Abstract
Context: Pycnogenol®, which is French maritime pine bark extract, is a potent antioxidant. It is used in medical conditions caused by oxidative stress. Cisplatin (cis-diamminedichloroplatinum II) is an antineoplastic agent. However, its serious side effects such as ototoxicity limit its usage.
Objective: Antioxidants can be used to prevent ototoxicity. We investigated the effect of Pycnogenol® on cisplatin-induced ototoxicity.
Materials and methods: Rats were randomly assigned to four groups of five. Distortion product-evoked otoacoustic emissions (DPOAE) test was performed for each rat. The experimental groups were as follows: Control Group, Pycnogenol® Group: 10 mg/kg Pycnogenol® intraperitoneally for 7 days, Cisplatin Group: intraperitoneally 15 mg/kg single injection of cisplatin on the fifth day, Cisplatin + Pycnogenol® Group: intraperitoneally 10 mg/kg Pycnogenol® treatment for 7 days, additionally on the fifth day, 15 mg/kg single injection of cisplatin was given. On the eighth day, DPOAE was re-performed and rats were sacrificed. Apoptosis was evaluated histopathologically.
Results: Mean percentage of apoptotic cells was 1.5, 3, 30 and 11% in organ of Corti and 2, 2, 40, 15% in spiral ganglion neurons in Control Group, Pycnogenol® Group, Cisplatin Group and Cisplatin + Pycnogenol® Group, respectively. Cisplatin Group and Cisplatin + Pycnogenol® Group were significantly different when compared to Control Group histopathologically both in organ of Corti and spiral ganglion neuron (p <0.001, p = 0.019, p = 0.001, p = 0.015). DPOAE results showed that Cisplatin + Pycnogenol® Group was significantly different when compared to Cisplatin Group at 3, 6 and 8 kHz (p < 0.05).
Conclusion: Pycnogenol protected against cisplatin ototoxicity. Also, pycnogenol is not ototoxic.
Introduction
Cisplatin (cis-diamminedichloroplatinum II) is a potent antineoplastic agent, widely used for a variety of malignancies (Rabik & Dolan Citation2007). Cisplatin has side effects limiting its usage like ototoxicity, nephrotoxicity and neurotoxicity. Patients treated with cisplatin develop bilateral and progressive hearing loss (Rybak Citation2007). Variations in high-frequency audiometry may be seen in nearly all cases. The severity of hearing loss is related to the dose and frequency of drug administration. The molecular factors in cisplatin ototoxicity are not determined exactly; however, reactive oxygen species (ROS) may play a role. Recent strategies to avoid the ototoxic effect of cisplatin are mostly about ROS (Deavall et al. Citation2012). Numerous studies have been conducted on prevention and restoration of the ototoxicity related to cisplatin (Chirtes & Albu Citation2014; Harrison et al. Citation2015). These studies have suggested that antioxidants prevent ototoxicity. One of these antioxidants is Pycnogenol®, which is a French maritime pine bark extract. It is a mixture of flavonoids and is used in multivitamins, dietary supplements, health products for its high antioxidant activity (Packer et al. Citation1999). Pycnogenol® is used for a wide variety of medical conditions caused by oxidative stress such as nephrotoxicity, diabetes, hepatotoxicity and tinnitus (Yang et al. Citation2008; Parveen et al. Citation2009; Luzzi et al. Citation2014).
To our knowledge, the effect of Pycnogenol® against ototoxicity is currently unknown. In this study, we evaluated the effect of Pycnogenol® on cisplatin-induced ototoxicity and the safety of Pycnogenol® on hearing.
Materials and methods
Experimental design
Male rats 4–6-month-old were taken from Experimental Animal Center of our University. All the experiments were done following the principles of Animal Ethical Committee’s approval (64583101/2013/037).
After a physical examination of the ears on the first day of study, the rats were weighed and randomly assigned to four groups with five rats per group. The distortion product-evoked otoacoustic emissions (DPOAE) test has been performed on each rat. The experimental groups were as follows:
Control Group: The rats in this group were administered 1 mL serum physiologic for 7 days intraperitoneally and served as the healthy animal group.
Pycnogenol® Group: Healthy animals with 10 mg/kg Pycnogenol® (gifted from Horpag Research Ltd., Geneva, Switzerland) treatment intraperitoneally for 7 days, from thefirst day of the study and the subsequent days. The Pycnogenol® dose was decided with a nephrotoxicity research (Parveen et al. Citation2009).
Cisplatin Group: The rats in this group were administered intraperitoneally15 mg/kg single injection of cisplatin (Platinol®, Bristol-Myers Squibb, Istanbul, Turkey) on the fifth day of study.
Cisplatin + Pycnogenol® Group: The rats in this group were administered intraperitoneally 10 mg/kg Pycnogenol® treatment for 7 days, from the first day of the study and the subsequent days. Additionally, on the fifth day of the study 15 mg/kg single injection of cisplatin was given.
On the eighth day of study, under ketamine/xylazine anaesthesia (50 and 5 mg/kg, respectively), the DPOAE test of each rat has been re-performed and rats were sacrificed. The cochlear bone samples were obtained with dissection by an Ear, Nose and Throat specialist. Then, they were fixed in 10% neutral buffered formalin solution.
Auditory assessment
First, the tympanic membranes and external auditory canals of the rats were examined. Rats with external or middle ear problems were excluded. All DPOAE tests were performed in a quiet room following ketamine/xylazine anaesthesia on the first day (before any medication), and they were re-performed on the eighth day. DPOAE test was applied to the left ear of each rat with the Otodynamics Echoport USB cochlear emission analyser, and it was calculated by the Otodynamics ILO software (MAICO MI 34, Berlin, Germany). The stimulus consisted of two pure tones (F1, F2; F1/F2 = 1.22) at 70 dB sound pressure level. DPOAE was considered as positive for signal-noise ratios of 6 dB sound pressure level, as specified by the company.
Histopathological procedures
Samples of inner ear tissues were prepared for light microscopy. Following fixation of the samples by immersion in 10% formalin, phosphate buffer (pH 7.4) for one night, they were dehydrated in increasing concentrations of ethanol (60, 70, 80 and 90% absolute ethanol) and xylene. Then, they were embedded in paraffin blocks. Serial sections (5 μm thick) were obtained from paraffin blocks in Leica RM2255 rotary microtome (Wetzlar, Germany). Deparaffinization, hydrating and staining with haematoxylin–eosin were done. The tissue damage of the inner ear tissue samples was assessed by light microscopy.
TUNEL procedure
All of the samples were stained with TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labelling) method. The apoptosis degree was assessed by the TUNEL procedure with an apoptosis detection kit. The procedure was done with the in situ Cell Death Detection Kit POD (Cat No. S7102, Merck Millipore, Germany) following company’s protocol. Serial 5-μm thick paraffin embedded sections were deparaffinized and rehydrated in graded alcohol. Then, they were washed with distilled water, followed by PBS. Deparaffinized tissue sections were incubated with 20 μg/mL proteinase K for 30 min at 37 °C. Then, the sections were rinsed and incubated with 1× equilibration buffer for 30 min at room temperature. Digoxigenin-labelled deoxynucleotide tail was incubated with terminal deoxynucleotidyl transferase for 60 min at 37 °C. The washing procedure was done in washing buffer for 10 min at room temperature. Next, tissue sections were incubated with anti-digoxigenin-peroxidase antibody for 30 min at room temperature. Then, they were stained with diaminobenzidine. Staining was assessed by light microscopy, following counterstaining with haematoxylin. For each slide, five fields were randomly chosen, and the TUNEL-positive cells were determined per field. Apoptotic index (apoptotic nuclei percentage) was calculated as follows: Apoptotic index = apoptotic nuclei/total nuclei count ×100. All counting evaluations were done blindly.
Statistical analyses
Data was analysed with Statistical Package for the Social Science (SPSS) 19.0 statistical software (SPSS, 10241440, Istanbul, TURKEY). The Wilcoxon signed rank and the Kruskal–Wallis tests were used. The Wilcoxon signed rank test was used to determine the median of the difference between DPOAE values obtained before the treatment and following the treatment. p < 0.05 was considered statistically significant.
Results
Histopathological results
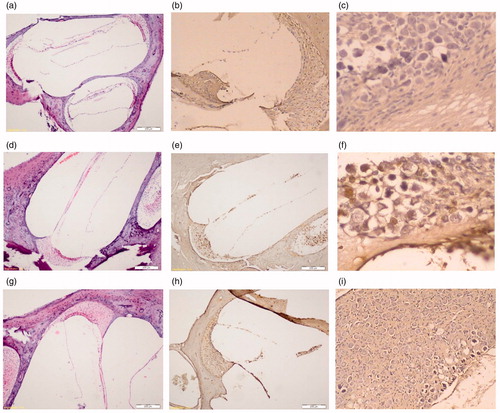
Histopathological analysis showed cisplatin-caused apoptotic cell death in the cochlear nucleus. The mean percentage of apoptotic cells was 1.5, 3, 30, 11% at the organ of Corti, and 2, 2, 40, 15% at the spiral ganglion neurons in Control Group, Pycnogenol® Group, Cisplatin Group and Cisplatin + Pycnogenol® Group, respectively (). The apoptosis ratio in the organ of Corti and the spiral ganglion neurons was significantly higher in Cisplatin Group. Control Group and Pycnogenol® Group had a smaller number of TUNEL-positive cells than the Cisplatin + Pycnogenol® group. The numbers of TUNEL-positive cells in Cisplatin Group and Cisplatin + Pycnogenol® Group were greater.
Table 1. The mean percentage of apoptotic cells for each group.
The TUNEL-positive cells were more in number in the organ of Corti and the spiral ganglion neurons in Cisplatin Group when compared to Cisplatin + Pycnogenol® Group; however, the difference was not statistically significant (p > 0.05). Cisplatin Group and Cisplatin + Pycnogenol® Group were statistically significantly different when compared to Control Group regarding both the organ of Corti and the spiral ganglion neurons (p < 0.001, p = 0.019, p = 0.001, p = 0.015). Additionally, Cisplatin Group was statistically significantly different when compared to Pycnogenol® Group regarding both the organ of Corti and the spiral ganglion neurons (p = 0.016, p = 0.009) (). The other group comparisons did not reveal any statistically significant results (p > 0.05). There was a positive relationship between the organ of Corti and the spiral ganglion neuron concerning TUNEL-positive cells (r = 0.89; p < 0.001).
Figure 1. The apoptosis with TUNEL and haematoxylin–eosin staining in Control Group, Cisplatin Group and Cisplatin + Pycnogenol® Group. (a) Control Group – Corti H&E (b) Control Group – Corti TUNEL (c) Control Group – spiral ganglion TUNEL (d) Cisplatin Group – Corti H&E. (e) Cisplatin Group – Corti TUNEL. (f) Cisplatin Group – spiral ganglion TUNEL (g) Cisplatin + Pycnogenol® Group – Corti H&E. (h) Cisplatin + Pycnogenol® Group – Corti TUNEL. (i) Cisplatin + Pycnogenol® Group – spiral ganglion TUNEL.
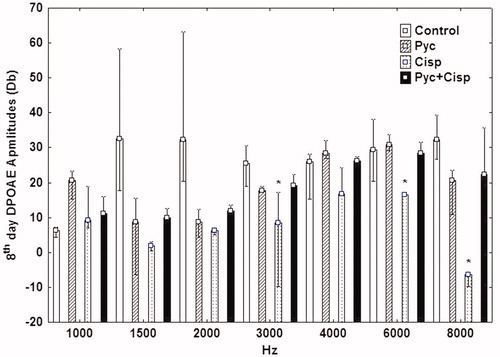
DPOAE test results
Significant statistical differences were recorded for all frequencies except 4 kHz between Cisplatin Group and Control Group at the eighth day DPOAE test (p < 0.05). Pycnogenol® Group and Cisplatin + Pycnogenol® Group had no statistically significantly difference in all frequencies when compared to Control Group at the eighth day DPOAE test (p > 0.05, p > 0.05). Cisplatin + Pycnogenol® Group was statistically significantly different when compared to Cisplatin Group at 3.6 and 8 kHz (p < 0.05), which may suggest that Pycnogenol® has a protective effect against cisplatin-caused ototoxicity ().
Discussion
Cisplatin is a well-known antineoplastic agent. One of the main dose-limiting side effects of cisplatin is ototoxicity, which is seen in 36% of patients receiving cisplatin. Cisplatin damages parts of the cochlea, such as outer hair cell from the organ of Corti, spiral ganglion and stria vascularis (Fetoni et al. Citation2004). Cisplatin creates damage by reactive oxygen radicals (ROS). However, the relationship of ROS and the antineoplastic effect of cisplatin has not been determined yet. Oxidative stress leads to apoptosis and aponecrosis in these parts of the cochlea. This apoptosis is related to caspase-3 activation (Labbé et al. Citation2005). Cisplatin and the cellular DNA make a complex and they avoid the cell cycle. In a study, the destroyed DNA in the spiral ganglion and stria vascularis was identified following administration of 10 mg/kg cisplatin.
However, in the same study, such findings were not present in the organ of Corti (Watanabe et al. Citation2000). In another study, the cisplatin dose was 20 mg/kg and the DNA damage was found in all parts of the cochlea (Alam et al. Citation2000). In our study, the mean percentage of apoptotic cells was the highest in Cisplatin Group (30%) and destruction was detected in all parts of the cochlea. We administered a single injection of cisplatin with a dose of 15 mg/kg. In the group that both cisplatin and Pycnogenol® were administered, we applied cisplatin to the left side and Pycnogenol® to the right side of the abdomen. However, considering that the peritoneum is a single space, we applied the drugs with an interval of nearly 12 h between them to avoid any interaction. Also, the eighth day DPOAE result in Cisplatin Group was statistically significantly different when compared to Control Group regarding all frequencies except 4 kHz. This result also showed the ototoxic effect of cisplatin. In DPOAE evaluation, we used the frequency range of 1–8 kHz. Although we cannot relate the rat ototoxicity to the high-frequency ototoxic effects in human ototoxicity, the deterioration was particularly detected in 6 and 8 kHz in rats (Olgun et al. Citation2014).
The cochlear apoptosis is associated with many pro-apoptotic proteins. Cisplatin ototoxicity seems to be associated with free radical production, which causes consumption of glutathione and antioxidant enzymes in the cochlea and lipid peroxidation. Activities of superoxide dismutase and catalase, together with malondialdehyde levels, were significantly increased in the cochleae of cisplatin-injected rats (Ravi et al. Citation1995).
Some natural antioxidants, such as Vitamin E, Gingko Biloba, pomegranate and resveratrol (Yazici et al. Citation2012; Olgun et al. Citation2014), block the ototoxic effect of cisplatin. The otoprotective effect of resveratrol was observed only in low doses; otherwise, it enhanced the ototoxic impact of cisplatin in higher doses (Olgun et al. Citation2014). However, there is no FDA-approved product for the treatment of cisplatin ototoxicity.
Pycnogenol®, which is a natural polyphenolic compound, is considered as an agent with potent antioxidant and anti-inflammatory properties. It protects against oxidative stress and it is an ROS scavenger (Taner et al. Citation2014). It contains procyanidins, phenolic acids, bioflavonoids and catechins. It is used in a wide variety of medical conditions caused by oxidative stress such as nephrotoxicity, diabetes mellitus, hepatotoxicity and tinnitus (Yang et al. Citation2008; Parveen et al. Citation2009; Luzzi et al. Citation2014). Moreover, it is used in a study of its anti-melanogenic effect, in addition to its antioxidant property (Kim et al. Citation2008). In our study, the TUNEL-positive cells were increased in Cisplatin Group when compared to Cisplatin + Pycnogenol® Group; this has suggested that Pycnogenol® reduced the apoptosis. Also supporting this suggestion, Cisplatin + Pycnogenol® Group was statistically significantly different when compared to Cisplatin Group regarding the eighth day DPOAE tests with 3, 6 and 8 kHz frequencies. The DPOAE results were better in Cisplatin + Pycnogenol® Group when compared to Cisplatin Group. These findings may suggest that Pycnogenol® has a protective effect against cisplatin-caused ototoxicity. Pycnogenol® Group was not statistically significantly different from Control Group. This result showed the safety of Pycnogenol® regarding ototoxicity. Our DPOAE results showed the safety of Pycnogenol®, also. There was no statistically significantly difference between Control Group and Pycnogenol® Group at the eighth day DPOA test regarding all frequencies.
To our knowledge, the otoprotective effect of Pycnogenol® has been studied by only a few researchers (Luzzi et al. Citation2014). In one of these studies, the group of patients who used Pycnogenol® for vertigo and tinnitus symptoms was found to be asymptomatic after 6 months of Pycnogenol® usage and in this group, the cochlear flow velocity was significantly better (Luzzi et al. Citation2014). Pycnogenol® inhibited apoptosis in various studies by suppressing ROS and caspase-3 activation (Siler-Marsiglio et al. Citation2004). Cisplatin increases caspase-3 and ROS within the cochlea (Cho et al. Citation2014; Olgun et al. Citation2014). Pycnogenol® may prevent the ototoxicity by suppressing ROS and caspase-3 activation. More studies are needed to determine the exact molecular pathways of Pycnogenol® during protection from cisplatin ototoxicity. In this study, our limitation was the small sample number. However, since similar sample number had been used in the study in which the effect of Pycnogenol® in dermatotoxicity was investigated, we preferred this number due to the high cost of animals (Lee et al. Citation2012). This small sample size might have affected the DPOAE data and the results of the statistical analysis; therefore, further studies are needed to evaluate the beneficial effects of Pycnogenol® in ototoxicity.
Conclusion
Our results suggested that Pycnogenol® has a protective role against cisplatin ototoxicity; Pycnogenol® protected against cisplatin-caused cochlear apoptosis. Additionally, our findings showed that Pycnogenol® is a safe product, which is not ototoxic.
Acknowledgements
We thank Horpag Research Ltd. for gifting us Pycnogenol®.
Disclosure statement
The research received no specific grant from any funding agency. All authors declare that there is no conflict of interest.
References
- Alam SA, Ikeda K, Oshima T, Suzuki M, Kawase T, Kikuchi T, Takasaka T. 2000. Cisplatin-induced apoptotic cell death in Mongolian gerbil cochlea. Hear Res. 141:28–38.
- Chirtes F, Albu S. 2014. Prevention and restoration of hearing loss associated with the use of cisplatin. Biomed Res Int. 2014:925485.
- Cho SI, Lee JH, Park JH, Do NY. 2014. Protective effect of (−)-epigallocatechin-3-gallate against cisplatin-induced ototoxicity. J Laryngol Otol. 15:1–6.
- Deavall DG, Martin EA, Horner JM, Roberts R. 2012. Drug-induced oxidative stress and toxicity. J Toxicol. 2012:645460.
- Fetoni AR, Quaranta N, Marchese R, Cadoni G, Paludetti G, Sergi B. 2004. The protective role of tiopronin in cisplatin ototoxicity in Wistar rats. Int J Audiol. 43:465–470.
- Harrison RT, DeBacker JR, Bielefeld EC. 2015. A low-dose regimen of cisplatin before high-dose cisplatin potentiates ototoxicity. Laryngoscope 125:E78–E83.
- Kim YJ, Kang KS, Yokozawa T. 2008. The anti-melanogenic effect of pycnogenol by its anti-oxidative actions. Food Chem Toxicol. 46:2466–2471.
- Labbé D, Teranishi M, Hess A, Bloch W, Michel O. 2005. Activation of caspase-3 is associated with oxidative stress in the hydropic guinea pig cochlea. Hear Res. 202:21–27.
- Lee IC, Kim SH, Shin IS, Moon C, Park SH, Kim SH, Park SC, Kim HC, Kim JC. 2012. Protective effects of pine bark extract on hexavalent chromium-induced dermatotoxicity in rats. Phytother Res. 26:1534–1540.
- Luzzi R, Belcaro G, Hu S, Dugall M, Hosoi M, Cacchio M, Ippolito E, Corsi M. 2014. Improvement in symptoms and cochlear flow with pycnogenol in patients with Meniere’s disease and tinnitus. Minerva Med. 105:245–254.
- Olgun Y, Kırkım G, Kolatan E, Kiray M, Bagriyanik A, Olgun A, Kizmazoglu DC, Elidokuz H, Serbetcioglu B, Altun Z, et al. 2014. Friend or foe? Effect of oral resveratrol on cisplatin ototoxicity. Laryngoscope. 124:760–766.
- Packer L, Rimbach G, Virgili F. 1999. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritima) bark, Pycnogenol. Free Radic Biol Med. 27:704–724.
- Parveen K, Khan MR, Siddiqui WA. 2009. Pycnogenol prevents potassium dichromate K2Cr2O7-induced oxidative damage and nephrotoxicity in rats. Chem Biol Interact. 18:343–350.
- Rabik CA, Dolan ME. 2007. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 33:9–23.
- Ravi R, Somani SM, Rybak LP. 1995. Mechanism of cisplatin ototoxicity: antioxidant system. Pharmacol Toxicol. 76:386–394.
- Rybak LP. 2007. Mechanisms of cisplatin ototoxicity and progress in otoprotection. Curr Opin Otolaryngol Head Neck Surg. 15:364–369.
- Siler-Marsiglio KI, Shaw G, Heaton MB. 2004. Pycnogenol and vitamin E inhibit ethanol-induced apoptosis in rat cerebellar granule cells. J Neurobiol. 59:261–271.
- Taner G, Aydın S, Bacanlı M, Sarigol Z, Sahin T, Basaran AA, Basaran N. 2014. Modulating effects of pycnogenol® on oxidative stress and DNA damage induced by sepsis in rats. Phytother Res. 28:1692–1700.
- Watanabe K, Hess A, Michel O, Yagi T. 2000. Nitric oxide synthase inhibitor reduces the apoptotic change in the cisplatin-treated cochlea of guinea pigs. Anti-Cancer Drugs. 11:731–735.
- Yang YS, Ahn TH, Lee JC, Moon CJ, Kim SH, Jun W, Park SC, Kim HC, Kim JC. 2008. Protective effects of Pycnogenol on carbon tetrachloride-induced hepatotoxicity in Sprague-Dawley rats. Food Chem Toxicol. 46:380–387.
- Yazici ZM, Meric A, Midi A, Arinc YV, Kahya V, Hafiz G. 2012. Reduction of cisplatin ototoxicity in rats by oral administration of pomegranate extract. Eur Arch Otorhinolaryngol. 269:45–52.