, Brazil to the south, and French Guiana to the east. Its surface area of 163,820 km2 makes it one of the smaller states within the continent. Suriname has a large area of undisturbed neotropical Amazonian forest. The interior of Suriname, with an area of approximately 150,000 km2, is largely uninhabited and covered with undisturbed neotropical Amazonian forest, making Suriname one of the largest places anywhere for conservation of this biome (CitationSchulz et al., 1977). Although some logging has occurred in the interior in recent years, this has so far been of limited scope, and Suriname remains a prime site for conservation efforts. It is listed as a “Group 2” country by the World Conservation Monitoring Centre, which places it in the top 50 countries in the world in richness of biodiversity, but not in the top 25 (CitationCaldecott et al., 1994). Suriname also has a diverse flora. It is estimated to contain approximately 5000 species of plants, and it has a higher percentage of intact, close-cover forest remaining than any other South American country (CitationMittermeier et al., 1990). The culture of Suriname is very different from the rest of South America, with both native Amerindian and Bushnegro populations in the interior and a blend of Creoles, Hindustanis, Javanese, Chinese, and Europeans in the coastal region. Finally, Suriname is politically stable. In contrast to some tropical countries with a rich biodiversity, Suriname has had a stable democratic government for the last several years, and recently held peaceful elections for the selection of a new government.
Madagascar is perhaps the number one conservation priority in the world. It is one of the highest priority Biodiversity Hotspots (CitationAnonymous, 1997), and one of only a handful that qualifies both as a hotspot and a mega-diversity country. The fourth largest island on earth and the largest oceanic island, it is about the size of France, covering 587,045 km2 or 0.4% of the planet’s land surface. In spite of being located only 400 km from the eastern coast of Africa, Madagascar (another piece of the supercontinent Gondwanaland) has been isolated from other land masses for more than 160 million years. Consequently, most of the plants and animals species found here evolved apart from the rest of the world and are unique to the island. The high endemism that has resulted is not just at the species level, but at the generic and family levels as well. In many respects, Madagascar is biologically a mini-continent, with levels of endemism that few other countries can match and with very high diversity in certain groups of organisms, especially given its relatively small size (CitationKingston, 2004). Madagascar is home to as many as 12,000 plant species, 70–80% of which are endemic. By comparison, perhaps 30,000–35,000 species occur in all of tropical Africa including the Sahel, which covers almost 35 times as much area. However, from 1950 to 1985, one-half of Madagascar’s forests disappeared, and in 1985 only 34% of the original forests existing in Madagascar remained. Hence, work is urgent there. As the poorest country that is both a Biodiversity Hotspot and in the top 25 countries in terms of biodiversity (CitationCaldecott et al., 1994), and a country with a very poorly known flora, Madagascar thus was an ideal opportunity for implementation of the goals of the ICBG program.
Suriname and Madagascar ICBG consortium partners and their roles
The research consortium consists of USA-based groups from the Missouri Botanical Garden, who carry out plant collection work, Conservation International, who focus on conservation and economic development work as well as plant collection in Suriname, and Virginia Polytechnic Institute and State University (VPISU). In addition to these groups, in-country teams from Bedrijf Geneesmiddelen Voorziening Suriname (BGVS) in Suriname and Centre National d’Application et des Recherches Pharmaceutiques (CNARP) and Centre National de Recherches Sur l’ Environment (CNRE) in Madagascar play key roles in coordinating the in-country work and preparing extracts for study. Finally, groups from Bristol-Myers Squibb Pharmaceutical Research Institute (BMS, 1993–2002), Eisai Research Institute (ERI, 2003–present), and Dow AgroSciences (DAS, 1998–present) carried out screening and isolation studies on extracts of interest to them.
Plant collection in Madagascar and Suriname
In Madagascar the basic procedure was for plant collections to be made by a team from the Missouri Botanical Garden, and for these collections to be ground and extracted with an ethanol/chloroform mixture by scientists at CNARP. The dried extracts were then shipped to VPISU, where they were bioassayed using either a yeast-based screen for DNA-damaging agents or a mammalian antiproliferative assay using the A2780 human ovarian cancer cell line. The collections were all made around the Zahamena protected area, which involved a challenging hike into and out of the collecting area (CitationKingston, 2004).
In Suriname the process was a little different, since collections were made both by Conservation International (CI) and by Missouri Botanical Garden (MBG). The CI team collected on an ethnomedical basis, after extensive discussions with the tribal healer of the village involved. The MBG team, on the other hand, collected on a botanical basis, which led to some overlap of the collections (CitationKingston, 2004).
Biodiversity conservation in Madagascar and Suriname
Primary responsibility for the biodiversity conservation aspects of this work was assumed by MBG and CI. MBG had the goal of contributing to a comprehensive botanical inventory of Suriname and preparing inventories of selected areas of Madagascar, while CI focused on strengthening the capabilities to plan and implement sustainable use policies and enhancing the value of biodiversity to local communities through benefit-sharing agreements, educational programs, and other approaches (CitationKingston et al., 2001). One of the many fruits of the MBG work in Madagascar has been the publication of guides to the ferns of Zahamena, the nature reserve near the MBG collection sites during the 1998–2003 period (Citationde Rasolohery, 2006).
Bioactive compounds of higher plants from Suriname and Madagascar rainforests and marine metabolites from Madagascar
The major biologically active components isolated from Suriname and Madagascar plants can be classified into (a) alkaloids, (b) aromatic compounds, (c) cardenolides, (d) terpenoids, (e) glycoresins, and (f) marine metabolites.
Alkaloids
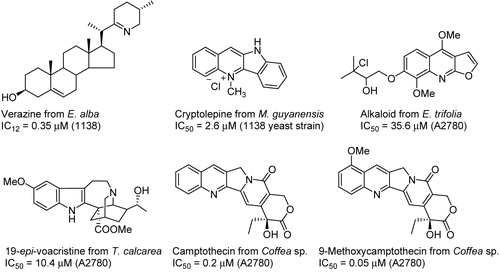
Bioassay-guided fractionation of the methanol extract of the leaves of Eclipta alba (L.) Hassk. (Asteraceae) yielded eight steroidal alkaloids (CitationAbdel-Kader et al., 1998) (). Verazine was active with an IC12 value of 0.38 μM; IC12 is the concentration required to inhibit the growth of a microorganism in a 12 mm diameter zone. The methanol extract of Microphilis guyanensis (A. DC.) Pierre (Sapotaceae) and Genipa Americana L. (Rubiaceae) collected at Asindopo village, Suriname, yielded the known cryptolepine hydrochloride (CitationYang et al., 1999a). From the aerial parts of Ertela (monniera) trifolia L. (Rutaceae) from Suriname, 10 alkaloids were purified (CitationCao et al., 2008). Bioassay-guided fractionation of the alkaloid portion of a CH2Cl2-MeOH extract of Tabernaemontana calcarea Pichon (Apocynaceae) from Madagascar resulted in the isolation of 15 alkaloids (CitationChaturvedula et al., 2003b). Camptothecin and 9-methoxycamptothecin were purified from a Coffea spp. (Rubiaceae) from Madagascar. It can be noted, however, that camptothecins have recently been shown to be produced by a fungus designated RJMEF001 isolated from Nothapodytes foetida (Wight) Sleumer (Icacinaceae), and it is thus very possible that the true source of these alkaloids is a fungal species (CitationCao et al., 2006a).
Aromatic compounds
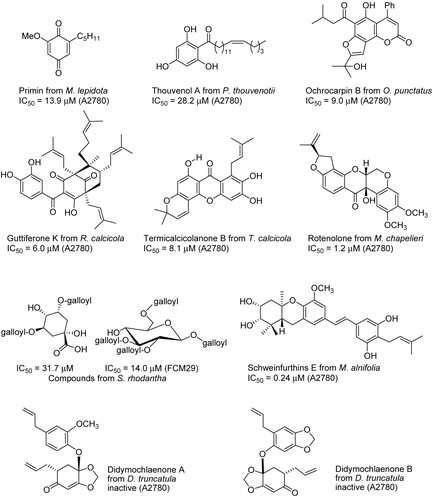
Several types of aromatic compounds such as quinones, phenols, coumarins, benzophenone derivatives, xanthones, flavonoids, stilbenes, lignans, etc. have been obtained as the active constituents from Suriname and Madagascar plants (). Two benzoquinones were isolated from the leaves of Miconia lepidota Schrank & Mart. ex DC. (Melastomataceae) collected in northern Suriname, and a limited structure–activity relationship (SAR) study was carried out on these compounds (CitationGunatilaka et al., 2001). Three alkylene phenolic compounds were isolated after chromatographic separation of the methanol extract of the dried fruits of Protorhus thouvenotii Lecomte (Anacardiaceae) from Madagascar (CitationCao et al., 2004b). Several cytotoxic coumarins and prenylated benzophenones were separated from the bark of Ochrocarpos punctatus H. Perrier (Clusiaceae) from Madagascar (CitationChaturvedula et al., 2002). Guttiferones of Rheedia calcicola Jum. & H. Perrier (Clusiaceae) from Madagascar showed mild activity against the A2780 human ovarian cancer cell line (CitationCao et al., 2007a). Two xanthones were obtained from the Madagascar plant Terminalia calcicola H. Perrier (Combretaceae) (CitationCao et al., 2007b). Rotenolone and rotenone were isolated from the methanol extract of Mundulea chapelieri (Baill.) R. Viguier ex Du Puy & Lab (Fabaceae), which was collected from the province of Toamasina in eastern Madagascar (CitationCao et al., 2004c). From the butanol-soluble portion of an extract of Sloanea rhodantha (Baker) Capuron var. rhodantha (Elaeocarpaceae) from Madagascar, seven compounds were purified, and five showed weak inhibitory activity against the drug-resistant FCM29 strain of Plasmodium falciparum Welch (CitationCao et al., 2006b). A2780 guided fractionation of an extract of the fruit of Macaranga alnifolia Baker (Euphorbiaceae) from Madagascar led to the isolation of four new prenylated stilbenes, schweinfurthins E–H, and the known prenylated stilbene vedelianin. Schweinfurthin E was also tested in the 60-cell human tumor cancer screen at the National Cancer Institute (NCI). The assay measure used by the NCI that most closely corresponds to the IC50 values for antiproliferative activity is the GI50 value, and schweinfurthin E exhibited a mean panel GI50 of 0.19 μM. All lines of the leukemia subpanel were found to be highly sensitive to schweinfurthin E, while all lines of the ovarian cancer subpanel (OVCAR-3, -4, and -8 and SK-OV-3) were somewhat resistant, with GI50 values averaging 2.2 μM. This result is somewhat surprising in view of the sensitivity of the A2780 ovarian cancer cell line to schweinfurthin E, but can be explained in part by the fact that the A2780 cell line is a drug-sensitive line (CitationYoder et al., 2007). A pair of lignans, didymochlaenones A and B, were isolated from a Madagascar fern Didymochlaena truncatula (Sw.) J. Sm. (Dryopteridaceae). The optical rotation and circular dichroism spectrum of didymochlaenone B were approximately the opposite of those of didymochlaenone A, indicating that didymochlaenone B most likely has the opposite stereochemistry to that of didymochlaenone A at the 2 and 4 positions. Although it is unusual for a plant to produce two similar compounds with opposite stereochemistry, it is not unprecedented; thus, various Pinus sp. (Pinaceae) produce both (+)- and (–)-α-pinene, and (+)- and (–)-limonene both occur in peppermint (CitationCao et al., 2006a).
Cardenolides
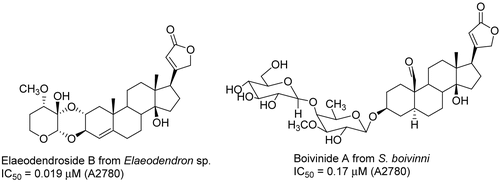
Bioassay-guided fractionation of an ethanol extract obtained from the Madagascar plant Elaeodendron sp. (Celastraceae) led to the isolation of six cardenolides (). Four compounds each with a 1,4-dioxane ring between rings A and A’ showed significant antiproliferative activity against A2780 human ovarian cancer cells with IC50 values of 0.019 to 0.19 μM (CitationCao et al., 2007c). The ethanol extract of Strophanthus boivinii Baill. (Apocynaceae) from the rainforest of Madagascar afforded 10 cardenolide glycosides. All compounds were tested against the A2780 human ovarian cancer cell line, and boivinide A was active with an IC50 of 0.17 μM (CitationKarkare et al., 2007).
Terpenoids – sesquiterpenoids
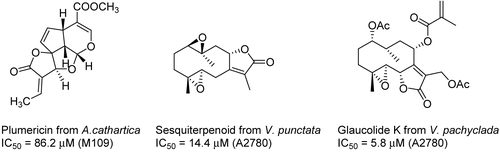
Bioassay-guided fractionation of the EtOAc extract of both Allamanda cathartica L. (Apocynaceae) and Himatanthus fallax (Müll. Arg.) Plumel (Apocynaceae) from Suriname using the Sc-7 yeast strain resulted in isolation of the weakly active isoplumericin and plumericin (CitationAbdel-Kader et al., 1997) (). Continuation of the chemical examination of the bioactive constituents of the wood of Vepris punctata (I. Verd.) Mziray (Rutaceae) from Madagascar resulted in the isolation of a new sesquiterpenoid lactone (CitationChaturvedula et al., 2004). The structure of this new compound was established on the basis of 1D and 2D nuclear magnetic resonance (NMR) spectroscopic data interpretation and chemical modifications. All the isolated compounds from V. punctata were tested against the A2780 human ovarian cancer cell line; the four sesquiterpenoids showed moderate activity. Bioassay-guided fractionation of the leaf extract of Vernonia pachyclada Baker (Asteraceae) from Madagascar led to the isolation of three new sesquiterpene lactones, designated glaucolides K–M. The structures of the new compounds were determined using 1D and 2D NMR spectroscopy, and the structure and stereochemistry of glaucolide K were confirmed by single-crystal X-ray diffraction. Glaucolide K showed moderate activity in the A2780 human ovarian cancer cell line, with an IC50 of 5.8 μM (CitationWilliams et al., 2005).
Terpenoids – diterpenoids
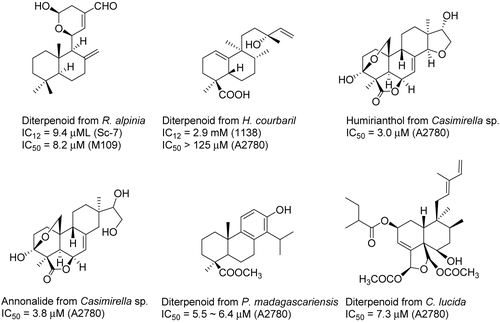
The plant Renealmia alpinia (Rottb.) Maas (Zingiberaceae) from Suriname was selected for investigation based on its ethnomedical use as a febrifuge, but testing in the yeast Sc-7 assay gave a positive response, indicative of cytotoxic activity. Using this bioassay, two new labdane diterpenes, 11-hydroxy-8(17),12(E)-labdadien-15,-16-dial 11,15-hemiacetal and 16-oxo-8(17),12(E)-labdadien-15-oic acid, and the known diterpene, 8(17),12(E)-labdadien- 15,16-dial, have been isolated (). The absolute configurations of 11-hydroxy-8(17),12(E)-labdadien-15,16-dial 11,15-hemiacetal and 16-oxo-8(17), 12(E)-labdadien-15-oic acid were established by circular dichroism (CD) spectroscopy and by the formation and subsequent NMR analysis of R-methoxyphenylacetyl esters. The hemiacetal 11-hydroxy-8(17), 12(E)-labdadien-15,16-dial 11,15-hemiacetal and was cytotoxic to M109 cells, with an IC50 value of 8.2 μM (CitationZhou et al., 1997; CitationYang et al., 1999b). Bioactivity-directed fractionation of a methanol extract of Hymenaea courbaril L. (Fabaceae) from Suriname afforded the three new diterpenoids (13R)-13-hydroxy-1(10),14-ent-halimadien-18-oic acid, (2S,13R)-2,13-dihydroxy-1(10)-14-ent-halimadien-18-oic acid, and (13R)-2-oxo-13-hydroxy-1(10),14-ent-halimadien-18-oic acid. The configurations of these compounds were determined from X-ray crystallography of (13R)-13-hydroxy-1(10)-14-ent-halimadien-18-oic acid, and CD of (2S,13R)-2,13-dihydroxy-1(10),-14-ent-halimadien-18-oic acid and (13R)-2-oxo-13-hydroxy-1(10),14-ent-halimadien-18-oic acid, in addition to spectral studies of prepared derivatives. (13R)-13-Hydroxy-1(10),14-ent-halimadien-18-oic acid exhibited weak activity toward the 1138 mutant yeast strain and the A2780 human ovarian cancer cell line (CitationAbdel-Kader et al., 2002). Bioassay-guided fractionation of the MeOH and EtOAc fractions of extracts of two lianas (Casimirella sp., Icacinaceae) collected in Suriname has led to the isolation of five new diterpenoids, humirianthone, 1-hydroxy-humirianthone, 15R-humirianthol, patagonol, and patagonal, and five known diterpenoids. All 10 diterpenoids showed some activity against the A2780 human ovarian cancer cell line. Humirianthol and annonalide were submitted to the NCI for testing in the 60 cell-line screen. With the exception of humirianthone, the compounds were not isolated in sufficiently large quantities for testing. Only after humirianthol and annonalide were tested was a large enough quantity of humirianthone isolated. The total growth inhibition (TGI) values for humirianthol in the 60 cell lines ranged from >100 to 2.5 μM, and the values for annonalide were between >100 and 4.8 μM (CitationAdou et al., 2005). These values were not sufficiently potent or selective to warrant further investigation. Bioassay-directed fractionation of an extract of the root and bark of Podocarpus madagascariensis Baker (Podocarpaceae) resulted in the isolation of a new totarol diterpenoid in addition to the three known cytotoxic diterpenoids 19-hydroxytotarol, totaradiol, and 4β-carboxy-19-nor-totarol (CitationReynolds et al., 2006). From a CH2Cl2-MeOH extract of the bark of Casearia lucida Tul (Flacourtiaceae) from Madagascar, 11 new clerodane diterpenes, namely, casearlucins A–K, were isolated (CitationPrakash et al., 2002).
Terpenoids – triterpenoids
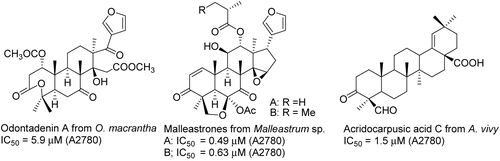
Bioassay-guided fractionation of the methanol extract of Odontadenia macrantha (Willd. ex Roem. & Schult.) Markgr. (Apocynaceae) from Suriname afforded a new limonoid, odontadenin A (). Odontadenin A was found to be moderately active against A2780 with an IC50 value of 3.2 μM (CitationChaturvedula et al., 2003a). Bioassay-guided separation of a dichloromethane fraction of Malleastrum sp. (Meliaceae) from Madagascar followed by silica gel column chromatography and diol high-performance liquid chromatography (HPLC) afforded three new limonoids, malleastrones A–C, respectively. The novel isolates malleastrones A and B displayed antiproliferative activity against a variety of tumor cell lines (A2780, MDA-MB-435 breast cancer cells, HT-29 colon cancer cells, H522-T1 non-small cell cancer cells, and U937 histiocytic lymphoma cells) and exhibited notable IC50 values ranging from 0.19 to 0.63 μM (CitationMurphy et al., 2008). Five new triterpenoids, acridocarpusic acids A–E, were isolated from the cytotoxic MeOH extract obtained from the leaves and flowers of Acridocarpus vivy Arènes (Malpighiaceae) collected in Madagascar (CitationCao et al., 2004a).
Triterpenoid glycosides (saponins)
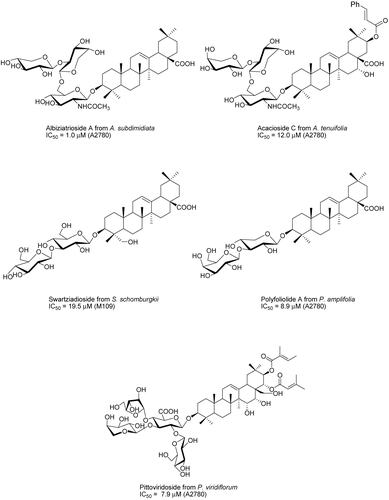
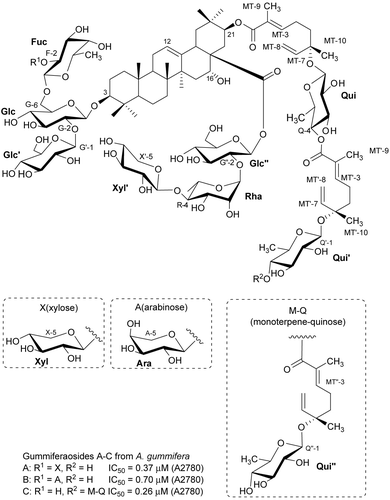
Bioassay-guided fractionation of a methanol extract of Albizia subdimidiata (Splitg.) Barneby & J.W. Grimes (Fabaceae) from Suriname using the engineered yeast strains 1138, 1140, 1353, and Sc-7 of Saccharomyces cerevisiae Meyen ex E.C. Hansen (Saccharomycetaceae) as the bioassay tool resulted in the isolation of two active saponins; one of these, albiziatrioside, is described for the first time (). Albiziatrioside was active against these yeast strains with IC50 values ranging from 32.5 to 37.9 μM (CitationAbdel-Kader et al., 2001). Six saponins were purified from the MeOH extract of Acacia tenuifolia (L.) Willd. (Fabaceae), which was collected at Botopasi, Sipaliwini District, Suriname. The new compounds, acaciosides B and C, exhibited weak activity against the engineered yeast strains 1138, 1140, 1353, and Sc-7 of Saccharomyces cerevisiae (CitationSeo et al., 2002b). The MeOH extract of Swartzia schomburgkii Benth. (Fabaceae) from Suriname was tested active against the 1138, 1140, and 1353 assays. Eight saponins, five active and three inactive, were separated, two of which, swartziadioside and swartziatrioside, were new. Five showed weak activity in our yeast bioassay, with IC50 values in the 0.1–1.5 mmol range. In a test at Bristol-Myers Squibb using the M-109 cell line, three compounds showed weak activity, with IC50 values against the M-109 cell line in the 25–50 μM range (CitationAbdel-Kader et al., 2000). Polyscias amplifolia (Baker) Harms (Araliaceae) from Madagascar gave four saponins, two of which were new oleanolic acid saponins. They were active against A2780 with IC50 values in the range 6.7–10.8 μg/mL (CitationPrakash et al., 2003). A novel triterpenoid saponin, pittoviridoside, which possesses an unusual 2,3,4-trisubstituted glycosidic linkage, has been isolated from Pittosporum viridiflorum Sims (Pittosporaceae) from Madagascar using the engineered yeast strains 1138, 1140, 1353, and Sc-7 for bioactivity-guided fractionation. Pittoviridoside showed weak activity (IC12 85, 80, 140, and 100 μg/mL) against the 1138, 1140, 1353, and Sc-7 strains in our yeast bioassay. In a test using the A2780 human ovarian cancer cell line, it also exhibited weak activity (IC50 7.9 μM) (CitationSeo et al., 2002a). A2780 guided fractionation of an EtOH extract obtained from the roots of the Malagasy plant Albizia gummifera (J.F. Gmel.) C.A. Sm. (Fabaceae) led to the isolation of three new cytotoxic oleanane-type triterpenoid saponins, gummiferaosides A–C. The structures of these new compounds were elucidated using 1D and 2D NMR experiments, including 1D total correlation spectroscopy (TOCSY) and heteronuclear single quantum coherence (HSQC)-TOCSY, and mass spectrometry. Gummiferaosides A–C showed activity against the A2780 human ovarian cancer cell line with IC50 values of 0.37, 0.70, and 0.26 μM, respectively (CitationCao et al., 2007d).
Glycoresins from Ipomoea squamosa
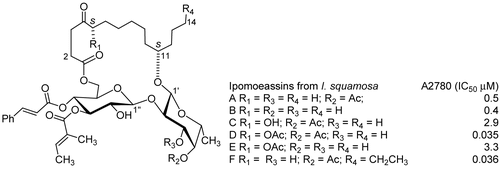
The pharmaceutical partner in our International Cooperative Biodiversity Group in Suriname, Bristol-Myers Squibb Pharmaceutical Research Institute, obtained an isolate (BMS-247181) from an Ipomoea sp. (Convolvulaceae) from Suriname that exhibited potent in vitro activity toward the M109 lung cancer cell line. The compound was identified as a resin glycoside, and a tentative structure was proposed, although the stereochemistry was not determined. BMS-247181 had a maximum ultraviolet (UV) absorption at 280 nm and a molecular mass of approximately 900 Da. A preliminary in vivo experiment indicated that it had a narrow therapeutic window, and work on the extract at BMS was discontinued. BMS withdrew from the Suriname ICBG in 2002, and it was subsequently decided to reinvestigate the cytotoxic constituents of Ipomoea sp. from Suriname, in the expectation that they might contain additional resin glycosides with potentially improved properties as compared with BMS-247181. The original extract from which BMS-24718 had been isolated was no longer available, but eight Ipomoea extracts were available from the National Cancer Institute and two Ipomoea extracts were available in-house, including one (E940631) that was a re-collection of the original plant that had furnished the extract provided to BMS. Analysis of all 10 extracts was carried out by liquid chromatography-mass spectrometry (LC-MS), using 280 nm as a monitoring wavelength and 800–1000 Da as a scanning range, and E940631 was chosen as the starting material since its LC-MS chromatogram showed peaks that had the same UV pattern and similar molecular weight(s) (MW) to BMS-247181. E940631 had an IC50 value of 8.0 μg/mL against the A2780 ovarian cancer cell line. The new glycoresins, ipomoeassins A–F, have been isolated from the leaves of Ipomoea squamosa Choisy (). The structures were elucidated by spectroscopic analyses and chemical transformations. The absolute configurations of C-5 (ipomoeassins C–E) and C-11 (ipomoeassins A and B) were determined by their derivatives with (R)- and (S)-MPA (methoxyphenylacetic acid). All the isolates were active in the A2780 human ovarian cancer cell line assay with an IC50 value of 0.5, 0.4, 2.9, 0.035, 3.3, and 0.036 μM, respectively. Interestingly, ipomoeassin D, which differs from ipomoeassin C only by an acetyl group, is almost two orders of magnitude more cytotoxic than ipomoeassin C, with IC50 values of 35 nM. The fully acetylated derivatives of the five natural products were significantly less active than the ipomoeassins, with IC50 values of 15.8 and 19.1 μM. All this suggests that relatively minor structural variations may make significant differences to activity and possibly other activities also. Ipomoeassin F exhibited potent activity against A2780 human ovarian cancer cell lines with an IC50 value of 0.036 μM, which was much more active than ipomoeassin A (0.5 μM). Here, we can see again that sometimes a slight modification of the structure will enhance the activity dramatically (CitationCao et al., 2005, Citation2007e).
Recently, a concise, flexible, and efficient total synthesis of the cytotoxic resin glycosides, ipomoeassin B and ipomoeassin E, was reported. While the analytical and spectroscopic data of the synthetic material nicely matched those of the natural products, the authors also noticed a concentration dependence of its 1H NMR spectrum in C6D6 (CitationFuerstner & Nagano, 2007).
Marine metabolites
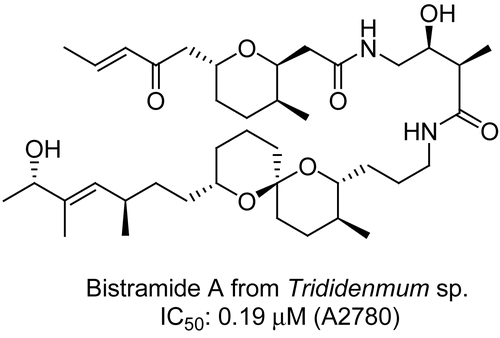
The crude extract from the marine tunicate (NB0405-31, Trididemnum sp., Didemnidae) had an IC50 value of approximately 3.9 μg/mL in the A2780 antiproliferative assay. Liquid–liquid partitioning of the crude led to a dichloromethane fraction with an IC50 value of 0.46 μg/ mL. Column chromatography over RP-C18 yielded two active fractions: 0.17 and 0.46 μg/mL. These fractions were subjected to RP-C18 HPLC and resulted in the isolation of two known antiproliferative agents, bistramides A and D. Bistramide A () has been reported to exhibit antiproliferative activity in the nanomolar range against a number of tumor cell lines in vitro (CitationRizvi et al., 2006), and displayed an IC50 value of 0.19 μM against the A2780 cancer cell line. Previous studies have shown that this marine metabolite exerts its antiproliferative activity by binding directly to monomeric G-actin and depolymerizing filamentous F-actin, an entity essential for cellular division and motility. Further isolation of additional bioactive molecules is currently under way, including a novel bistramide (CitationMurphy et al., 2007, Citation2009).
Conservation and development advances
The Suriname ICBG played a part in one significant conservation development, the establishment of the Central Suriname Nature Reserve. During the mid-1990s there was pressure on the Government of Suriname to approve logging concessions for much of central Suriname; the award of these concessions would have been devastating to the region and especially to the forest peoples who live there. The Suriname ICBG partner Conservation International worked closely with the government of Suriname to find a creative solution that would yield badly needed revenue while at the same time preserving the forest. The final outcome was the establishment of the Central Suriname Nature Reserve, which links three smaller protected areas in the center of Suriname into one contiguous area of over 1.6 million ha, and Conservation International entered into a 100-year lease of this Reserve. The lease fees provided welcome revenue to the government, and the forest was preserved for posterity; truly a win–win situation. The Suriname ICBG program provided a part of the justification for establishing this reserve, by showing the value of the forest as a sustainable source of biodiversity for future drug discovery (CitationKingston, 2001).
In addition to the establishment of the Nature Reserve, the Suriname ICBG program provided small but significant economic development opportunities to the forest peoples through a series of micro-loans and grants. The funds for these purposes came from upfront contributions provided by the group’s commercial partners BMS and DAS, and the grants and loans were channeled through the Forest Peoples’ Fund, administered by representatives of the Suriname people. Conservation International also established a training program for tribal healers, so that the ethnomedical knowledge would be preserved from the present generation to the next one. In the communities, those participating in the project developed an increased awareness of medicinal plant knowledge. Other tangible benefits of the program include direct and indirect employment, the establishment of popular organizations and micro-enterprises, and a noticeable decline in animosity between surrounding communities participating in the project. In addition, inter-country relations were improved as the Chiefs of the Saramaka and Tirio tribes of Suriname visited the King of the Naso tribe participating in the Panama ICBG. Finally, some impressive petroglyphs were discovered in the Tirio indigenous community of Kwamalasamutu, and plans for their protection and sustainable development have been prepared.
In Madagascar development work was again coordinated by Conservation International, with the direct assistance of MBG and CNARP. In the first phase of the program (1998–2003) the development work was focused around the Zahamena National Park, where the plant collections took place, and included projects such as construction of a simple bridge over a seasonal river, construction of grain storage buildings, and renovation and equipping of a primary school. Projects in the current phase of the program in the arid north of the country have focused on development of associations for market gardening and farming, building of watering places and wells with more than 2000 beneficiaries, reforestation, and dam construction. Funds for these projects were again supplied by the upfront contributions provided by the commercial partners DAS and ERI. In addition, after extensive studies and stakeholder involvement, the beautiful Montagne des Français was officially awarded the temporary status of a Protected Area, and the Ambodivahibe Bay area was granted temporary status as a new protected area in late 2008. Work is in progress to designate additional areas as Protected Areas.
Other benefits to Madagascar include the establishment of a laboratory at CNARP to carry out bioassays for antimalarial compounds, and training of several Malagasy scientists in botany, microbiology, and chemistry.
Conclusion
The Suriname and Madagascar ICBG programs have achieved many of their objectives. The local people in Suriname and Madagascar have benefited significantly from the ICBG program, as indicated above, and significant portions of the forest cover of both countries have been conserved for future generations to use and enjoy. Although the work described above has yet to yield a new drug, the ipomoeassins from Ipomoea squamosa have attracted attention from both the pharmaceutical industry and academia (CitationFuerstner & Nagano, 2007). In vitro, these compounds showed potent activity and good selectivity against various cancer cell lines, but the in vivo results did not yet meet the desired expectations. Continued investigation of these compounds is warranted, for past studies have proved that even slight structural modifications may help increase their in vivo efficacy, thus making them suitable for further development. In conclusion, it can be stated that the results and the accomplishments of the Suriname and Madagascar ICBG programs have had a significant impact on biodiversity conservation and on the cooperating institutions and the local communities in the host countries, and progress has been made in the discovery of anticancer agents from natural sources.
Acknowledgements
This article was presented at the Symposium: “Plants in the Service of Human Health: Continuing Search for Plant-based Therapies” – Society for Economic Botany 48th Annual Meeting in Chicago at Lake Forest College, June 4, 2007.
Declaration of interest: The work described in this review was carried out under an International Cooperative Biodiversity Group award from the National Institutes of Health, with support from the Fogarty International Center, the National Cancer Institute, the National Science Foundation, the National Heart, Lung and Blood Institute, the National Institute of Mental Health, the Office of Dietary Supplements, and the Office of the Director of NIH, under Cooperative Agreement U01 TW000313, and this support is gratefully acknowledged. We gratefully acknowledge courtesies extended by the Government of Madagascar (Ministère des Eaux et Forêts). Work in Madagascar was carried out in collaboration with colleagues from the Missouri Botanical Garden, Centre National de Recherches sur l’Environnement, Conservation International, Centre National d’Application des Recherches Pharmaceutiques, and Centre National de Recherches Océanographiques, and with generous financial support of conservation and development by Eisai Research Institute and Dow AgroSciences. Work in Suriname was carried out in collaboration with the Missouri Botanical Garden, Conservation International, and Bedrijf Geneesmiddelen Voorziening Suriname, and with generous financial support of conservation and development by Bristol-Myers Squibb. We are grateful to all these groups for their excellent collaborations, and to the many individual scientists and other colleagues who contributed to the success of this work, and whose names are included in the references cited below.
References
- Abdel-Kader M, Berger JM, Slebodnick C, Hoch J, Malone S, Wisse JH, Werkhoven MCM, Neddermann KM, Mamber S, Kingston DGI (2002): Isolation and absolute configuration of ent-halimane diterpenoids from Hymenaea courbaril from the Suriname rain forest. J Nat Prod 65: 11–15.
- Abdel-Kader M, Hoch J, Berger JM, Evans R, Miller JS, Wisse JH, Mamber SW, Dalton JM, Kingston DGI (2001): Two bioactive saponins from Albizia subdimidiata from the Suriname rainforest. J Nat Prod 64: 536–539.
- Abdel-Kader MS, Bahler BD, Malone S, Werkhoven MCM, van Troon F, David M, Wisse JH, Burkuser I, Neddermann KM, Mamber SW, Kingston DGI (1998): DNA-damaging steroidal alkaloids from Eclipta alba from the Suriname rain forest. J Nat Prod 61: 1202–1208.
- Abdel-Kader MS, Bahler BD, Malone S, Werkhoven MCM, Wisse JH, Neddermann KM, Bursuker I, Kingston DGI (2000): Bioactive saponins from Swartzia schomburgkii from the Suriname rainforest. J Nat Prod 63: 1461–1464.
- Abdel-Kader MS, Wisse J, Evans R, van der Werff H, Kingston DGI (1997): Bioactive iridoids and a new lignan from Allamanda cathartica and Himatanthus fallax from the Suriname rain forest. J Nat Prod 60: 1294–1297.
- Adou E, Williams RB, Schilling JK, Malone S, Meyer J, Wisse JH, Frederik D, Koese D, Werkhoven MCM, Snipes CE, Werk TL, Kingston DGI (2005): Cytotoxic diterpenoids from two lianas from the Suriname rainforest. Bioorg Med Chem 18: 6009–6014.
- Anonymous (1997): New map of “Biodiversity Hotspots” aids targeting of conservation efforts. Diversity 13(1): 27–29.
- Bhakuni DS, Rawat DS (2005): Bioactive Marine Natural Products.New Delhi, Anamaya Publisher, pp. 1–382.
- Caldecott JO, Jenkins MD, Johnson T, Groombridge B (1994): Priorities for conserving global species richness and endemism. In: Collins NM, ed., World Conservation Monitoring Centre, Biodiversity Series No. 3. Cambridge, UK, World Conservation Press, p. 17.
- Cao S, Al-Rehaily AJ, Brodie P, Wisse JH, Moniz E, Malone S, Kingston DGI (2008): Furoquinoline alkaloids of Ertela (monnieria) trifolia (L.) Kuntze from the Suriname rainforest. Phytochemistry 69: 553–557.
- Cao S, Brodie P, Miller JS, Ratovoson F, Birkinshaw C, Randrianasolo S, Takotobe E, Rasamison VE, Kingston DGI (2007a): Guttiferones K and L, antiproliferative compounds of Rheedia calcicola from the Madagascar rain forest. J Nat Prod 70: 686–688.
- Cao S, Brodie PJ, Miller JS, Randrianaivo R, Ratovoson F, Birkinshaw C, Andriantsiferana R, Rasamison VE, Kingston DGI (2007b): Antiproliferative xanthones of Terminalia calcicola from the Madagascar rain forest. J Nat Prod 70: 679–681.
- Cao S, Brodie PJ, Miller JS, Ratovoson F, Callmander MW, Randrianasolo S, Rakotobe E, Rasamison VE, Suh EM, TenDyke K, Kingston DGI (2007c): Antiproliferative cardenolides of an Elaeodendron sp from the Madagascar rain forest. J Nat Prod 70: 1064–1067.
- Cao S, Guza RC, Wisse JH, Miller JS, Evans R, Kingston DGI (2005): Ipomoeassins A-E, cytotoxic macrocyclic glycoresins from the leaves of Ipomoea squamosa from the Suriname rainforest. J Nat Prod 68: 487–492.
- Cao S, Norris A, Miller JS, Ratovoson F, Razafitsalama J, Andriantsiferana R, Rasamison VE, TenDyke K, Suh T, Kingston DGI (2007d): Cytotoxic triterpenoid saponins from Albizia gummifera from the Madagascar rain forest. J Nat Prod 70: 361–366.
- Cao S, Norris A, Wisse JH, Miller JS, Evans R, Kingston DGI (2007e): Ipomoeassin F, a new cytotoxic macrocyclic glycoresin from the leaves of Ipomoea squamosa from the Suriname rainforest. Nat Prod Res 21: 872–876.
- Cao S, Radwan MM, Norris A, Miller JS, Ratovoson F, Mamisoa A, Andriantsiferana R, Rasamison VE, Rakotonandrasana S, Kingston DGI (2006a): Cytotoxic and other compounds from Didymochlaena truncatula from the Madagascar rain forest. J Nat Prod 69: 284–286.
- Cao S, Ranarivelo L, Ratsimbason M, Randrianasolo S, Ratovoson F, Andrianjafy M, Kingston DGI (2006b): Antiplasmodial activity of compounds from Sloanea rhodantha (Baker) Capuron var. rhodantha from the Madagascar rain forest. Planta Med 72: 1438–1439.
- Cao S, Schilling JK, Guza RC, Miller JS, Andriantsiferana R, Rasamison VE, Kingston DGI (2004a): New cytotoxic triterpenoids from Acridocarpus vivy from the Madagascar rain forest. J Nat Prod 67: 986–989.
- Cao S, Schilling JK, Miller JS, Randrianasolo A, Andriantsiferana R, Rasamison VE, Kingston DGI (2004b): New cytotoxic alkyl phloroglucinols from Protorhus thouvenotii. Planta Med 70: 683–684.
- Cao S, Schilling JK, Miller JS, Andriantsiferana R, Rasamison VE, Kingston DGI (2004c): Cytotoxic compounds from Mundulea chapelieri from the Madagascar rainforest. J Nat Prod 67: 454–456.
- Chaturvedula VSP, Schilling JK, Kingston DGI (2002): New cytotoxic coumarins and prenylated benzophenone derivatives from the bark of Ochrocarpus punctatus from the Madagascar rainforest. J Nat Prod 65: 965–972.
- Chaturvedula VSP, Schilling JK, Miller JS, Andriantsiferana R, Rasamison VE, Kingston DGI (2004): New cytotoxic terpenoids from the wood of Vepris punctata from the Madagascar rainforest. J Nat Prod 67: 895–898.
- Chaturvedula VSP, Schilling JK, Wisse JH, Miller JS, Evans R, Kingston DGI, (2003a): A new cytotoxic limonoid from Odontadenia macrantha from the Suriname rainforest. Mag Res Chem 41: 139–142.
- Chaturvedula VSP, Sprague S, Schilling JK, Kingston DGI (2003b): New cytotoxic indole alkaloids from Tabernaemontana calcarea from the Madagascar rainforest. J Nat Prod 66: 528–531.
- Cragg GM, Newman DJ (2001): Natural product drug discovery in the next millennium. Pharm Biol 39(Suppl.): 8–17.
- Cragg GM, Newman DJ (2005a) Biodiversity: a continuing source of novel drug leads. Pure Appl Chem 77: 7–24.
- Cragg GM, Newman DJ (2005b): International collaboration in drug discovery and development from natural sources. Pure Appl Chem 77: 1923–1942.
- Cragg GM, Newman DJ, Snader K (1997): Natural products in drug discovery and development. J Nat Prod 60: 52–60.
- Dabydeen DA, Burnett JC, Bai R, Verdier-Pinard P, Hickford SJH, Pettit GR, Blunt JW, Munro MHG, Gussio R, Hamel E (2006): Comparison of the activities of the truncated halichondrin B analog NSC 707389 (E7389) with those of the parent compound and a proposed binding site on tubulin. Mol Pharmacol 70: 1866–1875.
- de Rasolohery A (2006): Inventaire des Fougeres de Zahamena, Madagascar. Antananarivo, Madagascar, Missouri Botanical Garden.
- Fuerstner A, Nagano T (2007): Total syntheses of ipomoeassin B and E. J Am Chem Soc 129: 1906–1907.
- Gunatilaka AAL, Berger JM, Evans R, Miller JS, Wisse JH, Neddermann KM, Bursuker I, Kingston DGI (2001): Isolation, synthesis, and structure-activity relationships of bioactive benzoquinones from Miconia lepidota from the Suriname rain forest. J Nat Prod 64: 2–5.
- Karkare S, Adou E, Cao S, Brodie PJ, Miller JS, Ratovoson F, Andriantsiferana R, Rasamison VE, Kingston DGI (2007): Antiproliferative cardenolide glycosides of Strophanthus boivinii from Madagascar rainforest. J Nat Prod 70: 1766–1770.
- Kingston DGI (2001): Biodiversity conservation and drug discovery in Suriname. Explorations in nature’s combinatorial library. Pure Appl Chem 73: 595–599.
- Kingston DGI (2004): Biodiversity conservation and drug discovery in Madagascar and Suriname: explorations in Nature’s combinatorial library. In: Yayli N, Küçük M, eds., Proceedings of the 1st International Conference on the Chemistry of Natural Products (ICNP-2002), Trabzon, Turkey, Karadeniz Technical University, pp. 1–7.
- Kingston DGI, Abdel-Kader M, Zhou B-N, Yang S-W, Berger JM, van der Werff H, Evans R, Mittermeier R, Malone S, Famolare L, Guerin-McManus M, Wisse JH, Miller JS (2000): Biodiversity conservation, economic development, and drug discovery in Suriname. In: Cutler SJ, Cutler HG, eds., Biologically Active Natural Products: Pharmaceuticals. Boca Raton, CRC Press, pp. 39–49.
- Kingston DGI, Abdel-Kader M, Zhou B-N, Yang S-W, Berger JM, van der Werff H, Miller JS, Evans R, Mittermeier R, Famolare L, Guerin-McManus M, Malone S, Nelson R, Moniz E, Wisse JH, Vyas DM, Wright JJK. Aboikonie S (1999): The Suriname International Cooperative Biodiversity Group program: lessons from the first five years. Pharm Biol 37: 22–34.
- Klein LE, Freeze BS, Smith ABIII, Horwitz SB (2005): The microtubule stabilizing agent discodermolide is a potent inducer of accelerated cell senescence. Cell Cycle 4: 501–507.
- Mittermeier RA, Plotkin MJ, Werner TB, Malone SA, Baal F, MacKnight J, Mohadin K, Werkhooven M (1990): Conservation action plan for Suriname. Report prepared by Conservation International, Suriname Forest Service, World Wildlife Fund, Foundation for Nature Preservation in Suriname, and University of Suriname.
- Murphy BT, Slebodnick C, Brodie PJ, Miller JS, Rasamison VE, Tendyke K, Suh EM, Kingston DGI (2008): Antiproliferative compounds of Malleastrum sp. from the Madagascar rainforest. J Nat Prod 71: 235–329.
- Murphy BT, Cao SG, Brodie P, Maharavo J, Kingston DGI (2007): Antiproliferative bistramides from the Ascidian Trididemnum sp. from Madagascar. Presented at: The 48th Annual Meeting of the American Society of Pharmacognosy, Portland, ME, USA, July 14–18.
- Murphy BT, Cao SG, Brodie P, Maharavo J, Andriamanantoanina H, Ravelonandro P, Kingston DGI (2009): Antiproliferative bistramides from a Trididemnum sp. from Madagascar. J Nat Prod In Press.
- Newman, S (2007): Eribulin, a simplified ketone analog of the tubulin inhibitor halichondrin B, for the potential treatment of cancer. Curr Opin Invest Drugs 8: 1057–1066.
- Newman DJ (2008): Natural products as leads to potential drugs: an old process or the new hope for drug discovery? J Med Chem 51: 2589–2599.
- Newman DJ, Cragg GM (2004): Marine natural products and related compounds in clinical and advanced preclinical trials. J Nat Prod 67: 1216–1238.
- Newman DJ, Cragg GM (2007): Natural products as sources of new drugs over the last 25 years. J Nat Prod 70: 461–477.
- Prakash CVS, Hoch JM, Kingston DGI (2002): Structure and stereochemistry of new cytotoxic clerodane diterpenoids from the bark of Casearia lucida from the Madagascar rainforest. J Nat Prod 65: 100–107.
- Prakash CVS, Schilling JK, Miller JS, Andriantsiferana R, Rasamison VE, Kingston DGI (2003): New cytotoxic oleanane saponins from the infructescences of Polyscias amplifolia from the Madagascar rainforest. Planta Med 69: 440–444.
- Reynolds M, Chaturvedula VSP, Ratovoson F, Andriantsiferana R, Rasamison VE, Guza RC, Kingston DGI (2006): Cytotoxic diterpenoids from Podocarpus madagascariensis from the Madagascar rainforest. Nat Prod Res 20: 606–610.
- Rizvi S, Tereshko V, Kossiakoff AA, Kozmin SA (2006): Structure of actin-bistramide A complex at 1.35 å resolution. J Am Chem Soc 128: 3882–3883.
- Schulz JP, Reichert H, Mittermeier RA (1977): Wildlife in Suriname. Oryx 14(2): 133–144.
- Seo Y, Berger JM, Hoch J, Neddermann KM, Bursuker I, Mamber SW, Kingston DGI (2002a): A new bioactive triterpenoid saponin from Pittosporum viridiflorum from the Madagascar rainforest. J Nat Prod 65: 65–68.
- Seo Y, Hoch J, Abdel-Kader M, Malone S, Derveld I, Adams H, Werkhoven MCM, Wisse JH, Mamber SW, Dalton JM, Kingston DGI (2002b): Bioactive saponins from Acacia tenuifolia from the Suriname rainforest. J Nat Prod 65: 170–174.
- Simmons TL, Andrianasolo E, McPhail K, Flatt P, Gerwixk WH (2005): Marine natural products as anticancer drugs. Mol Cancer Ther 4: 333–342.
- Williams RB, Norris A, Slebodnick C, Merola J, Miller JS, Andriantsiferana R, Rasamison VE, Kingston DGI (2005): Cytotoxic sesquiterpene lactones from Vernonia pachyclada from the Madagascar rainforest. J Nat Prod 68: 1371–1374.
- Yang S-W, Abdel-Kader M, Malone S, Werkhoven MCM, Wisse JH, Bursuker I, Nedermann K, Fairchild C, Raventos-Suarez C, Menendez AT, Lane K, Kingston DGI (1999a): Synthesis and biological evaluation of analogues of cryptolepine, an alkaloid isolated from the Suriname rainforest. J Nat Prod 62: 976–983.
- Yang S-W, Zhou B-N, Malone S, Werkhoven MCM, van Troon F, Wisse JH, Kingston DGI (1999b): A new labdane diterpenoid from Renealmia alpinia collected in the Suriname rain forest. J Nat Prod 62: 1173–1174.
- Yoder BJ, Cao, S, Norris A, Miller JS, Ratovoson F, Razafitsalama J, Andriantsiferana R, Rasamison VE, Kingston DGI (2007): Antiproliferative prenylated stilbenes and flavonoids from Macaranga alnifolia from the Madagascar rainforest. J Nat Prod 70: 342–346.
- Zhou B-N, Baj NJ, Glass TE, Malone S, Werkhoven MCM, van Troon F, David M, Wisse JH, Kingston DGI (1997) Bioactive labdane diterpenoids from Renealmia alpinia collected in the Suriname rain forest. J Nat Prod 60: 1287–1293.