Abstract
Context: Paclitaxel (PTX) is widely used in chemotherapy for cancer treatment; however, it has some serious side effects. Andrographolide (Andro) is a potential cancer therapeutic agent isolated from Andrographis paniculata (Burm. f.) Nees (Acanthaceae).
Objective: The objective of this study is to evaluate the effects of PTX combined with Andro against A549 cells.
Materials and methods: The effects of 24–48 h treatment with 0.48–60.75 nM PTX and 5.10–328.0 μM Andro on cellular proliferation, apoptosis, cell cycle and intracellular reactive oxygen species (ROS) were determined by sulphorhodamine B assay, Annexin V-FITC/PI apoptosis detection, PI staining and ROS assay, respectively. Synergy was determined using combination index. The antitumour efficacy of 20 mg/kg PTX with 100 mg/kg Andro was studied in a xenograft murine model.
Results: IC50 value of the PTX combined with Andro against A549 cells was 0.5–7.4 nM, which was significantly lower than that of PTX (15.9 nM). PTX with 10 μM Andro caused (1.22–1.27)-fold apoptosis and 1.7-fold ROS accumulation compared with PTX alone. N-Acetylcysteine, a ROS scavenger, blocked this synergy in vitro. In contrast, G2/M phase cell cycle arrest resulting from PTX was not potentiated by Andro. Moreover, PTX in combination with Andro inhibited the growth of A549 transplanted tumours by 98%.
Discussion and conclusion: The results indicate that the combination of PTX and Andro exert significant synergistic anticancer effect on A549 cells in vitro and in vivo. The synergy may be the result of the accumulation of ROS. The combination of Andro and PTX represents a potential strategy for the treatment of A549 cells.
Introduction
Lung cancer, including small-cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC), is the leading cause of cancer-related death worldwide (Parkin et al. Citation2005). NSCLC is the most prevalent lung cancer with high morbidity and mortality (Jakel et al. Citation2013). Paclitaxel (PTX) is one of the most effective drugs used for the treatment of cancer and is often used in NSCLC chemotherapy. Its remarkable anticancer activities induce cellular apoptosis, stabilization of microtubules, and a mitotic arrest in the G2/M phase of the cell cycle (Crosasso et al. Citation2000; Hou et al. Citation2015). However, PTX is associated with serious side effects due to its toxicity. In clinical therapeutic regimens, drug combination is effective in the prevention of drug resistance, reduction of toxicity and improvement of efficacy. The synergy of combinational use has attracted considerable attention in biomedical fields, especially with regard to cancer therapy. Recently, several plant-derived compounds, such as curcumin and phenylpropanoid in combination with PTX, have been found to exhibit synergistic antitumour effects against some cancers (In et al. Citation2011; Boztas et al. Citation2013). Therefore, identification of naturally occurring compounds that have relatively low toxicity for combinational use may be a potential strategy to promote the effective utilization of PTX.
Andrographolide (Andro), a major labdane diterpenoid constituent of Andrographis paniculata (Burm. f.) Nees (Acanthaceae), is known for its antibacterial and anti-inflammatory activities. Moreover, Andro inhibits cell migration, cell invasion and expression of hypoxia-inducible factor-1α (HIF-1α) in A549 cells. It is worth mentioning that HIF-1α plays an important role in tumour growth, angiogenesis and lymph node metastasis of NSCLC (Lin et al. Citation2011). Andro also causes cell-cycle arrest in the G0/G1 phase, upregulates intracellular reactive oxygen species (ROS) levels and induces TRAIL (TNF-related apoptosis-inducing ligand)-mediated apoptosis in some cancer cell lines (Yang et al. Citation2010; Parveen et al. Citation2014). Hence, Andro may represent a good candidate for the potentiation of PTX in NSCLC treatment.
The purpose of the present study was to explore the synergistic antitumour effect of PTX and Andro on A549 human NSCLC cells in vitro and in vivo. Cellular proliferation, apoptosis, cell cycle and intracellular ROS were evaluated to analyze the underlying mechanisms of synergic effect of PTX and Andro on A549 cells. Additionally, antitumour effects were also investigated in a xenograft murine model.
Materials and methods
Chemicals and reagents
Andro, PTX and N-acetylcysteine (NAC) were purchased from Aladdin Industrial Corporation (Shanghai, China). Fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM) and 0.25% (w/v) Trypsin were obtained from Gibco-BRL (Gaithersburg, MD). Sulphorhodamine B (SRB) was purchased from Sigma-Aldrich (St Louis, MO). Trichloroacetic acid (TCA) and dimethyl sulphoxide (DMSO) were purchased from Shanghai Lingfeng Chemical Reagent (Shanghai, China).
Preparation of solutions
Andro and PTX were dissolved in DMSO to the desired stock concentrations and then prepared for a secondary solution in DMEM, in which the final DMSO content was no higher than 0.1%. Similarly, NAC was dissolved in PBS to 500 mM as a stock solution, which was diluted to the desired concentration with DMEM.
Cell culture and proliferation studies
A549 NSCLC cells, purchased from the Cell Bank of the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China), were cultured in DMEM containing 10% (v/v) FBS, 0.37% (w/v) NaHCO3, 100 units/mL penicillin (HyClone, Logan, UT), and 100 μg/mL streptomycin (HyClone, Logan, UT) at 37 °C in a humidified incubator with a 5% CO2 atmosphere. The effects of Andro and PTX on cell proliferation were evaluated using a SRB assay (Monks et al. Citation1991). Exponentially growing A549 cells were seeded into each well of 96-well plates containing 200 μL of fresh growth medium, allowed to attach for 24 h, and then subjected to 200 μL of Andro (5.10–328.05 μM) and/or PTX (0.48–60.75 nM). Cells treated with medium containing 0.1% DMSO were used as a control. After incubation for 48 h, the cells were fixed with 10% trichloroacetic acid (TCA) at 4 °C for 1 h, stained with 100 μL of SRB solution in 1% acetic acid for 0.5 h and then washed with 1% acetic acid to remove the unbound dye. The bound dye was extracted with 10 mM Tris-HCl and the optical density (OD) value of each well was measured at 540 nm using a microplate reader (SpectraMax M2, Molecular Devices, Silicon Valley, CA). The cell survival rate was calculated as follows:
Additionally, A549 cells were subjected to different concentrations of PTX (0.48–60.75 nM) in the presence of Andro (10 μM) and/or NAC (5 mM) to evaluate the effect of ROS levels on the proliferation of treated cells. There were three replicate wells for each experimental and control group. The results of three independent experiments are shown as the mean ± SD.
Combination studies
Combinations of PTX at different concentrations with a fixed Andro concentration were utilized in the cell treatments. A549 cells were exposed to solutions containing 10, 20 and 30 μM Andro combined with PTX (0.48–60.75 nM). The median effect analysis was employed to study the combined effect; the combination index (CI) was calculated based on the median effect equation of Chou (Chou Citation2010; Almohaimeed & Donev Citation2014):
where D1 and D2 represent the concentrations of compounds 1 and 2 in combination to achieve x% inhibition, respectively, whereas D1x and D2x represent concentrations of compounds 1 and 2 to achieve x% inhibition when used alone, respectively. CI <1, CI =1 and CI >1 indicate synergism, additivity and antagonism, respectively.
Quantification of apoptosis
Apoptosis was determined using an Annexin V-FITC/PI apoptosis detection kit (Signalway Antibody, College Park, MD). A549 cells were treated with 13 and 40 nM PTX with or without 10 μM Andro for 48 h. Cells treated with medium plus 0.1% DMSO were used as a control. After treatment, cells were collected, washed twice with cold PBS, centrifuged for 5 min at 1000 r/min and re-suspended with 400 μL of Annexin V binding buffer. The cell suspension was incubated with 5 μL of Annexin V-FITC for 15 min and 10 μL of propidium iodide (20 μg/mL) for 5 min in the dark. A Becton–Dickinson FACScan flowcytometer (Becton, Dickinson and Company, Franklin Lakes, NJ) was used to detect apoptotic cells at an excitation wavelength 488 nm and an emission wavelength of 530 nm, and FlowJo 7.6.1 software (Tree Star, Ashland, OR) was used to calculate the extent of apoptosis. The results of three replicated tests are shown as the mean ± SD.
Cell-cycle analysis
Cell-cycle analysis was conducted using a cell cycle detection kit (Signalway Antibody, College Park, MD). A549 cells were treated by 13 and 40 nM PTX with or without 10 μM Andro for 24 h. Cells treated with medium plus 0.1% DMSO were used as a control. After treatment, the cells were collected, washed twice with cold PBS, centrifuged for 5 min at 1000 r/min, and re-suspended and fixed using 500 μL of 70% (v/v) frozen ethanol at −20 °C for 1 h. Fixed cells were washed twice with cold PBS, centrifuged for 5 min at 1000 rpm, and re-suspended in 200 μL of cool PBS, followed by RNase treatment in water bath at 37 °C for 30 min. Next, the cells were incubated with 400 μL of propidium iodide (50 μg/mL) for 1 h in the dark at 4 °C. At least 10,000 cells in each group were analyzed using a Becton-Dickinson FACScan flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ) at an excitation wavelength of 488 nm and a test wavelength of 620 nm. The results of three replicated tests are shown as the mean ± SD.
ROS measurement
Levels of intracellular ROS were determined using a ROS assay kit (Beyotime, Haimen, China) with the fluorescent marker 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA). Approximately 5 × 105 A549 cells seeded in 6-well plates were exposed to 13 nM PTX with or without 10 μM Andro for 48 h. The cells treated with medium plus 0.1% DMSO were used as a control. After treatment, cells in 6-well tissue culture plates were washed three times with PBS, and incubated with 10 μL of DCFH-DA (10 μM) in FBS-free medium for 30 min in the dark. Then, cells were collected, washed three times with PBS, centrifuged for 5 min at 1000 rpm and re-suspended in 1 mL of PBS for measurement. Fluorescence intensity indicating ROS accumulation in cells was immediately measured by flow cytometry at an excitation wavelength of 488 nm and an emission wavelength of 525 nm. The results of three replicated tests are shown as the mean ± SD.
Animal experimental protocols
A total of 16 specific pathogen-free, 6-week-old female BALB/c nude mice, weighing 20–25 g, were purchased from Slac Laboratory Animal (Shanghai, China). All the mice were housed under standard conditions with sterile distilled water and rodent laboratory food. A total of 107 A549 cells in 200 μL of 0.9% normal saline were injected subcutaneously into the left oxter. When the tumour volume reached approximately 100 mm3, the mice were randomized into four treatment groups: I (Control), II (100 mg/kg Andro), III (20 mg/kg PTX) or IV (100 mg/kg Andro and 20 mg/kg PTX). Andro and/or PTX were administered on days 1, 3 and 5 via intraperitoneal injection. Tumour volume was measured with a Vernier calliper every 2 d. Mice in each group were euthanized on the fifteenth day, and the tumours were excised. Tumour weight was measured with an electronic balance with an accuracy of 0.1 mg. Tumour volume (V) was calculated using the formula:
where length is the largest diameter of the tumour and width is its perpendicular measurement (Razi et al. Citation2014).
Statistical analysis
Statistically significant differences between various drug treatments were determined by one-way analysis of variance and Student’s t-test using Prism 5.0 software (GraphPad Inc., San Diego, CA). A value of p< 0.05 was considered significant.
Results
Effects of PTX and Andro on A549 cell proliferation
The antiproliferative effects of PTX and Andro against NSCLC A549 cells were assessed with a SRB assay after 48 h of treatment. Andro (5.10–328.0 μM) inhibited the proliferation of A549 cells in a dose-dependent manner (). Concentrations of 10–30 μM Andro enhanced the antiproliferative effect of PTX in three combinations (). IC50 value of PTX was 15.9 nM. However, when PTX was combined with Andro (10, 20 or 30 μM), the IC50 value of the combination was significantly lower than PTX alone in the range of 7.4–0.5 nM (). The CI values were all lower than 1 when Fa was between 0.1 and 0.6 (). These results suggest significant synergy between Andro and PTX when 10–60% of the cells were inhibited.
Figure 1. Effects of PTX and Andro on A549 cell proliferation. Viability percentage of A549 cells subjected to Andro (A), PTX or PTX + Andro (B). (C) CIs of PTX with Andro. Fa (= cell inhibition %) indicates the fraction affected.
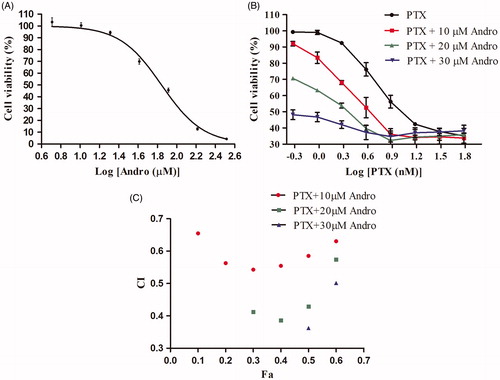
Table 1. IC50 values of PTX alone and in combination with Andro.
Effects of PTX combined with Andro on A549 cell apoptosis and cell cycle
Total apoptosis induced by PTX (13, 40 nM) was potentiated by treatment with 10 μM Andro (). The concentration of Andro utilized for all the following experiments was 10 μM unless otherwise stated. Neither treatment with Andro nor 13 nM PTX resulted in significant differences from the control for the level of induced apoptosis (p > 0.05). However, apoptosis induced by PTX in combination with Andro was significantly increased compared with that caused by PTX alone (p < 0.001).
Figure 2. Apoptosis and cell cycle arrest induced by PTX and/or Andro. (A) A549 cells were treated with 13 or 40 nM PTX with or without 10 μM of Andro for 48 h. Annexin V-FITC and PI were used to determine apoptosis. **p < 0.001 compared with the control. ##p < 0.001 compared with the corresponding PTX group. (B) A549 cells were treated with 13 or 40 nM PTX with or without 10 μM Andro for 24 h. Values are shown as the mean ± SD (n = 3).
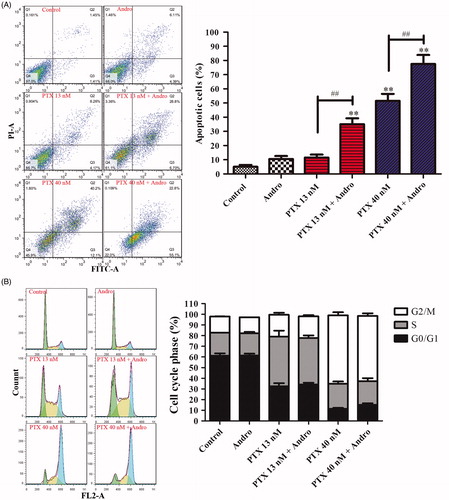
PTX induced A549 cell-cycle arrest at the G2/M phase in a dose-dependent manner (). Treatment with 10 μM Andro barely induced cell-cycle arrest compared with the control. Furthermore, PTX caused almost the same level of cell-cycle arrest regardless of whether it was applied in combination with Andro.
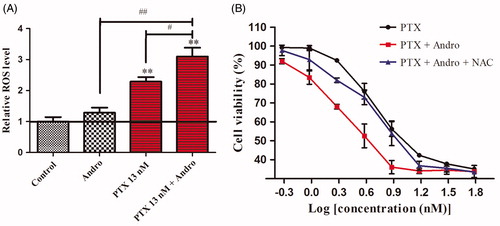
ROS accumulation caused by the combination of PTX with Andro and its effect on synergistic antiproliferation Intracellular ROS were not significantly increased by Andro compared with the control. However, the combination of PTX and Andro increased ROS accumulation by 1.7- and 7.8-fold () compared with either agent alone. These results suggest that Andro can not only promote the antiproliferative effect of PTX in A549 cells but can also increase the intracellular ROS accumulation induced by PTX. This synergistic antiproliferative effect may be associated with intracellular ROS accumulation. Thus, in this experiment, 5 mM NAC was used to eliminate ROS (Chairuangkitti et al. Citation2013). A549 cells seeded in a 96-well plate were treated with PTX in the presence of Andro and/or NAC. Five millimolar NAC did not affect the proliferation of A549 cells (data not shown). However, the growth inhibition of cells treated by PTX and Andro was reduced upon the addition of NAC (). The results suggest that synergistic antiproliferative effects of PTX and Andro were blocked by NAC.
Figure 3. ROS accumulation caused by the combination of PTX with Andro and its effect on synergistic antiproliferation. (A) ROS levels increased by Andro and PTX. Control: untreated A549 cells. Andro: A549 cells were exposed to 10 μM Andro. PTX 13 nM: A549 cells were exposed to 13 nM PTX. PTX 13 nM + Andro: A549 cells were exposed to 13 nM PTX with 10 μM Andro. **p < 0.001 compared with the control. #p < 0.01, ##p < 0.001 compared with the PTX 13 nM or Andro. (B): A549 cells were subjected to different concentrations of PTX in the presence of Andro and/or NAC. Values are shown as the mean ± SD (n = 3).
Antitumour effect of PTX and Andro
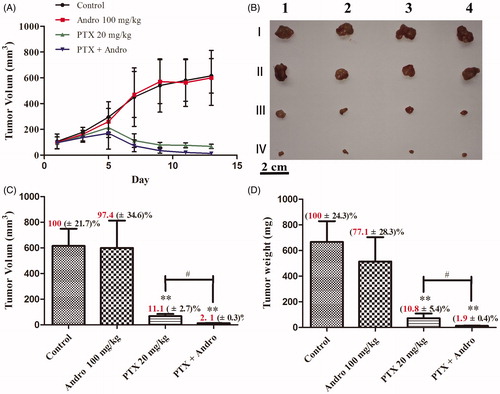
To evaluate the antitumour effect of the combination treatment in vivo, a xenograft murine model with A549 cell was used. shows that a dose of 100 mg/kg Andro did not significantly reduce tumour volume within 13 d compared with the controls (p > 0.05), but it did potentiate the antitumour effect of PTX. The same results are shown in . The volume and weight of the tumours in the Andro group were approximately the same as those in the control group. The mice treated with a combination of PTX and Andro had significantly smaller tumours (p < 0.01; ). Inhibition of tumour volume and weight reached as high as 97.9% () and 98.1% (), respectively.
Figure 4. Antitumour effects of PTX and Andro. (A) Tumour volume changes of BALB/c nude mice bearing A549 tumours after the first administration during the 13 days. (B, C and D) The morphology, volume and weight, respectively, of the tumours isolated from mice at day 15. Mice were randomized into four treatment groups: I (Control), II (100 mg/kg Andro), III (20 mg/kg PTX) and IV (100 mg/kg Andro and 20 mg/kg PTX). Values are shown as the mean ± SD (n = 4). **p < 0.001 compared with the control. #p < 0.01 compared with PTX.
Discussion
PTX is a common and effective chemotherapy drug used for a variety of cancers. However, PTX will simultaneously injure normal tissues and inhibit tumour growth during the course of treatment. The combination of low toxicity compounds and PTX is a promising strategy to reduce toxicity caused by PTX and to maintain, or even increase, curative efficacy. Andro, a relatively low toxicity compound extracted from Andrographis paniculata, has several bioactivities including antibacterial, anti-inflammatory and anticancer activities. In the present study, A549 cells were introduced in vitro for anticancer research to investigate the possible synergistic effect of PTX and Andro. A xenograft murine model with A549 cells was used to evaluate antitumour efficacy in vivo. The preliminary mechanism of synergy was also proposed based on several well-established biomarkers, including cellular proliferation, cell apoptosis, cell cycle and intracellular ROS.
The results of the SRB assay suggest that PTX, over a wide range of concentrations, has a synergistic antiproliferative effect on A549 cells when it is applied in combination with Andro (10, 20 or 30 μM). PTX, along with 10 μM Andro, synergistically induced apoptosis of A549 cells. Common synergistic mechanisms involved in the anticancer activities of PTX combination treatment have previously been elucidated as follows: induction of cell-cycle arrest (Liu et al. Citation2013) and increased intracellular ROS accumulation (Alexandre et al. Citation2006; Wang et al. Citation2012). In the present study, PTX caused cell-cycle arrest at the G2/M phase in a dose-dependent manner, which suggests that PTX exerts its anticancer effect in the form of mitotic arrest at the G2/M phase of the cell cycle. Andro causes cell-cycle arrest in the G0/G1 phase, and induces apoptosis in some types of cancer cell lines, including A549 (Lee et al. Citation2010; Yang et al. Citation2010; Lin et al. Citation2011; Parveen et al. Citation2014). However, shows that 10 μM Andro had minimal influence on the A549 cell cycle compared with the control. The results of this study indicate that the cell-cycle arrest effect of PTX was not synergistic with Andro. This may be due to the different cell-cycle phases that PTX and Andro arrest in A549 cells, i.e., G2/M and G0/G1, respectively.
ROS was proposed to both accelerate and delay cancer initiation, as well as progression, because of its multiple roles during the evolution of cancer (Chandel & Tuveson Citation2014). ROS can promote cancer by oxidizing specific intracellular chemical moieties, causing genetic mutations and stimulating proliferation and neoplastic transformation (Finkel Citation2011). Increased mitochondrial ROS are produced in cancer cells, which further stimulate neoplastic transformation (Chandel & Tuveson Citation2014). In the present study, intracellular ROS in A549 cells were not significantly increased by Andro compared with the control, but the combination of PTX and Andro greatly increased ROS accumulation compared with either agent alone. Furthermore, 5 mM NAC, a ROS scavenger was used to investigate the association of ROS with the synergistic effect. The low concentration of NAC was demonstrated to have no effect on the viability of A549 cells (Chairuangkitti et al. Citation2013). Our results showed that the anticancer synergy between PTX and Andro was blocked by NAC. Therefore, ROS may play a vital role in the synergistic antiproliferative effect of PTX and Andro. High levels of ROS are toxic. The therapeutic effect of ionizing radiation and many common chemotherapeutic agents in the treatment of cancer depends on the cytotoxic action of ROS (Chandel & Tuveson Citation2014).
Additionally, Andro caused mild side effects in tumour-bearing mice (Rajagopal et al. Citation2003) and barely reduced the tumour volume in the treated mice compared with the control. However, the treatment in which Andro synergized with PTX was capable of significantly reducing A549 tumour volume in mice.
The synergistic anticancer activity of PTX and Andro against A549 cells both in vitro and in vivo may result from mechanisms other than ROS accumulation. It has been reported that PTX activates the NF-kB-signalling pathway and that Andro inhibits the PI3K/Akt-signalling pathway (Fitzpatrick & Wheeler Citation2003; Lee et al. Citation2010). The different signalling pathways affected by Andro and PTX in the regulation of apoptosis may also contribute to the apparent synergy between them. Interestingly, Andro was shown to inhibit MMP-7 expression via suppression of the PI3K/Akt/AP-1 signalling pathway, which led to a reduction in the invasiveness of A549 cells (Lee et al. Citation2010), and NF-κB signalling activated by PTX induced the expression of pro-inflammatory genes. Thus, the synergistic antitumour efficacy of the two agents may also benefit from their inflammatory effects.
Conclusions
The present study demonstrates synergy of PTX and Andro against A549 cells both in vitro and in vivo. Such potentiation may be attributed to the elevation of ROS and apoptosis. The results suggest that the combination of Andro and PTX may represent a potential strategy in the treatment of A549 cells. However, the underlying molecular mechanisms of this synergy need further clarification.
Disclosure statement
The authors report that they have no conflicts of interest. This work was financially supported by the Science and Technology Commission of Shanghai Municipality (STCSM, Contract no. 11nm0505700).
References
- Alexandre J, Batteux F, Nicco C, Chéreau C, Laurent A, Guillevin L, Weill B, Goldwasser F. 2006. Accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo. Int J Cancer. 119:41–48.
- Almohaimeed B, Donev AN. 2014. Experimental designs for drug combination studies. Comput Stat Data Anal. 71:1077–1087.
- Boztas AO, Karakuzu O, Galante G, Ugur Z, Kocabas F, Altuntas CZ, Yazaydin AO. 2013. Synergistic interaction of paclitaxel and curcumin with cyclodextrin polymer complexation in human cancer cells. Mol Pharm. 10:2676–2683.
- Chairuangkitti P, Lawanprasert S, Roytrakul S, Aueviriyavit S, Phummiratch D, Kulthong K, Chanvorachote P, Maniratanachote R. 2013. Silver nanoparticles induce toxicity in A549 cells via ROS-dependent and ROS-independent pathways. Toxicol In Vitro. 27:330–338.
- Chandel NS, Tuveson DA. 2014. The promise and perils of antioxidants for cancer patients. N Engl J Med. 371:177–178.
- Chou TC. 2010. Drug combination studies and their synergy quantification using the Chou–Talalay method. Cancer Res. 70:440–446.
- Crosasso P, Ceruti M, Brusa P, Arpicco S, Dosio F, Cattel L. 2000. Preparation, characterization and properties of sterically stabilized paclitaxel-containing liposomes. J Control Release. 63:19–30.
- Finkel T. 2011. Signal transduction by reactive oxygen species. J Cell Biol. 194:7–15.
- Fitzpatrick F, Wheeler R. 2003. The immunopharmacology of paclitaxel (Taxol), docetaxel (Taxotere), and related agents. Int Immunopharmacol. 3:1699–1714.
- Hou ZH, Zhao WC, Zhang Q, Zheng W. 2015. Effect of paclitaxel-loaded nanoparticles on the viability of human hepatocellular carcinoma HepG2 cells. Asian Pac J Cancer Prev. 16:1725–1728.
- In LL, Azmi MN, Ibrahim H, Awang K, Nagoor NH. 2011. 1′S-1′-Acetoxyeugenol acetate: a novel phenylpropanoid from Alpinia conchigera enhances the apoptotic effects of paclitaxel in MCF-7 cells through NF-κB inactivation. Anticancer Drugs. 22:424–434.
- Jakel A, Plested M, Dharamshi K, Modha R, Bridge S, Johns A. 2013. A systematic review of economic evaluations in second and later lines of therapy for the treatment of non-small cell lung cancer. Appl Health Econ Health Policy. 11:27–43.
- Lee YC, Lin HH, Hsu CH, Wang CJ, Chiang TA, Chen JH. 2010. Inhibitory effects of andrographolide on migration and invasion in human non-small cell lung cancer A549 cells via down-regulation of PI3K/Akt signaling pathway. Eur J Pharmacol. 632:23–32.
- Lin HH, Tsai CW, Chou FP, Wang CJ, Hsuan SW, Wang CK, Chen JH. 2011. Andrographolide down-regulates hypoxia-inducible factor-1α in human non-small cell lung cancer A549 cells. Toxicol Appl Pharmacol. 250:336–345.
- Liu K, Cang S, Ma Y, Chiao JW. 2013. Synergistic effect of paclitaxel and epigenetic agent phenethyl isothiocyanate on growth inhibition, cell cycle arrest and apoptosis in breast cancer cells. Cancer Cell Int. 13:10.
- Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A, et al. 1991. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst. 83:757–766.
- Parkin DM, Bray F, Ferlay J, Pisani P. 2005. Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108.
- Parveen R, Ahmad FJ, Iqbal Z, Samim M, Ahmad S. 2014. Solid lipid nanoparticles of anticancer drug andrographolide: formulation, in vitro and in vivo studies. Drug Dev Ind Pharm. 49:1206–1212.
- Rajagopal S, Kumar RA, Deevi DS, Satyanarayana C, Rajagopalan R. 2003. Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. J Exp Ther Oncol. 3:147–158.
- Razi SS, Rehmani S, Li X, Park K, Schwartz GS, Latif MJ, Bhora FY. 2014. Antitumor activity of paclitaxel is significantly enhanced by a novel proapoptotic agent in non-small cell lung cancer. J Surg Res. 194:622–630.
- Wang L, Liu X, Wu Y, Wu W, Wu Y. 2012. Involvement of ROS in the inhibitory effect of thermotherapy combined with chemotherapy on A549 human lung adenocarcinoma cell growth through the Akt pathway. Oncol Rep. 28:1369–1375.
- Yang S, Evens AM, Prachand S, Singh AT, Bhalla S, David K, Gordon LI. 2010. Mitochondrial-mediated apoptosis in lymphoma cells by the diterpenoid lactone andrographolide, the active component of Andrographis paniculata. Clin Cancer Res. 16:4755–4768.
