Abstract
In 1999, the Department of Medicinal Chemistry and Pharmacognosy at the College of Pharmacy, University of Illinois (UIC) at Chicago was funded to establish a Botanical Dietary Supplements Research Center from the National Institutes of Health (NIH). The emphasis of the UIC/NIH Center for Botanical Dietary Supplements Research (CBDSR) is botanical dietary supplements (BDS) for women’s health. The Center’s research has focused on BDS that may improve women’s health and quality of life, specifically in the areas of menopause, premenstrual syndrome, and persistent urinary tract infections. Center investigators have overcome many challenges associated with botanical dietary supplements research, including acquiring and identifying plant species for investigation, isolating and identifying active constituents, elucidating the mechanisms of action of these botanicals, and conducting Phase I and Phase II clinical studies. Black cohosh [Actaea racemosa L. (Ranunculaceae)] has been used as a model to illustrate the steps involved in taking a botanical dietary supplement from the field, all the way to clinical trials. Bioassays are described that were necessary to elucidate the pertinent biological studies of plant extracts and their mechanisms of action. The Center has used an innovative multidisciplinary approach to this type of research, and thus has been very successful in fulfilling its specific aims.
Introduction
Botanical dietary supplements are widely used by many Americans despite a lack of safety and evidence for most of these products. In 2002, the National Health Interview Survey conducted by the Centers for Disease Control and Prevention indicated that approximately 38.2 million (19%) American adults use natural products, primarily botanical supplements (CitationNCCAM, 2009). Recognition of these statistics led the US Congress to the formation of the Office of Dietary Supplements (ODS) and the National Center for Complementary and Alternative Medicine (CitationNCCAM, 2009), which later established the botanical centers program. The Centers for Botanical Dietary Supplements Research (CBDSR) identify and characterize botanicals, assess bioavailability and bioactivity, explore mechanisms of action, conduct preclinical and clinical evaluations, help select botanicals to be tested in clinical trials, and provide a rich environment for training and career development. The goals of the Centers are to advance the scientific base of knowledge about botanicals, including issues of their safety, efficacy, and biological action. The University of Illinois at Chicago/National Institutes of Health (UIC/NIH) Center for Botanical Dietary Supplements Research was established in 1999 during the first round of funding from a grant from the NIH via the Office of Dietary Supplements (ODS), the National Center for Complementary and Alternative Medicine (NCCAM), the National Institute of General Medicine Sciences, and the Office of Research in Women’s Health. The center focuses on three areas of concern to women: menopause, premenstrual symptoms, and urinary tract infections.
The UIC/NIH CBDSR uses a multidisciplinary approach to its research, and had from its inception the specific aim of taking a plant from the field to the clinic, ensuring the processing of a safe and effective botanical dietary supplement for human consumption. The Center research team includes faculty from the College of Pharmacy (Departments of Medicinal Chemistry and Pharmacognosy, Biopharmaceutical Sciences, and Pharmacy Practice), the College of Medicine (Departments of OB/GYN and Psychiatry), and the College of Liberal Arts and Sciences (Department of Math and Statistics). The collaboration also involved Northwestern University (Obstetrics and Gynecology) in conjunction with two industrial partners, Pharmavite LLC and Naturex, Inc. The Center faculty and staff selected 12 botanical dietary supplements that were widely used by women in the United States to treat the three specific areas of concern. These botanicals include Angelica sinensis (Oliv.) Diels (Apiaceae; dong quai), Trifolium pratense L. (Fabaceae; red clover), and Actaea racemosa L. (syn: Cimicifuga racemosa (L.) Nutt.; Ranunculaceae; black cohosh), which were all used for the management of menopausal symptoms. Viburnum prunifolium L. (Caprifoliaceae; black haw) and Vitex agnus-castus L. (Verbenaceae; chasteberry) were commonly used to treat premenstrual syndrome (PMS), dysmenorrhea, and cyclic mastalgia. Ginkgo biloba L. (Ginkgoaceae; ginkgo), Glycyrrhiza glabra L. (Fabaceae; licorice), Humulus lupulus L. (Cannabaceae; hops), Panax ginseng C.A. Meyer (Araliaceae; Asian ginseng), and Panax quinquefolius L. (Araliaceae; American ginseng) were all reported to have estrogenic effects and were thought to be of potential use for the treatment of menopause or PMS. Vaccinium macrocarpon Ait. (Ericaceae; cranberry) was commonly used for the prevention or treatment of urinary tract infections, and Valeriana officinalis L. (Valerianiaceae; valerian) was often used by menopausal women to improve sleep (CitationFarnsworth, 2009). As each of these botanicals was investigated, the results determined whether the plants were appropriate for further studies or, where inactive, they were to be removed from the list.
Center organization
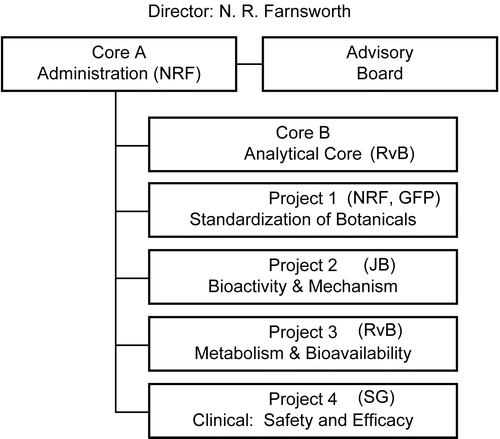
The UIC/NIH CBDSR is organized into five research components consisting of three project areas and two clinical evaluation teams (). All research projects are supported by an administrative core (Core A) and a technology utilization core (Core B), which supplies expertise in mass spectrometry-liquid chromatography/mass spectrometry. Work flows naturally from the most basic science research in botanicals, which includes selecting the plant species, collection (primarily wild crafting), and isolation and elucidation of bioactive compounds, through established and novel methods of phytochemistry (Project 1) to the biological evaluation and mechanism of action studies (Project 2); to the characterization of metabolism, bioavailability, safety, and pharmacokinetics of active species contained in these botanicals (Project 3); and finally to safety and efficacy determined by Phase I and Phase II clinical trials (Clinical Evaluation Group). A continuous feedback mechanism is in place between projects and cores. The Center by this structural scheme promotes continuous interactivity between basic science researchers and the clinicians administering the Center’s clinical trials, thus following the philosophical construct of translational science.
Chemical and biological standardization
The taxonomic identities of plants to be used in the Center research are verified with voucher materials that are stored for future reference. If the plant is collected in the wild, the process for acquiring the plant, including necessary permits, follows good collection (wild crafting) practices. If the plants are cultivated, good agricultural standards are followed according to guidelines (CitationNCCAM, 2009). Processors and final formulation producers must follow good manufacturing practices to ensure that heavy metals, toxic materials, and chemical and microbial contaminants are not present in the product. All these steps are necessary to ensure traceability, should questions arise during the manufacturing and supply chain process, and are supported in documents published by CitationNCCAM (2009). For human use, botanical dietary supplements should also undergo both chemical and biological standardization. Chemical standardization is common with supplements. The compound to which the supplement is standardized is the predominant chemical but it may not be the biologically active constituent. For active constituents, the Center employs bioassay-directed fractionation using several separation methods, including countercurrent chromatography, which resulted in the isolation and structure characterization using ultraviolet, infrared, proton magnetic resonance, carbon magnetic resonance, mass spectrum, optical rotatory dispersion, and X-ray analyses of > 150 pure entities, including such diverse chemical groups as triterpenes, flavonoids, chalcones, phenolic acids, iridoids, alkaloids, depsides, phthalides, diterpenes, lactones, and sterols (CitationBooth et al., 2006; CitationBurdette et al., 2002a, Citation2002b; CitationChadwick et al., 2004; CitationChen et al., 2002a, Citation2002b, Citation2002c; CitationDeng et al., 2006; CitationDietz et al., 2005a; CitationFabricant et al., 2005; CitationHe et al., 2006; CitationLi et al., 2000, Citation2003; CitationLiu et al., 2001, Citation2004, Citation2005; CitationNikolic et al., 2005; CitationPiersen et al., 2004; CitationSheng-Hong et al., 2002; CitationTurner et al., 2005, Citation2007).
In contrast to chemical standardization, biological standardization reflects the biological activity of the plant (or extract) in vitro or in vivo with the use of receptor- and cellular-based or animal assays that identify the biological activities, and may identify and filter out potentially toxic substances. The Center researchers anticipated that the alleviation of hot flashes as well as symptoms associated with premenstrual syndrome are partially mediated by activity at estrogen receptors. Estrogenicity seemed to be common to many of the 12 botanicals, with the exception of cranberry and valerian. Numerous bioassays were developed as data accumulated, which indicated that not all of the plants acted through estrogen mechanisms. Bioassays used in our studies included assays for estrogen receptors (CitationBurdette et al., 2002b, Citation2003; CitationDeng et al., 2006; CitationOverk et al., 2005; CitationTurner et al., 2005), serotonin receptors (CitationDeng et al., 2006; CitationDietz et al., 2005b), opioid receptors (CitationRhyu et al., 2006; CitationWebster et al., 2006; CitationXu et al., 2002), bacterial antiadherence (CitationTurner et al., 2005, Citation2007), and antioxidants (CitationBurdette et al., 2002a; CitationLiu et al., 2005). Additionally, the Center developed and conducted studies of metabolism (CitationNikolic et al., 2005). Three of the plants were not active in our estrogen receptor (ER)-α and -β in vitro screening assays. Because we were unable to verify an estrogen-binding effect of ginkgo, licorice, and the ginsengs, we dropped them from additional work. Black cohosh, while not estrogenic, was retained because of its extensive history of use in women’s health, primarily on the basis of several German clinical trials that showed presumptive evidence of the alleviation of hot flashes in menopause.
Biological activities of black cohosh
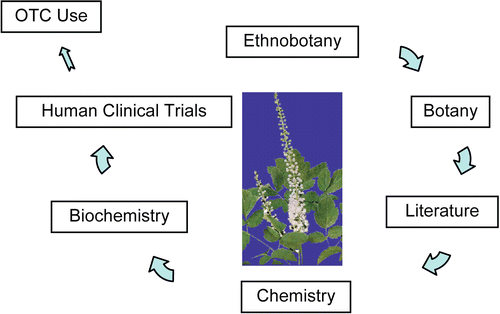
To determine whether any plant extract should be tested in clinical trials, a safety and efficacy profile must be established. For black cohosh, our first step was to perform a literature search using databases such as PUBMED (National Library of Medicine, Bethesda, MD) and the UIC-based NAPRALERT database (CitationFarnsworth, 2009), a searchable database of scientific and ethnomedical botanical and folkloric literature dating from the early 19th century and exclusively focused on natural products and their derivatives. Our literature searches determined that there were sufficient data indicating that black cohosh extracts had significant potential for the treatment of menopausal hot flashes. However, the process of taking an extract of black cohosh into a clinical trial presented many challenges. Scientific and medical literature searches provided many examples of well-characterized studies of black cohosh and were correlated with substantial ethnomedical use of the plant for menopause, especially hot flashes. At the time of initiation of our work, published clinical studies showed no significant adverse effects associated with the ingestion of black cohosh preparations, and no adverse events were observed in any of our black cohosh extract studies to date.
The quality control process of preparing any botanical dietary supplement for an in vivo study must begin with properly collected and identified raw materials. Before any kind of biological or chemical studies are begun on a botanical, quality must be assured. To commence our Phase I study of safety and determination of the appropriate dose, researchers from the Center were sent to work closely with botanist G. W. Ramsey, expert in the North American genus of black cohosh, to collect raw material in the Blue Ridge Parkway and Great Smokey Mountains National Park of North Carolina, Pennsylvania, Tennessee, and Virginia using good field collection practices. Voucher specimens were then housed off-campus in two private herbaria – the Searle Herbarium at the Field Museum of Natural History (Chicago) and the Ramsey-Freer Herbarium at Lynchburg College (Lynchburg, VA). Roots were subjected to macroscopic, microscopic, and DNA techniques to identify and validate the plant materials. The plant materials were dried and extracted (CitationLiu et al., 2001) and tested in numerous bioassays (). Since the early mechanism of action for black cohosh presumed that these extracts acted as estrogen receptor agonists, the first step was to clarify the estrogen receptor binding and determine if indeed black cohosh extracts were estrogenic in vivo. No estrogen binding of the extract was found in either ERα or ERβ (CitationBurdette et al., 2003). Center researchers confirmed that a 40% isopropanol and 75% ethanol extract of black cohosh were not estrogenic in an animal study. The extract was administered to ovariectomized rats at doses of 4, 40, and 400 mg/kg body weight (b.w.) per day with or without estradiol at 50 μg/kg b.w. per day. No estrogenic, antiestrogenic, or additive effects were found (CitationBurdette et al., 2003). Having confirmed the lack of estrogenicity of the black cohosh extract, the Center researchers used a series of bioassays for the serotonin (5-HT) receptors 5-HT7, 5-HT1A, and 5-HT1D (CitationBurdette et al., 2003). We confirmed that the 75% ethanol extract bound to the serotonin receptors, and acted as a partial agonist of 5-HT7 (CitationBurdette et al., 2003). Center investigators also showed that black cohosh acts as a mixed competitive ligand and partial agonist of the human mu opiate receptor (CitationRhyu et al., 2006). Confirming this activity was significant as there is an association of the locus of temperature control in the central nervous system and the endogenous opiate system. Thus, the Center research has shown that black cohosh could potentially modulate the vasomotor symptoms associated with menopause through these two mechanisms. The black cohosh extract was standardized based on the chemical and biological data for use in the clinical trials.
Table 1. Biological standardization and mechanism of action.
The Center then conducted a Phase I clinical trial of its black cohosh and red clover extracts. The Phase I trial was used to assess preliminary safety of the black cohosh extract, determine the highest nontoxic dose for each botanical, and obtain pharmacokinetic and metabolism data. With these data, we proceeded to perform a Phase II study. The Phase II study was a four-arm trial including black cohosh, red clover, the hormone therapy Prempro (Wyeth Pharmaceuticals, Inc., Philadelphia, PA) as a positive control, and placebo. More than 1500 female volunteers were interviewed for this study, and 88 women were randomized into the 12-month clinical trial. Subjects in the red clover arm were treated with a single oral daily dose of 120 mg of a standardized extract, and the black cohosh arm were treated with a single oral daily dose of 128 mg of a standardized extract (5.5% triterpenes, IC50 13 μg/mL in the 5-HT7 assay). The Phase II trial experienced recruiting impediments due to the adverse publicity resulting from findings of the Women’s Health Initiative; women interested in participating were reluctant to be in the Prempro arm and opted out during initial screening. The phase II trial has since been completed and is currently undergoing data analysis for the results. This study will provide preliminary data indicating whether black cohosh and red clover are safe and effective for treating hot flashes associated with menopause.
The UIC/NIH CBDSR was established in 1999, and was refunded in 2005. During this time we have identified several promising botanicals used in dietary supplements for women’s health using a multidisciplinary collaborative approach (). All of the steps have been defined and implemented and require botanical, chemical, and biological assessment as well as mechanism-of-action studies. Multidisciplinary research on botanical dietary supplements is highly collaborative and necessary for success, as the challenges to this type of work are significant. However, we have been able to overcome these challenges through collaboration and the use of novel chemical, biological, and recruiting techniques. The significant amount of research, training, and publications that have resulted from the UIC/NIH Center shows that this approach to botanical research is very effective.
Acknowledgements
This work was supported by a research grant from NIH (P50 AT000155) jointly funded by the Office of Dietary Supplements, the National Center for Complementary and Alternative Medicine (NCCAM), the Office for Research on Women’s Health, and the National Institute for General Medical Sciences. The black cohosh clinical trial product was developed in collaboration with Naturex, Inc. (South Hackensack, NJ) and Pharmavite LLC (Northridge, CA). A secondary clinical study, an additional study included in the Center’s Phase II clinical trial, was supported by grants (R21AT001868 and K01AT002321) from NIH/NCCAM.
This article was presented at the Symposium: “Plants in the Service of Human Health: Continuing Search for Plant-based Therapies” – Society for Economic Botany 48th Annual Meeting in Chicago at Lake Forest College, June 4, 2007.
Declaration of interest: The contents are the responsibility of the authors and do not necessarily represent the views of the funding agencies.
References
- Booth NL, Overk CR, Yao P, Burdette JE, Nikolic D, Chen SN, Bolton JL, van Breemen RB, Pauli GF, Farnsworth NR (2006): The chemical and biologic profile of a red clover (Trifolium pratense L.) phase II clinical extract. J Altern Complement Med 12: 133–139.
- Burdette JE, Chen SN, Lu ZZ, Xu H, White BE, Fabricant DS, Liu J, Fong HH, Farnsworth NR, Constantinou AI, Van Breemen RB, Pezzuto JM, Bolton JL (2002a): Black cohosh (Cimicifuga racemosa L.) protects against menadione-induced DNA damage through scavenging of reactive oxygen species: bioassay-directed isolation and characterization of active principles. J Agric Food Chem 50: 7022–7028.
- Burdette JE, Liu J, Lantvit D, Lim E, Booth N, Bhat KP, Hedayat S, Van Breemen RB, Constantinou AI, Pezzuto JM, Farnsworth NR, Bolton JL (2002b): Trifolium pratense (red clover) exhibits estrogenic effects in vivo in ovariectomized Sprague-Dawley rats. J Nutr 132: 27–30.
- Burdette JE, Liu J, Chen SN, Fabricant DS, Piersen CE, Barker EL, Pezzuto JM, Mesecar A, Van Breemen RB, Farnsworth NR, Bolton JL (2003): Black cohosh acts as a mixed competitive ligand and partial agonist of the serotonin receptor. J Agric Food Chem 51: 5661–5670.
- Chadwick LR, Nikolic D, Burdette JE, Overk CR, Bolton JL, van Breemen RB, Fröhlich R, Fong HH, Farnsworth NR, Pauli GF (2004): Estrogens and congeners from spent hops (Humulus lupulus). J Nat Prod 67: 2024–2032.
- Chen SN, Fabricant DS, Lu ZZ, Zhang H, Fong HH, Farnsworth NR (2002a): Cimiracemates A-D, phenylpropanoid esters from the rhizomes of Cimicifuga racemosa. Phytochemistry 61: 409–413.
- Chen SN, Fabricant DS, Lu ZZ, Fong HH, Farnsworth NR (2002b): Cimiracemosides I-P. New 9,19-cyclolanostane triterpene glycosides from Cimicifuga racemosa. J Nat Prod 65: 1391–1397.
- Chen SN, Li W, Fabricant DS (2002c): Isolation, structure elucidation, and absolute configuration of 26-deoxyactein from Cimicifuga racemosa and clarification of nomenclature associated with 27-deoxyactein. J Nat Prod 65: 601–605.
- Deng S, Chen SN, Yao P, Nikolic D, van Breemen RB, Bolton JL, Fong HH, Farnsworth NR, Pauli GF (2006): Serotonergic activity-guided phytochemical investigation of the roots of Angelica sinensis. J Nat Prod 69: 536–541.
- Dietz BM, Kang YH, Liu G, Eggler AL, Yao P, Chadwick LR, Pauli GF, Farnsworth NR, Mesecar AD, van Breemen RB, Bolton JL (2005a): Xanthohumol isolated from Humulus lupulus inhibits menadione-induced DNA damage through induction of quinone reductase. Chem Res Toxicol 18: 1296–1305.
- Dietz BM, Mahady GB, Pauli GF, Farnsworth NR (2005b): Valerian extract and valerenic acid are partial agonists of the 5-HT5a receptor in vitro. Brain Res Mol Brain Res 138: 191–197.
- Fabricant DS, Nikolic D, Lankin DC (2005): Cimipronidine, a cyclic guanidine alkaloid from Cimicifuga racemosa. J Nat Prod 68: 1266–1270.
- Farnsworth NR, ed. (2009): NAPRALERT [Natural products relational database housed at University of Illinois at Chicago]. Available online at: http://www.napralert.org/ (accessed March 15, 2009).
- He K, Pauli GF, Zheng B (2006): Cimicifuga species identification by high performance liquid chromatography-photodiode array/mass spectrometric/evaporative light scattering detection for quality control of black cohosh products. J Chromatogr A 1112: 241–254.
- Li W, Gu C, Zhang H, Fong HHS, Fitzloff JF (2000): Use of high-performance liquid chromatography-tandem mass spectrometry to distinguish Panax ginseng C.A. Meyer (Asian ginseng) and Panax quinquefolius (North American ginseng). Anal Chem 72: 5417–5422.
- Li Wenkui, Sun Yongkai, Liang Wenzhong, Fitzloff JF, van Breeman RB (2003): Identification of caffeic acid derivatives in Actea racemosa (Cimicifuga racemosa, black cohosh) by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 17: 978–982.
- Liu J, Burdette JE, Sun Y, Deng S, Schlecht SM, Zheng W, Nikolic D, Mahady G, van Breemen RB, Fong HH, Pezzuto JM, Bolton JL, Farnsworth NR (2004): Isolation of linoleic acid as an estrogenic compound from the fruits of Vitex agnus-castus L. (chaste-berry). Phytomedicine 11: 18–23.
- Liu G, Eggler AL, Dietz BM, van Breeman RB (2005): Screening method for the discovery of potential cancer chemoprevention agents based on mass spectrometric detection of alkylated Keap1. Anal Chem 77: 6407–6414.
- Liu J, Burdette JE, Xu H, Gu C, van Breemen RB, Bhat KP, Booth N, Constantinou AI, Pezzuto JM, Fong HH, Farnsworth NR, Bolton JL (2001): Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J Agric Food Chem 49: 2472–2479.
- NCCAM (2009): Product Quality: Biologically Active Agents Used in Complementary and Alternative Medicine (CAM) and Placebo Materials. Interim Applicant Guidance. Notice number NOT-AT-05-004. Available online at: http://grants.nih.gov/notice-files/NOT-AT-004.html (accessed March 8, 2009).
- Nikolic D, Li Y, Chadwick LR, Pauli GF, van Breemen RB (2005): Metabolism of xanthohumol and isoxanthohumol, prenylated flavonoids from hops (Humulus lupulus L.), by human liver microsomes. J Mass Spectrom 40: 289–299.
- Overk CR, Yao P, Chadwick LR, Nikolic D, Sun Y, Cuendet MA, Deng Y, Hedayat AS, Pauli GF, Farnsworth NR, van Breemen RB, Bolton JL (2005): Comparison of the in vitro estrogenic activities of compounds from hops (Humulus lupulus) and red clover (Trifolium pratense). J Agric Food Chem 53: 6246–6253.
- Piersen CE, Booth NL, Sun Y, Liang W, Burdette JE, van Breemen RB, Geller SE, Gu C, Banuvar S, Shulman LP, Bolton JL, Farnsworth NR (2004): Chemical and biological characterization and clinical evaluation of botanical dietary supplements: A phase I red clover extract as a model. Curr Med Chem 11: 1361–1374.
- Rhyu MR, Lu J, Webster DE, Fabricant DS, Farnsworth NR, Wang ZJ (2006): Black cohosh (Actaea racemosa, Cimicifuga racemosa) behaves as a mixed competitive ligand and partial agonist at the human mu opiate receptor. J Agric Food Chem 54: 9852–9857.
- Sheng-Hong Li, Hong-Jie Zhang, Qui Sheng-Xiang Qui (2002): Vitexlactam A, a novel labdane diterpene lactam from the fruits of Vitex agnus-castus. Tetrahedron Lett 43: 5131–5134.
- Turner A, Chen SN, Joike MK, Pendland SL, Pauli GF, Farnsworth NR (2005): Inhibition of uropathogenic Escherichia coli by cranberry juice: a new antiadherence assay. J Agric Food Chem 53: 8940–8947.
- Turner A, Chen SN, Nikolic D, Breemen R, Farnsworth NR, Pauli GF (2007): Coumaroyl iridoids and a depside from cranberry (Vaccinium macrocarpon). J Nat Prod 70: 253–258.
- Webster DE, Lu J, Chen SN, Farnsworth NR, Wang ZJ (2006): Activation of the mu-opiate receptor by Vitex agnus-castus methanol extracts: implication for its use in PMS. J Ethnopharmacol 106: 216–221.
- Xu H, Fabricant DS, Piersen CE (2002): A preliminary RAPD-PCR analysis of Cimicifuga species and other botanicals used for women’s health. Phytomedicine 9: 757–762.