Abstract
Context: Diabetic patients have a higher risk of colorectal cancer (CRC). The role of metformin in CRC incidence among type 2 diabetes mellitus (T2DM) remains controversial.
Objective: A meta-analysis was performed to evaluate the role of metformin treatment in the occurrence of CRC among T2DM patients.
Methods: Search was performed throughout PubMed, Embase, Springer databases up to November 2014. The search terms were (biguanides or metformin) and (bowel or colon or rectal or colorectal) and (cancer or neoplasm or neoplasia). Relative risk (RR) and 95% confidence interval (CI) was pooled using random-effects model or fixed-effect model basing on heterogeneity, which was calculated basing on Q statistics and χ2 test. In addition, subgroup analyses were performed according to region, study design and control treatment. Finally, publication bias was evaluated using Egger’s regression test and trim and fill analysis.
Results: A total of 11 studies, including eight cohort studies and three case-control studies, were enrolled in the meta-analysis. Obvious heterogeneity was noted, and a 25% lower CRC incidence was found among diabetic patients treated with metformin (pooled RR=0.75, 95% CI: 0.66-0.86), using the random-effects model. Subgroup analyses showed that CRC incidence significantly reduced among T2DM in different regions, non-metformin treatment and cohort studies. Evidence supported significant publication for studies investigating from Egger’s regression test. Conversely, no missing data were found using trim and fill analysis.
Conclusion: In conclusion, the meta-analysis suggests metformin may reduce CRC incidence among diabetics, which is useful medical information for clinicians.
Introduction
Colorectal cancer (CRC) has become the third leading cause of cancer death in the United States (Siegel et al. Citation2014), and is the sixth most common cancer in China (Zheng et al. Citation2014). Increasing evidence has shown that diabetic patients have an increased risk of suffering CRC because of metabolic syndromes, such as insulin resistance, hyperinsulinaemia and hyperglycaemia (Kim Citation1998; Larsson et al. Citation2005). Metformin, as the most widely used antidiabetic drug (Ben Sahra et al. Citation2010), performs its therapeutic benefits through activating AMP-activated protein kinase, and have a potential role as anticancer drugs (Hardie Citation2007).
Recently, numerous studies are focusing on the association of metformin and CRC risk among type 2 diabetes mellitus (T2DM). Several epidemiological studies reported significantly decreased CRC incidence among T2DM treated with metformin (Oliveria et al. Citation2008; Currie et al. Citation2009; Libby et al. Citation2009; Lee et al. Citation2011; Hsieh et al. Citation2012; Ruiter et al. Citation2012). However, other studies did not detect the relationship between metformin treatment and CRC risk among diabetes (Yang et al. Citation2004; Bodmer et al. Citation2012; Cardel et al. Citation2014; Tsilidis et al. Citation2014; Xu et al. Citation2014). Several meta-analyses were conducted to evaluate the relationship, while the tissue still remains controversial (Decensi et al. Citation2010; Zhang et al. Citation2011; Noto et al. Citation2012; Soranna et al. Citation2012; Gandini et al. Citation2014).
By pooling five observational studies, the meta-analysis by Zhang et al. (Citation2011) supports the conclusion that metformin therapy was associated with a significantly lower risk of colorectal cancer in T2DM patients. Notably, one study investigated the association between metformin and colorectal adenoma (Chung et al. Citation2008). Gandini et al. (Citation2014) performed a new meta-analysis, which shows that metformin may reduce cancer incidence and mortality in patients with diabetes, including the colon cancer. Thereafter, several further studies were conducted to explore the relationship between metformin therapy and CRC risk among T2DM (Tsilidis et al. Citation2014; Xu et al. Citation2014). Accordingly, we performed the meta-analysis through enrolling the studies focusing on the tissue associated with the relationship exposition.
Materials and methods
The paper did not involve any humans or animals. Therefore, ethical approval was not required.
Search strategy and study selection
Search was carried out using PubMed, Embase, Springer link databases to identify eligible studies published up to November 2014. The following keywords were used: (biguanides or metformin) and (bowel or colon or rectal or colorectal) and (cancer or neoplasm or neoplasia). In order to explore data related to this issue, printed articles and the reference lists of reviews were also hand-checked.
Inclusion and exclusion criteria
Studies were included if they (a) enrolled T2DM as study objects; (b) investigated exposure of metformin; (c) assessed the risk of colorectal cancer; (d) provided values estimating the association between outcomes and exposure, including Hazard risk (HR), Relative risk (RR), Odds risk (OR) and its 95% confidence intervals (CIs), or provided sufficient data to calculate these values; (e) obtained ethical approval from ethics committee. The most recent and complete publication would be considered when data were studied more than once. Studies such as reviews, letters and comments were excluded.
Data extraction and quality assessment
Data extraction was independently performed by two investigators following a standard form designed in advance. Details on the first author’s name, publication year, country, study design, essential characteristics of participates (sample size, age, sex ratio), HR/OR/RR and the corresponding 95% CI, and correction factor. After finishing data extraction, the extraction form would be interchanged for verification. The disagreement would be solved through discussing with the third investigator.
The Newcastle-Ottawa Scale was used to perform the quality assessment (Wells et al. Citation2011). A total of 9 stars were enrolled in the scale, and three aspects were included including selection, comparability and exposure/outcomes. Studies scored more than 7 stars were defined as high quality; the studies scored stars ranged 4-6 were good quality; the studies scored below 3 stars were low quality study.
Statistical analysis
Heterogeneity among individual studies was tested using Cochrane Q and χ2 test. p < 0.05/I2> 50% would be recognized as a significant heterogeneity and the random-effects model would be used to pool the effect size, otherwise, the fixed-effect model would be used.
The source of heterogeneity was investigated by subgroup analyses. Subgroup analyses would be performed according to geographic area, study design and non-metformin treatment in the control. Finally, publication bias was evaluated by the Egger’s regression test and trim, and fill analysis. All of the statistical analysis was carried out by Stata software program version 11.0 (STATA, College Station, TX).
Results
Publication selection
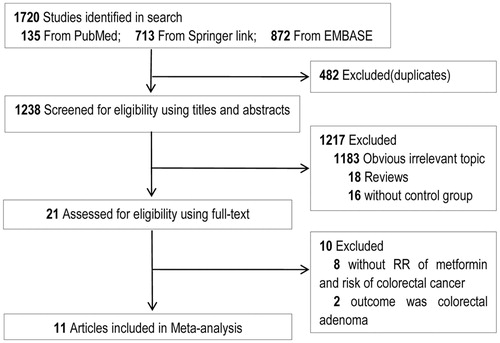
As shown in , originally 1720 were selected in the publication search. After removing duplicate publications, 1238 studies were left. On the basis of title and abstract, 1217 studies were removed, including 1183 irrelevant studies, 18 reviews, and 16 studies without a control group. The remaining 21 articles were all full-reviewed. Finally, 11 studies (Yang et al. Citation2004; Oliveria et al. Citation2008; Currie et al. Citation2009; Libby et al. Citation2009; Lee et al. Citation2011; Bodmer et al. Citation2012; Hsieh et al. Citation2012; Ruiter et al. Citation2012; Cardel et al. Citation2014; Tsilidis et al. Citation2014; Xu et al. Citation2014) were enrolled in the meta-analysis. No study was included after hand-checking.
Study characteristics
shows that eight cohort studies (Oliveria et al. Citation2008; Currie et al. Citation2009; Libby et al. Citation2009; Lee et al. Citation2011; Hsieh et al. Citation2012; Ruiter et al. Citation2012; Tsilidis et al. Citation2014; Xu et al. Citation2014) including 175,432 cases and 126,967 controls, and three case-control studies (Yang et al. Citation2004; Bodmer et al. Citation2012; Cardel et al. Citation2014) including 3133 cases and 15,774 controls were retrieved in the meta-analysis. Two studies were based on American (Oliveria et al. Citation2008; Xu et al. Citation2014), seven studies were based on European (Yang et al. Citation2004; Currie et al. Citation2009; Libby et al. Citation2009; Bodmer et al. Citation2012; Ruiter et al. Citation2012; Cardel et al. Citation2014; Tsilidis et al. Citation2014), and two studies were based on Chinese (Taiwan) (Lee et al. Citation2011; Hsieh et al. Citation2012). All the studies were published from 2004 to 2014. Drugs chosen as therapy for control included insulin in three studies, sulfonylurea in four studies, and non-metformin in six studies. As shown in , all the studies were scored more than 6, suggesting that the studies were of high quality.
Table 1. Characteristics of studies included in the meta-analysis.
Table 2. Methodological quality of cohort/case-control studies included in the meta-analysis.Table Footnotea
CRC incidence in diabetic patients treated with metformin
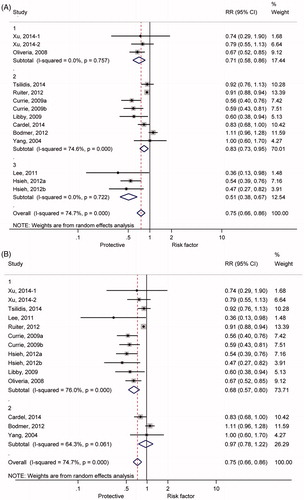
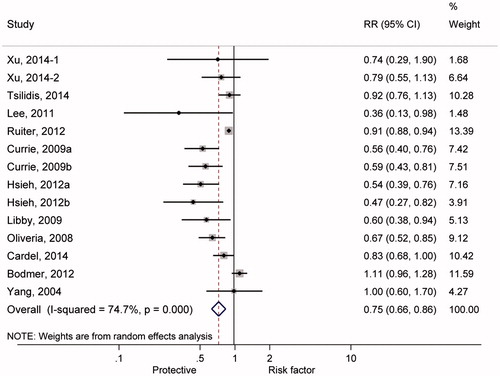
shows the summary RRs of colorectal cancer incidence associated with metformin treatment for T2DM patients. Significant heterogeneity was found across the individual studies enrolled in the meta-analysis (I2 = 74.7%, p < 0.01). Therefore, the effect size was pooled using the random-effects model, and metformin treatment significantly reduced CRC incidence among T2DM patients by 25% (RR =0.75, 95% CI: 0.66-0.86).
Figure 2. Forest plot of pooling estimate of relative risk and 95% confidence interval of colorectal cancer incidence associated with metformin therapy among type 2 diabetic mellitus.
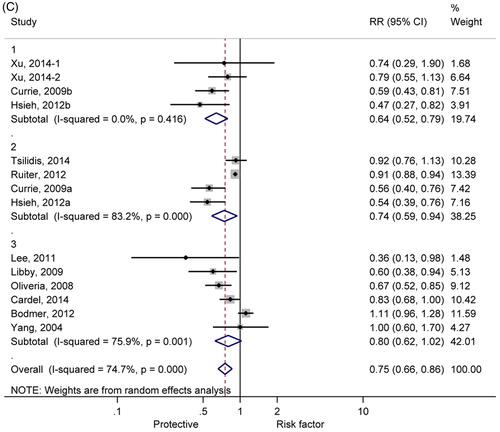
Subgroup analyses are shown in . As shown in , 29, 17 and 49% reduced incidences of CRC were found among diabetic patients of America (RR = 0.71, 95% CI: 0.58-0.86), Europe (RR = 0.83, 95% CI: 0.73-0.95) and China (RR = 0.51, 95% CI: 0.38-0.67), respectively. Moreover, significant heterogeneity appeared among individual studies distributed in Europe (I2 = 74.6%, p< 0.01). shows significant relationship according to the retrospective cohort studies (RR =0.68, 95% CI: 0.57-0.80), but no relationship according to the case-control studies (RR = 0.97, 95% CI: 0.78-1.22), respectively. Additionally, in order to evaluate bias derived from treatment in control, we performed subgroup analysis to compare the CRC incidence in diabetic patients treated with metformin with insulin, sulfonylurea and non-metformin, respectively. Significantly reduced CRC incidence was found in diabetes treated with metformin, compared with other treatments ().
Figure 3. Forest plot of colorectal cancer risk estimates for the relationship between use of metformin and CRC incidence among type 2 diabetic mellitus in different regions, study designs, and other anti-diabetes treatments. (A) different regions (1, America; 2, Europe; 3, China); (B) study design (1, retrospective cohort study design; 2, case-control study design); (C) anti-diabetes treatments (1, metformin versus insulin; 2, metformin versus sulfonylurea; 3, metformin versus non-metformin).
Publication bias
Evidence supported significant publication bias of studies, suggesting by Egger’s test (p = 0.015). Conversely, trim and fill analysis demonstrated that no missing data were found corresponding to the software, suggesting the conclusion basing on the enrolled studies is stable.
Discussion
In the present meta-analysis, metformin was associated with a 25% significantly lower incidence of CRC in T2DM patients compared with no use of metformin. However, significant heterogeneity among individual studies reminded us that the conclusion might be disturbed by other influence factors. Thus, subgroup analysis according to regions, study design and non-metformin treatment was conducted. Reduced CRC incidence was found among diabetic patients in America, Europe and China. However, different results appeared in the subgroup analysis according to retrospective cohort studies and case-control studies. In addition, significantly reduced CRC incidence was also found in diabetes treated with metformin compared with other treatments.
Direct and indirect mechanisms might be associated with CRC occurrence related to metformin treatment. Metabolic syndrome is largely accepted as risk factors for CRC occurrence, including hyperinsulinemia and high glycemic levels (Giovannucci Citation2007; Hsu et al. Citation2007). Metformin plays anti-diabetic roles through targeting adenosine monophosphate activated protein kinase (AMPK) system and acts as a sensor of cellular energy status (Towler & Hardie Citation2007). Moreover, tuberous sclerosis 2 (TSC2), as a tumor suppressor gene, which is phosphorylated by AMPK could protect cells from energy deprivation-induced apoptosis (Inoki et al. Citation2003). In line with the mechanism exploration, the conclusion that metformin is related to the decreased CRC incidence among T2DM patients is also supported by our meta-analysis.
Previous meta-analyses mostly supported reduced CRC risk associated with diabetes treated with metformin (Zhang et al. Citation2011; Soranna et al. Citation2012; Gandini et al. Citation2014). The recent meta-analysis performed by Zhang et al. (Citation2011), including five studies, suggested a 37% reduction (95% CI: 0.50-0.79) of colon cancer incidence, which was consistent with our results. Nonetheless, Decensi et al. (Citation2010) concluded that metformin has no influence on the risk for colon cancer (RR =0.64, 95% CI: 0.38-1.08), which is inconsistent with the results of our study (RR =0.75, 95% CI: 0.66–0.86). The difference might come from the individual studies included in the meta-analysis. Decensi et al. (Citation2010) included three articles in the meta-analysis of colon cancer incidence (Currie et al. Citation2009; Libby et al. Citation2009), which were all included in our study and in addition we also included eight more studies. Meta-analysis for all cancer risk by Gandini et al. (Citation2014) also found an association between metformin and colon cancer (RR=0.80, 95% CI: 0.64–1.00) by including 12 included studies without three new studies in 2014 (Cardel et al. Citation2014; Tsilidis et al. Citation2014; Xu et al. Citation2014), which were included in our study. These results imply the potential effects of metformin against the occurrence of colon cancer in T2DM patients.
In the present meta-analysis, significant heterogeneity was observed among the individual studies. The heterogeneity might come from the populations in different area (America: p = 0.757; Europe: p < 0.001; China: p = 0.722). In addition, from the performance of subgroup analysis, reduced incidence and no significant relationship were calculated among retrospective cohort study and case-control studies, respectively. Thus, we suggest study design would influence the conclusion. There was no heterogeneity (p < 0.001) among the cohort study while significant heterogeneity (p < 0.061) among the case-control study. Follow-up in cohort studies provided us the detail that metformin treatment influenced CRC occurrence, and the ignorance information in case-control studies might weaken the relationship between metformin treatment and CRC incidence. Moreover, different control drugs also introduce heterogeneity. Finally, other factors like the different dosage of drugs may also result in the higher heterogeneity. For instance, in the study of Cardel et al. (Citation2014), the dose of the metformin use was approximately 450 DDD (defined daily dose) while it was 1000 DDD in the study of Lee et al. (Citation2011).
Several limitations during the meta-analysis were encountered. First, the study designed to assess the relationship between metformin use and CRC incidence was based on previous data in the retrospective studies, which might cause bias induced by incomplete information including details on the dose of drug use, duration time for metformin treatment, and other adjunctive therapy. Second, although the basic characteristics have been adjusted in the individual study, the different basic characteristics among the individual studies, such as sex ratio and body max index might contribute to the bias and the heterogeneity in the meta-analysis.
Conclusions
In summary, the meta-analysis supported reduced CRC incidence related to metformin treatment among T2DM, which would be helpful for medicine suggestion for T2DM. However, due to the relatively lower power of the retrospective study, further better-designed study is needed to confirm the potential benefit through enrolling long-term randomized controlled studies.
Disclosure statement
All authors declare that they have no conflict of interests to state.
References
- Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, Bost F. 2010. Metformin in cancer therapy: a new perspective for an old antidiabetic drug? Mol Cancer Ther. 9:1092–1099.
- Bodmer M, Becker C, Meier C, Jick SS, Meier CR. 2012. Use of metformin is not associated with a decreased risk of colorectal cancer: a case-control analysis. Cancer Epidemiol Biomarkers Prev. 21:280–286.
- Cardel M, Jensen SM, Pottegard A, Jorgensen TL, Hallas J. 2014. Long-term use of metformin and colorectal cancer risk in type II diabetics: a population-based case-control study. Cancer Med. 3:1458–1466.
- Chung YW, Han DS, Park KH, Eun CS, Yoo KS, Park CK. 2008. Insulin therapy and colorectal adenoma risk among patients with Type 2 diabetes mellitus: a case-control study in Korea. Dis Colon Rectum. 51:593–597.
- Currie CJ, Poole CD, Gale EA. 2009. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 52:1766–1777.
- Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, Gandini S. 2010. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila). 3:1451–1461.
- Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, Decensi A, Szabo E. 2014. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila). 7:867–885.
- Giovannucci E. 2007. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 86:s836–s842.
- Hardie DG. 2007. AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol. 47:185–210.
- Hsieh MC, Lee TC, Cheng SM, Tu ST, Yen MH, Tseng CH. 2012. The influence of type 2 diabetes and glucose-lowering therapies on cancer risk in the Taiwanese. Exp Diabetes Res. 2012:413782.
- Hsu IR, Kim SP, Kabir M, Bergman RN. 2007. Metabolic syndrome, hyperinsulinemia, and cancer. Am J Clin Nutr. 86:s867–s871.
- Inoki K, Zhu T, Guan KL. 2003. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 115:577–590.
- Kim YI. 1998. Diet, lifestyle, and colorectal cancer: is hyperinsulinemia the missing link? Nutr Rev. 56:275–279.
- Larsson SC, Orsini N, Wolk A. 2005. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 97:1679–1687.
- Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. 2011. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 11:20.
- Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. 2009. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 32:1620–1625.
- Noto H, Goto A, Tsujimoto T, Noda M. 2012. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One. 7:e33411.
- Oliveria SA, Koro CE, Ulcickas Yood M, Sowell M. 2008. Cancer incidence among patients treated with antidiabetic pharmacotherapy. Diabetes Metab Syndr. 2:47–57.
- Ruiter R, Visser LE, van Herk-Sukel MP, Coebergh JW, Haak HR, Geelhoed-Duijvestijn PH, Straus SM, Herings RM, Stricker BH. 2012. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care. 35:119–124.
- Siegel R, Desantis C, Jemal A. 2014. Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
- Soranna D, Scotti L, Zambon A, Bosetti C, Grassi G, Catapano A, La Vecchia C, Mancia G, Corrao G. 2012. Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: a meta-analysis. Oncologist. 17:813–822.
- Towler MC, Hardie DG. 2007. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 100:328–341.
- Tsilidis KK, Capothanassi D, Allen NE, Rizos EC, Lopez DS, van Veldhoven K, Sacerdote C, Ashby D, Vineis P, Tzoulaki I, et al. 2014. Metformin does not affect cancer risk: a cohort study in the U.K. Clinical practice research datalink analyzed like an intention-to-treat trial. Diabetes Care. 37:2522–2532.
- Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. (2011). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses; [cited 2014]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Xu H, Aldrich MC, Chen Q, Liu H, Peterson NB, Dai Q, Levy M, Shah A, Han X, Ruan X, et al. 2014. Validating drug repurposing signals using electronic health records: a case study of metformin associated with reduced cancer mortality. J Am Med Inform Assoc. 22:179–191.
- Yang YX, Hennessy S, Lewis JD. 2004. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 127:1044–1050.
- Zhang ZJ, Zheng ZJ, Kan H, Song Y, Cui W, Zhao G, Kip KE. 2011. Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: a meta-analysis. Diabetes Care. 34:2323–2328.
- Zheng ZX, Zheng RS, Zhang SW, Chen WQ. 2014. Colorectal cancer incidence and mortality in china, 2010. Asian Pac J Cancer Prev. 15:8455–8460.