Abstract
The antifungal and antibacterial effects of the stem bark extracts of Harungana madagascariensis Lam. ex Poir. (Clusiaceae) were examined against nine microbial pathogens causing infections in both man and animals. Hexane (H), dichloromethane (D), chloroform (C), ethyl acetate (E), acetone (A), methanol (M), and water (W) extracts were tested in vitro through bioautography and minimum inhibitory concentrations (MIC) determination using the serial micro-dilution assays. Bioautographic results revealed the presence of eight different spots. Extract A exhibited the lowest MIC of 0.04 mg/mL against Microsporum canis, while water extract (W) and methanol (M) showed the highest MIC of 2.5 mg/mL against at least one of the tested fungi when compared to amphotericin B with 0.0625–1 g/mL. Sporotrichum schenckii was the most susceptible fungal pathogen with average MIC of 0.06 mg/mL, while the acetone extract (A) was the most active against three fungal organisms when compared with other extracts. Similarly, extracts D, C, E and A exhibited very high activity with low MIC values of 0.156–0.62 mg/mL, while M and W gave the highest values of 0.31–2.5 mg/mL on bacterial pathogens as compared to gentamicin (0.02–0.62 8 g/mL). The dichloromethane extract is the most active against bacteria with average MIC of 0.19 mg/mL, while Staphylococcus aureus is the most sensitive organism; that shows susceptibility at an average MIC of 0.34 mg/mL. These results provide promising information for the potential use of the crude extracts from the stem-bark of H. madagascariensis in the treatment of bacterial and fungal infections similar to what was obtained in the leaves.
Introduction
Plant-based products contain a variety of chemical constituents and have been identified as possible sources to control microbial infections. In South Africa, several species of medicinal plants are used by many ethnic groups for the treatment of various ailments in both humans and domestic animals (CitationMasika & Afolayan, 2002). The treatment of livestock diseases using traditional remedies is widely practiced in the rural communities and this practice dates back for centuries. Harungana madagascariensis Lam. ex Poir. (Clusiaceae) is a native of Madagascar and Mauritius, and is used in folk medicine for treating a wide variety of disorders in animals and humans (CitationGurib-Fakin, 2007). Traditionally, it is used as an abortifacient and antiseptic, in the treatment of anemia, asthma, tuberculosis, fever, angina, diarrhea, dysentery, syphilis, gonorrhea, malaria, parasitic skin diseases, and wounds, as a natural source of dermatological agents and cosmetics (CitationTona et al., 1998; CitationEMEA, 1999; CitationLukwa et al., 2001; CitationErah et al., 2003; CitationKamanzi Atindehou et al., 2004). According to the literature, H. madagascariensis shows excellent analgesic and anti-inflammatory activities (CitationNwodo, 1989). Similarly, the plant inhibits the activity of G-glucosidase and was found to have antioxidant properties (CitationKouam et al., 2006a, Citation2006b). CitationKouam et al. (2007) have isolated a prenylated 1, 4-anthraquinone from the hexane extract of the stem-bark of H. madagascariensis and have shown it to possess G-glucosidase inhibition and antioxidant activities. Most of the investigations on the pharmacological efficacies of H. madagascariensis are based on the leaves (CitationMadubunyi et al., 1995; CitationKamanzi Atindehou et al., 2002; CitationOkoli et al., 2002; CitationMoulari et al., 2006a, Citation2006b; CitationKouam et al., 2007).
We therefore investigated the effectiveness of different extracts from the stem-bark of H. madagascariensis against five important animal fungi and the four most important nosocomial bacterial pathogens.
Materials and methods
Plant materials and extract preparation
Freshly peeled stem-bark of Harungana madagascariensis was obtained from the main university campus, Ile-Ife, in September 2006. The plant was identified by O.A. Oladele of the Department of Pharmacognosy, Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife and a voucher specimen with voucher number FHI 107392 was kept at the herbarium of the Forestry Research Institute of Nigeria, Ibadan.
Extraction and preparation of plant extracts
The extraction procedure was carried out in accordance with the method of extraction that has been developed in the Phytomedicine Programme (CitationEloff, 1998). Separate samples of finely ground plant material (1 g) were extracted with 10 mL of hexane (H), dicloromethane (D), chloroform (C), ethyl acetate (E), water (W), acetone (A), and methanol (MeOH), and separately in a centrifuge tube shaken in a Labotec 20.2 shaking machine at moderate speed for 30 min. The extracts were decanted after centrifuging at 3500 g for 5 min. The whole process was repeated three times to exhaustively extract the plant material and the extracts were combined. The solvent was removed under a stream of air in a fume cupboard at room temperature to quantify the extraction. The extracts were individually resuspended in acetone to a concentration of 10 mg/mL.
Test microorganisms
The plant extracts were assayed for antimicrobial activity against four important nosocomial bacterial organisms obtained from the American Type Culture Collection (ATCC, USA and the Pretoria Academic Hospital (South Africa). They include Pseudomonas aeruginosa (ATCC 27853), Staphylococcus aureus (ATCC 29213), Enterococcus faecalis (ATCC 29212) and Escherichia coli (ATCC 25922). The fungal organisms were isolated from clinical cases that were not treated prior to sampling in the Department of Veterinary Tropical Diseases, Faculty of Veterinary Science, University of Pretoria. Aspergillus fumigatus was isolated from a chicken, Candida albicans from a Gouldian finch, Cryptococcus neoformans from a cheetah, M. canis from a cat suffering from dermatophytosis and S. schenckii from a horse with cutaneous lymphangitis. The cultures were a generous gift from J. Picard of the Department of Veterinary Tropical Diseases, University of Pretoria.
TLC analysis of extracts
Silica thin layer chromatography (TLC) plates (ALGRIMS® SIL g/UV 254-MACHEREYNAGEL, Merck) were used to visualize the chemical composition of the extracts. An aliquot of each extract (10 μL) was spotted onto the TLC plates which were developed using three solvent systems of different polarities. The solvent system mixtures that were used are as follows:
Ethyl acetate:methanol:water (EMW)/(40:5.4:4)/(polar/neutral)
Chloroform:ethyl acetate:formic acid (CEF)/(5:4:1)/(intermediate polarity)
Benzene:ethanol:ammonia hydroxide (BEA)/(90:10:1)/(non-polar/basic)
Development of the TLC plates was done in a pre-saturated glass TLC tank lined with filter paper. The chromatograms were then sprayed with vanillin-sulfuric acid spraying agent (0.1 g vanillin dissolved in 28 mL methanol plus 1 mL sulfuric acid) and heated in an oven at 110°C for approximately 5 min until color development. Acetone stem bark extract alone was further analyzed by TLC using two different solvent systems: hexane/ethyl acetate and hexane/acetone (19:1, 9:1, and 4:1) as eluents. This was done to have an insight into the possible constituents present in the stem bark. Plates were sprayed using standard phytochemical methods (CitationWagner & Bladt, 1996). The spots were examined under UV and developed by the following reagents: 5% ethanol KOH and 1% ethanol vanillin + 10% ethanol sulfuric acid which was followed by heating at 110°C for 5 min and the plates observed under UV light at 365 nm ().
Table 1. Phytochemical investigations of acetone extract of Harungana madagascariensis stem bark.
Bioassays
Minimum inhibitory concentrations (MIC) determination
MIC is defined as the lowest concentration of extract that inhibited visible growth of the organism on the microtiter plate. MIC values of each plant extract against the test organisms were determined by a two-fold serial dilution on a microplate assay (CitationEloff, 1998; CitationMartini & Eloff, 1998). An overnight culture of bacteria grown on Mueller Hinton broth 2.3% (w/v) (Oxoid, Basingstoke, UK) was diluted with fresh broth (1:100) and used for the assay. For fungal assay, sterile swabs were used to transfer one-week fungal cultures on agar to fresh Sabouraud dextrose (SD) agar 6.5% (w/v) (Oxoid, Basingstoke, UK) and 100 μL of the broth was added to each well. An aliquot of 40 μL of p-iodonitrotetrazolium violet INT (0.2 mg/mL) was added to each of the microtiter wells to serve as an indicator of microbial growth. Because of their slow growth, the growth indicator was added to fungal assays before incubation at 37°C, while in the case of bacteria, the indicator was added after 24 h of incubation at 37°C. Gentamicin and amphotericin B were used as the reference drugs for the bacterial and fungal assays, respectively. Acetone served as an untreated control agent in both assays. The two-fold serial dilutions of plant extracts were prepared; 100 μL of fungi and bacteria culture (overnight culture diluted 1:100 with fresh Sabouraud dextrose (SD) broth for fungi and MH broth for bacteria respectively) was added to each well. The covered microplates were incubated overnight at 37°C. In both assays a pinkish coloration is indicative of actively dividing microorganisms because of their ability to convert INT to red formazan.
Bioautography
TLC plates spotted with the extracts were eluted in BEA, EMW, and CEF, and were dried for at least 40 h to ensure adequate removal of the eluting solvents. Chromatograms were then sprayed with a suspension of actively growing cells of fungi and bacteria and incubated overnight at 37°C in a chamber at 100% relative humidity. The plates were then sprayed with a 2 mg/mL solution of INT after an overnight incubation. Inhibition of growth was indicated by clear zones on the chromatogram (CitationBegue & Kline, 1972).
Total activity of each extract
The total activity (TA) gives an indication to what volume the bioactive compounds present in one gram of the dried plant material can be diluted and still inhibit the growth of the test organism (CitationEloff, 2000). The TA is obtained by dividing the amount of extract yield from 1 g of plant materials by the MIC. Higher values of total activity indicate increased usefulness and economic value of the plant.
Results
Extracts yield, TLC fingerprinting, bioautography and phytochemical analysis
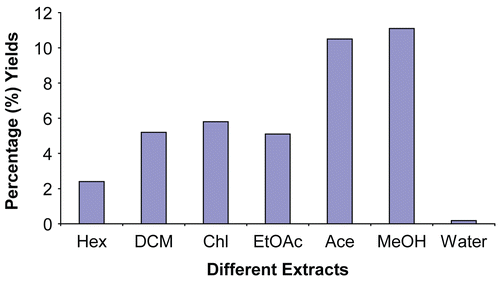
In the extraction process using solvents of varying polarities it was observed that M extracted the largest quantity (11.1%) followed by A (10.5%), C (5.8%), D (5.2%), E (5.1%), H (2.4%), and W (0.18%), respectively (). TLC fingerprinting using acidified vanillin showed 8 different colors (). Different colors are indicative of different types of chemical classes of compounds present in the test extracts (CitationMasoko, 2006). Bioautographic results of TLC plates eluted in BEA revealed the presence of several different active spots (colorless spots), which are indicative of the active compounds against the different strains of fungi and bacteria. denotes the active spots of the different extracts against S. aureus. Phytochemical analysis indicated the presence of anthraquinone, anthranoids, coumarins, and triterpenoids ().
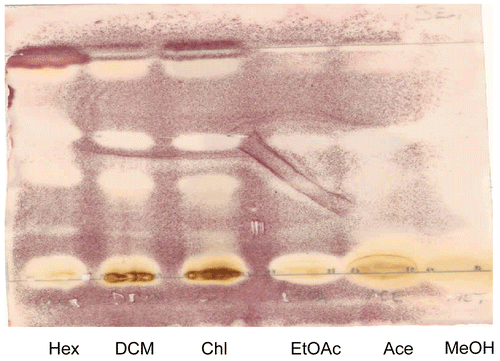
Figure 2. TLC Finger-print chromatogram developed in benzene: ethanol:ammonia hydroxide (BEA)/(90:10:1) and ethyl acetate: methanol:water (EMW)/(40:5.4:4) dried, sprayed with vanillin and heated at 110°C. The different colors denote various compounds. Lanes from left to right are hexane, acetone, chloroform, ethyl acetate, methanol and dichloromethane extracts.
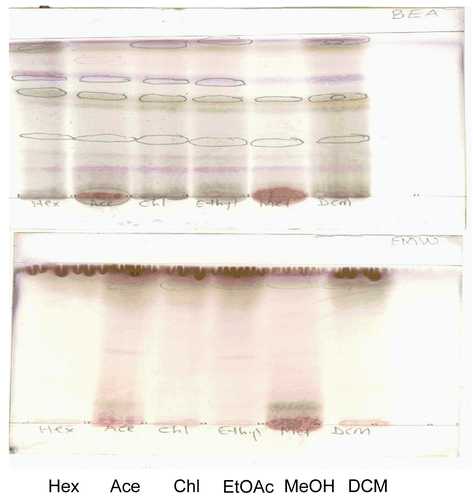
Figure 3. Bioautogram of different extracts of H. madagascariensis stem bark. TLC chromatogram developed in benzene:ethanol: ammonia hydroxide(BEA)/(90:10:1)/ (non-polar/basic) dried, sprayed with S. aureus cell suspension, incubated and sprayed with INT. Colorless areas denote inhibition of bacterial growth. Lanes from left to right are hexane, dichloromethane, chloroform, ethyl acetate, acetone and methanol extracts.
Antifungal activity
All the extracts (H, D, C, E, A, M and W) showed inhibition against all the fungi tested in this study in various degrees, however, methanol showed no activity against C. albicans, while the water extract also showed no activity against S. schenckii, and A. fumigatus (). Except for the methanol and water extracts against C. albicans, S. schenckii, and A. fumigatus, all other extracts showed more than 50% inhibition with activity ranging from 52.5% to 92.5% on different fungi pathogens. The MIC results of the extracts against the fungal pathogens are shown in . It shows that acetone gave the lowest MIC values in all the strains of fungi used, while methanol and water extracts exhibited the highest values. Therefore the order of activities demonstrated by the extracts on the fungi pathogens is as follows: A > D = C = E > H >M > W. However, the most and the least sensitive strains of pathogen are M. canis and A. fumigatus respectively. The sensitivity occurs in the following order: M. canis > C. neoformans > C. albicans > S. schenckii > A. fumigatus.
Table 2. The mean MIC values of seven different extracts of Harungana madagascariensis stem bark (mg/mL) and Amphotericin B (μg/mL) and the total activity (TA) of the extracts (ml)on fungi pathogens (n = 3).
Antibacterial activity
The antibacterial results of the all the extracts are presented in . With the exception of the water extract, all the extracts demonstrated activity against the Gram-positive and negative bacteria tested in this study, and the various effects decrease with time. The extracts were more active against the Gram-positive bacteria (Enterococcus faecalis and Staphylococcus aureus) than the Gram negative strains (Escherichia coli and Pseudomonas aeruginosa). As was observed in fungi, (except for the water extract at all time, hexane and methanol at 24 h, all other extracts showed more than 50% inhibition with activity ranging from 50% to 90% on different bacteria pathogens. The MIC ranged from 0.156-0.62 mg/mL except for the M and W that gave the highest values of 0.31-2.5 mg/mL. The sensitivity MIC results of the extracts showed that D, C, A and E extracts gave the equipotent lowest MIC values in all the strains of bacteria used, while water extract exhibited the highest values (). Therefore, the order of activities demonstrated by the extracts on the strains is as follow: D = C = A = E > H > M > W. However, the most and the least sensitive strains of the pathogens are S. aureus and E. coli, respectively. The sensitivity occurs in this order: S. aureus > E. faecalis > P. aeruginosa > E. coli.
Table 3. The mean MIC values of seven different extracts of H. madagascariensis stem bark (mg/mL) and Gentamicin (μg/mL) and the total activity (TA) of the extracts (mL) on bacteria pathogens (n = 3).
Total activity results
The total activity of all extracts except water gave an indication of a very high index of material medium dilution ratio, and this decreased with time. Acetone extract possessed the most outstanding total activity against fungal pathogens, it has the highest total activity value of 2600 mL/kg against M. canis and S. schenckii after 24 h of incubation. On average, the acetone extract showed total activity of 1566.8 mL/kg against all the tested pathogens (). Methanol extract (M) and dichloromethane extract (D) have the highest total activity of 739 and 631 mL/kg against E. coli respectively, after 2 h of incubation. The highest average total activity of 415.75 mL/kg was shown by methanol extract (). All the extracts except water extract exhibited equipotent total activity against bacterial pathogens as was observed in their MIC.
Discussion
Both fungi and bacteria are the major life threatening pathogens in human and animal diseases, especially in tropical regions and in immunocompromised or immunodeficient patients. Despite the existence of potent antibiotic and antifungal agents, resistant or multi-resistant strains are continuously appearing, imposing the need for a permanent search and development of new drugs (CitationSilver & Bostian 1993). Most available drugs are either fungistatic or mostly bacteriostatic. The actions of these agents, however, rely heavily on the host’s immune response to counter the infections. The antimicrobial agents currently used to treat or prevent bacterial and fungi infections in animals are essentially the same classes of compounds that are used in human medicine (CitationSchwarz et al., 2001).
Ethnoveterinary usefulness of Harungana madagascariensis (dragon blood tree) has been documented in Africa and in Europe for the treatment of animals with anemia and various infections (CitationEMEA, 1999). Most of the pharmacological studies and reports on H. madagascariensis were on aqueous extracts of leaves. Therefore, in this study the antimicrobial activity of hexane, dichloromethane, chloroform, ethyl acetate, acetone, methanol and water extracts of H. madagascariensis stem bark were determined with a serial dilution microplate assay. From the results presented in this study, it still confirms that fungal pathogens respond better than the bacteria, even though fungi infection is difficult to treat because of their ubiquitous nature and slow response to chemotherapy.
The fungal pathogens used (Aspergillus fumigatus, Candida albicans, Cryptococcus neoformans, Microsporum canis and Sporotrix schenckii) are known to cause infections of the mucous membrane of the respiratory, enteric and reproductive tracts, skin and meninges. The most effective extract, acetone, was less potent than the positive agent amphotericin B, while the most sensitive strain M. canis was more sensitive to amphotericin B than all the extracts. The extracts also had good activity against Staphylococcus aureus, Enterococcus faecalis, Pseudomonas aeruginosa, and Escherichia coli. These microbes are known to be responsible for infections of the respiratory, enteric, reproductive and urinary tracts, skin and wound contaminants. The extracts D, C, E and A, equipotently inhibit these bacteria pathogens, but were less potent than the positive agent gentamicin, while the most sensitive strain S. aureus was more sensitive to gentamicin than all the extracts. The action of these extracts tends to be fungistatic and bacteriostatic. This is because their activities on microbes generally decreased with time.
In a recent study of microbes’ susceptibility tests, it was indicated that the susceptibility profile of synthetic and common antibiotics on most of the microbes tested in this study showed a very high level of antibiotic resistance by the clinical and environmental microbes (CitationAladesanmi et al., 2007). There was a high level of antibiotic resistance to the commonly used antibiotics such as penicillin V (100%), cephalothin (98%), tetracycline (82%), and amoxicillin + potassium clavulanate- (Augmentin®) (77%). Some resistance was also demonstrated against fluoroquinolones, e.g. ofloxacin (6.3%), ciprofloxacin (21%) and erythromycin (100%). Generally, 64% of the microbes were resistant to more than 50% of the antibiotics tested (CitationAladesanmi et al., 2007). Therefore, the activities of the extracts against these microbes given in and could support the efficacy and the treatment of various diseases caused by the microorganisms.
To the best of our knowledge and to date, no study of antifungal activity of H. madagascariensis stem bark has been reported. Our antibacterial data compared favorably with earlier reports on the leaves of H. madagascariensis showing greater MIC values (CitationMadubunyi et al., 1995; CitationKamanzi Atindehou et al., 2002; CitationOkoli et al., 2002; CitationMoulari et al., 2006a).Quantification of total activity of plant materials in various therapeutic models have been established, particularly in antioxidant activity of many plants extracts (CitationMiller et al., 1995). Apart from MIC, the total antimicrobial activity (TA) of the extracts can be applied as an index (measurement) of potency among various extracts examined and their time of incubation with microorganisms. The entire total activity values of extracts, except that of water, in this study were very high and decrease with time. Acetone extract produced the most outstanding total activity against fungi pathogens, while all the extracts except water exhibited equipotent high activity against bacterial pathogens as observed in their MIC.
These data indicate that TA, besides a mere quantification in MIC, is a reliable indicator of the antimicrobial activity of extracts. It is also of interest for estimating the amount in volume of the medium in which 1 g of the extract will be diluted and still produce inhibition of microbes as antifungal and antibacterial agents. This type of information will therefore be very beneficial to the rural populace that uses this plant material for both human and animal diseases. The choice of different solvents for extraction in this study were based on our previous observations and other reports that water extracts of the plants generally showed little or no antimicrobial activities (CitationEloff, 1998; CitationMasika & Afolayan, 2002). However, water extract was added to prove the point. Observations on very weak or no activity of aqueous plant extracts on Gram-negative bacteria are well documented (CitationEloff, 1998; CitationAfolayan, 2003).
In this study, water extract did not show activity to both the Gram-positive and -negative bacteria. Generally, Gram-negative bacteria have been reported to be more resistant to plant extracts than the Gram-positive strains (CitationRabe & Van Staden, 1997). This is also confirmed in this study. The study therefore showed that antimicrobial compounds also reside in the stem bark like the leaves (CitationOkoli et al., 2002; Moulari et al., 2006a, 2006b) with the highest activities found in the acetone, dichloromethane, chloroform and ethyl acetate extracts. H. madagascariensis has been used traditionally for the treatment of various respiratory, gastro-intestinal, sexual transmission and parasitic diseases by the people of east and west Africa for a long time (CitationKamanzi Atindehou et al., 2004). These activities on fungi and bacteria pathogens could then justify the usefulness of the plant in the treatment of tuberculosis, diarrhea, dysentery, syphilis, gonorrhea, parasitic skin diseases, and wounds.
From the phytochemical studies, anthraquinone, anthranoids, coumarins, and triterpenoids are the compounds that were detected. The family Clusiaceae is well known for the production of various phenolic compounds such as anthraquinones, xanthones, coumarins, biflavonoids, and anthrone derivatives (CitationGunatilaka et al., 1984; CitationIinuma et al., 1995; CitationRitchie & Taylor, 1964) which are known to exhibit antimicrobial activities (CitationKitanov & Blinova, 1987; CitationRabanal et al., 2002). The ability of the extracts of this plant to inhibit the growth of several bacteria and fungi is an indication of the broad-spectrum antimicrobial potential that further validates the use of this plant for the treatment of various ailments in Africa.
In conclusion, the plant extracts of Harungana madagascariensis showed great promise for microbial infections. We are in the process of isolating and characterizing the compounds detected from the acetone extract of the stem bark that exhibited the highest antimicrobial activity.
Declaration of interest: The authors are grateful for the financial support given through the South African National Research Foundation. This work was also supported by the Obafemi Awolowo University, Ile-Ife, Nigeria. The authors alone are responsible for the content and writing of the paper.
References
- Afolayan AJ (2003): Extracts from the shoot of Arctotis arctotoides inhibits the growth of bacteria and fungi. Pharm Biol 41: 22–25.
- Aladesanmi AJ, Iwalewa EO, Adebajo AC, Akinkunmi EO, Taiwo BJ, Olorunmola FO, Lamikanra A (2007): Antimicrobial and antioxidant activities of some medicinal plants Afr J Trad Compl Alter Med 4: 173–184.
- Begue MJ, Kline RM (1972): The use of tetrazolium salts in bioautographic procedures. J Chromatog 64: 182–184.
- EMEA (1999): Committee for Veterinary Medical Product Harungana madagascariensis. The European Agency for the Evaluation of Medicinal Products. Available at http://www.eudra.org/emea.html.
- Eloff JN (1998): Which extractant should be used for the screening and isolation of antimicrobial components from plants? J Ethnopharmacol 60: 1–8.
- Eloff JN (2000): On expressing the antibacterial activity of plant extracts – A small first step in applying scientific knowledge to rural primary health care. South Afr J Sci 96: 116–118.
- Erah PO, Asonye CC, Okhamafe AO (2003): Response of Trypanosoma brucei brucei-induced anaemia to a commercial herbal preparation. Afr J Biotech 2: 307–311.
- Gunatilaka AAL, Silvia AMYD, Sostheeswaran S, Balasubramaniam S, Wazeer MIM (1984): Terpenoid and biflavonoid constituents of Calophyllum calaba and Garcinia spicata from Sri Lanka. Phytochemistry 23: 323–328.
- Gurib-Fakin A (2007): Harungana madagascariensis (Clusiaceae), in: Gurib-Fakin A, ed., Medicinal Plants of Mauritius and the World. CRC Press, pp. 152–153.
- Iinuma M, Hideki T, Tetsuro I, Toshiyuki T, Mohammad A (1995): Two prenylated anthrones in Harungana madagascariensis. Phytochemistry 40: 267–270.
- Kamanzi Atindehou K, Koné M, Terreaux C, Traore D, Hostettmann K, Dosso M (2002): Evaluation of the antimicrobial potential of medicinal plants from the Ivory Coast. Phytother Res 16: 497–502.
- Kamanzi Atindehou K, Schmid C, Brun R, Kone MW, Traore D (2004): Antitrypanosomal and antiplasmodial activity of medicinal plants from Cote d’Ivoire. J Ethnopharmacol 90: 221–227.
- Kitanov GM, Blinova KF (1987): Modern state of the chemical study of species of the genus Hypericum. Chem Nat Comp 23: 151–166.
- Kouam SF, Khan SN, Krohn K, Ngadjui BT, Lapche DG, Yapna DB, Zareem S, Moustafa AM, Choudhary MI (2006a): Alpha-glucosidase inhibitory anthranols, kenganthranols A-C, from the stem bark of Harungana madagascariensis. J Nat Prod 69: 229–233.
- Kouam SF, Ngadjui BT, Krohn K, Wafo P, Ajaz A, Choudhary MI (2006b): Prenylated anthronoid antioxidants from the stem bark of Harungana madagascariensis. Braz J Med Biol Res 38: 1087–1094.
- Kouam SF, Yapna DB, Krohn K, Ngadjui BT, Ngoupayo J, Choudhary MI, Schulz B (2007): Antimicrobial prenylated anthracene derivatives from the leaves of Harungana madagascariensis. J Nat Prod 70: 600–603.
- Lukwa N, Mutambu SL, Makaza N, Molgaard P, Furu P (2001): Perceptions about malaria transmission and control using anti-malaria plants in Mola, Kariba Zimbabwe. Nig J Nat Prod Med 5: 4–7.
- Madubunyi II, Obi SKC, Nwebube NI, Chime AB (1995): Antihepatotoxic and antimicrobial activities of Harungana madagascariensis leaf extracts. Int J Pharmacog 33: 129–134.
- Martini N, Eloff JN (1998): The preliminary isolation of several antibacterial compounds from Combretum erythrophyllum. J Ethnopharmacol 62: 255–263.
- Masika PJ, Afolayan AJ (2002): Antimicrobial activity of some plants used for the livestock diseases in the Eastern Cape. South Afr J Ethnopharmacol 84: 129–134.
- Masoko P (2006): Characterization of antifungal compounds isolated from Combretum and Terminalia species (Combretaceae). Unpublished PhD thesis, University of Pretoria, South Africa.
- Miller NJ, Diplock AT, Rice-Evans C (1995): Evaluation of the total antioxidant activity as a marker of the deterioration of apple juice on storage. J Agric Food Chem 43: 1794–1801.
- Moulari B, Pellequer Y, Lboutounne H, Girard C, Chaumont JP, Millet J, Muyard F (2006a): Isolation and in vitro antibacterial activity of astilbin, the bioactive flavanone from the leaves of Harungana madagascariensis Lam. Ex Poir. (Hypericaceae) J Ethnopharmacol 106: 72–278.
- Moulari B, Lboutounne H, Chaumont JP, Guillaume Y, Millet J, Pellequer Y (2006b): Potentiation of the bactericidal activity of Harungana madagascariensis Lam. Ex Poir. (Hypericaceae) leaf extract against oral bacteria using poly (D, Llactidecoglycolide) nanoparticles: In vitro study. Acta Odontol Scandinavica 64: 153–158.
- Nwodo OFC (1989) Antibiotic and anti-inflammatory analgesic activities of Harungana madagascariensis stem bark. Int J Crude Drug Res 27: 137–140.
- Okoli AS, Okeke MI, Iroegbu CU, Ebo PU (2002): Antibacterial activity of Harungana madagascariensis leaf extracts. Phytother Res 16: 174–179.
- Rabanal RM, Arias A, Prado B, Hernández-Pérez M, Sánchez-Mateo CC (2002): Antimicrobial studies on three species of Hypericum from the Canary Islands. J Ethnopharmacol 81: 287–292.
- Rabe T, Van Staden J (1997): Antibacterial activity of South African plants used for medicinal purposes. J Ethnopharmacol 56: 81–87.
- Ritchie E, Taylor WC (1964): The constituents of Harungana madagascariensis Poir. Tetrahedron Lett 23: 1431–1436.
- Schwarz S, Kehrenberg C, Walsh TR (2001): Use of antimicrobial agents in veterinary medicine and food animal production. Int J Antimicrob Agents 17: 431–437.
- Silver LL, Bostian KA (1993): Discovery and development of new antibiotics: The problem of antibiotic resistance. Antimicrob Agents Chemother 37: 377–383.
- Tona L, Kambu K, Ngimbi N, Cimanga K, Vlietinck AJ (1998): Antiamoebic and phytochemical screening of some Congolese medicinal plants. J Ethnopharmacol 61: 57–65.
- Wagner H, Bladt S (1996). Plant Drug Analysis. A Thin Layer Chromatography Atlas second edition. Berlin, Heidelberg, New York, Tokyo: Springer Verlag, pp. 347–357.