Abstract
Context: Various studies have shown that the leaf extracts of Spondias mombin Linn (Anacardiaceae) possess pharmacological properties such as antioxidant and antiviral effects. However, no biological activity from its essential oil has been reported in literature.
Objective: To analyse the chemical constituents, cytotoxic activity and antioxidant capability of the essential oils from fresh and dried leaves of S. mombin.
Materials and methods: Hydrodistillation using Clevenger-type apparatus was employed to obtain the essential oil. Oil analysis was performed using an HP 6890 Gas Chromatograph coupled with an HP 5973 Mass Selective Detector. The cytotoxicity bioassay was carried out using the brine shrimp lethality test (10,000–0.01 μg/mL). Additionally, the reactive oxygen species scavenging potential of the two S. mombin oils (1000–200 μg/mL) were investigated using a hydroxyl radical scavenging and ferric iron reducing system.
Results: Chemical analysis of essential oils from S. mombin revealed the presence of 41 compounds, with predominance of monoterpenoids, sesquiterpenoids and non-terpenoids derivatives. In both fractions, the principal component was β-caryophellene (27.9–30.9%), followed by γ-cadinene (9.7–12.3%). There was an increase in the oxygenated monoterpenoid contents and a concomitant decrease in the amounts of sesquiterpenoids hydrocarbons observed on drying the leaves. The oil obtained from the fresh leaves was more active than that obtained from dried leaves, with LC50 values (from the brine shrimp lethality assay) of 0.01 and 4.78 μg/mL, respectively. The two oils (from fresh and dried leaves) at 1.0 mg/mL scavenged hydroxyl radical by 83% and 99.8%, respectively. Moreover, they reduced ferric ion significantly and compared favourably with vitamin C.
Conclusions: Essential oil derived from the leaves of S. mombin could hold promise for future application in the treatment of cancer-related diseases.
Introduction
Essential oils extracted through hydrodistillation from aromatic and medicinal plants are well known to possess therapeutic properties (Baratta et al. Citation1998; Bozin et al. Citation2006; Mukazayire et al. Citation2011). Their usage is influenced by the nature of their constituents, which has a widespread application in the pharmaceutical, agricultural, flavour and fragrance industries.
Spondias mombin Linn (Anacardiaceae) is a fructiferous tree found in Nigeria, Brazil, India, Sri Lanka and other tropical forests in the world. The fruit usually yellow when ripe has a leathery skin and a thin layer of pulp with exotic taste. It is known as ‘Isada’ among the Hausas of the North-central Nigeria. Other common names are Iyeye (Yoruba), ngulungwu (Igbo), pauda tapera (Brazil) and ubo (Peru). The concoction from the leaves is widely used for the treatment of diarrhea, dysentery, stomach ache and inflammation (Ayoka et al. Citation2008). Various studies have shown that the leaf extracts possess pharmacological properties such as anti-inflammatory, antimalaria, antibacterial, anxiolytic, antioxidant and antiviral effects (Ayoka et al. Citation2008). Despite the ethnobotanical usage of the plants, no biological activity from its essential oil has been reported in literature to support this claim. Herein, we report a comparison of constituents between the fresh and air-dried leaf oils of S. mombin, cytotoxic activity and their antioxidant capability for the first time.
Materials and methods
Chemicals used
All analytical grade chemicals and standard drug used were supplied by Sigma-Aldrich Inc., St. Louis, MO.
Plant material
Fresh leaves of S. mombin were collected in September 2012 from Offa, North-central zone of Nigeria. The plant was identified and authenticated by Mr. Bolu S. Ajayi of Herbarium section, Plant Biology Department, University of Ilorin, Nigeria. A voucher specimen (UIL: 106132) was deposited in the herbarium of the university.
Isolation of the leaf oil
Fresh and air-dried leaves (500 g each) were separately hydrodistilled using distilled water (1000 mL) for 3 h in an all glass apparatus constructed to the specifications of the British Pharmacopoeia using hexane as the collecting solvent. The essential oils collected were separated from water by drying with anhydrous sodium sulphate and stored in well-capped bottles at 4 °C prior to analysis.
Cytotoxicity assay
The cytotoxicity bioassay was carried out using the brine shrimp lethality test (BST) protocol of McLaughlin and Rogers (Citation1998). The brine shrimp (Artemia salina Leach) eggs were hatched in sea water for 48 h at room temperature. Solution of the essential oil was made in DMSO, at various concentrations (10,000, 1000, 100, 10, 1, 0.1, 0.01 μg/mL) and incubated in triplicates test tubes with the 10 brine shrimp larvae per test tube (30 shrimp per concentration). Control brine shrimp larvae were placed in a mixture of sea water and DMSO only. The setup was left for 24 h, after which the average number of larvae that survived in each test tube was determined. The probit analysis to determine the LC50 values of 95% confidence intervals for statistically significant comparison of potencies were calculated using a computer Finney program.
Hydroxyl radical (OH•−) scavenging assay
The OH•− scavenging activity of S. mombin oils was measured as described previously by Smirnoff and Cumbes (Citation1989). Briefly, 2 mL of essential oil extract at various concentrations (200–1000 μg/mL), 0.6 mL of 8 mM ferrous sulphate, 0.5 mL of 20 mM H2O2 and 2 mL of 3 mM salicylic acid were mixed and incubated at 37 °C for 30 min. Thereafter, 0.9 mL of distilled water was added to each vial. The final solution was centrifuged at 10,000 rpm for 10 min after which the absorbance was read at 510 nm. The percentage OH•− scavenging activity of essential oil extract was calculated by using the formula:
where Acontrol is the absorbance of the mixture without oil, Asample is the absorbance of the mixture with oil and Aextract is the absorbance of the oil alone.
Reducing power
The reducing power of essential oil extract was determined using the procedure described by Oyaizu (Citation1986). Briefly, 2.5 mL of 1% potassium hexacyanoferrate [K3Fe(CN)6], 2.5 μL of 0.2 M phosphate buffer (pH 6.6) and various concentrations (200–1000 μg/mL) of essential oil extract suspended in 1 mL of distilled water and incubated at 50 °C for 20 min. Thereafter, 2.5 μL of trichloroacetic acid was added to the mixture. This was centrifuged at 400 rpm for 10 min after which 2.5 μL of the supernatant was mixed with an equal amount of distilled water and 0.5 mL of 0.1% FeCl3. Absorbance of the resulting solution was read at 700 nm.
Gas chromatography–mass spectrometry
Oil analyses were performed using an HP 6890 coupled with an HP 5973 mass selective detector. Separation was carried out with an HP 5MS column (30 m × 0.25 mm ×0.25 μm). GC operating conditions were as follows: injector: split ratio 20:1; carrier gas: hydrogen, at a flow rate of 1.0 mL/min; temperature: 250 °C. The GC oven temperature was kept at 40 °C and programmed to reach 200 °C at a rate of 5 °C/min, then kept constant at 220 °C for 2 min. The inlet temperature was set at 150 °C. Mass spectra were acquired at 70 eV with mass range 50–300 m/z. The data are reported as mean value of two injections and analysed using HP ChemStation software (Karachi, Pakistan).
Identification of constituents
The compounds were identified by comparing the retention times and mass spectra of the chromatographic peaks with those of standards analysed under the same conditions. The assignment of the peaks of other volatile constituents was based on computer matching of the mass spectra obtained with the WILEY 275, NIST and ADAMS libraries, taking to accounts the coherence of the retention indices of the analysed compounds with those reported by Adams and NIST08 libraries.
Statistical analysis
All experimental data were expressed as means ± SD (n = 3). Two-way analysis of variance was used and p< 0.05 was considered statistically significant.
Results
Hydrodistillation of fresh and air-dried leaves of S. mombin yielded greenish oil with lemon aroma. The yields of fresh and dried leaves were 0.24% (v/w) and 0.35% (v/w), respectively. A total of 41 compounds were identified from the fresh and dried leaf oils, representing 100% of the oil. Constituents were listed in order of elution from HP-5 capillary column (). The major components in the fresh leave oil were β-caryophellene (27.9%), γ-cadinene (12.3%), α-humulene (8.1%), β-cadinene (7.8%), caryophyllene oxide (6.9%), 5-isocedranol (6.4%), α-gurjenene (6.4%), neral (6.2%), α-muurolene (5.9%), β-elemene (4.2%), γ-muurolene (4.0%) and geranial (3.7%). In the dried leaf oil, 41 compounds were also identified. The major constituents in the dried leave oil were β-caryophellene (30.9%), γ-cadinene (9.7%), 5-isocedranol (9.5%), neral (9.4%), α-gurjenene (7.4%), β-cadinene (6.6%), caryophyllene oxide (6.2%), α-humulene (5.4%), α-muurolene (4.2%), β-elemene (3.2%) and geranial (3.8%). Other constituents present were less than 3% in amounts.
Table 1. Percentage composition of essential oils of fresh and dried leaves of S. mombin.
The two oils were analysed with the BST to determine their relative potencies. The two oil fractions examined were highly active, with LC50 values of 0.01 and 4.78 μg/mL from fresh and dried leaves, respectively.
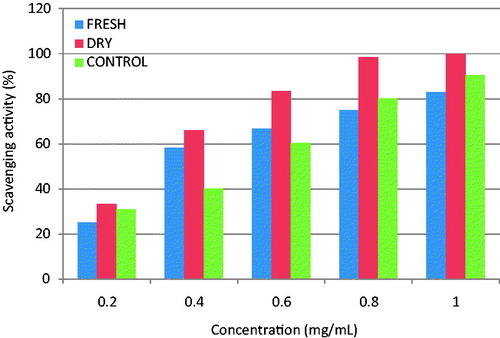
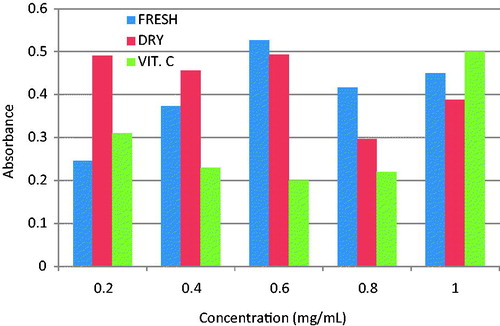
The S. mombin’s volatile oils (fresh and dried leaves) dependently scavenged OH•−, with the highest concentration (1 mg/L) in each case producing 83% and 99.8% scavenging effects, respectively (). This was similar to reference antioxidant (vitamin C) used in this study. The capability of the two oils to reduce K3Fe(CN)6 significantly (p < 0.005) shows its effectiveness to halt the oxidation of cellular macromolecules by oxidizing molecules that could arise from the metabolism of either drugs or toxins (Ajiboye et al. Citation2013). Also, the reducing effect of the oils was similar to that produced by vitamin C (), but the greatest activity was observed in fresh leaf oil.
Discussion
A comparative analysis of volatile chemical profiles of the fresh and dried leaves of S. mombin showed no significant change in the constituents of the essential oils (). β-Caryophellene (30.9% and 27.9%, respectively) was found to be most abundant in S. mombin oil. This result agrees with those of other studies, for which β-caryophellene was found as the main component of dried leaves oil (Moronkola et al. Citation2003; Ogunwande et al. Citation2012). However, β-elemene and nerol were not reported in the previous study and 2-pentyl furan, o-cymene, p-cymene, valencene and abiraterone were not detected in this study. The variation in the composition could be attributed to the source, cultivation, vegetative stage and growing season of the plant under investigation (Sari et al. Citation2006; Smith et al. Citation2010).
Generally, there was an increase in the oxygenated monoterpenoid contents and a concomitant decrease in the amounts of sesquiterpenoids hydrocarbons was observed on drying the leaves. About a quarter of the amount of γ-cadinene and α-humulene was lost through drying and similar trends were observed for β-cadinene, α-muurolene and caryophyllene oxide. Percentage composition of neral, 5-isocedranol and bicyclogermacrene increased (about 30%) drastically after air drying; and smaller increments were recorded in β-caryophellene, geranial and α-gurjenene. The contents of β-bisabolene, geraniol, eugenol, borneol, β-selinene, torreyol, α- selinene, α-copane, α-thujene, γ-terpene and terpinen-4-ol were stable in the two oils, though they were present in small amounts (<1%). The occurrence of geranial and neral in appreciable quantities (>3.7%) in the two oils could be responsible for the lemon aroma (Ekundayo et al. Citation1988).
A positive correlation between brine shrimp toxicity and 9KB (human epidermoid carcinoma of nasopharynx) cytotoxicity (p = 0.036 and kappa=0.56) has been established, sparing the need for higher animals or their serum; and many novel antitumour and pesticide natural products have been discovered using this bioassay (BST) (Anderson et al. Citation1988; McLaughlin & Rogers Citation1998). The two oil fractions examined were highly active, though oils obtained from fresh leaves yielded the most potent fraction with LC50 value as low as 0.01 μg/mL, compared to oils obtained from dried leaves with LC50 value of 4.78 μg/mL. The significant disparity in their potency could be as a result of higher percentage composition of γ-cadinene, α-humulene β-cadinene, α-muurolene and caryophyllene oxide present in oils from fresh leaves. However, plants having LC50 values greater 1000 μg/mL are considered inactive. Plants having LC50 values less than 200 μg/mL in case of extracts and 5 μg/mL in case of pure compounds are considered as highly active (Anderson et al. Citation1988; Tawaha Citation2006). Various reports showed that the major constituents in the oils such as β-caryophellene, α-humulene, caryophyllene oxide and β-elemene exhibited cytotoxic activity against different cell lines. Their potency could be as a result of higher percentage of β-caryophellene and α-humulene present in the fractions, since (E)-β-caryophellene and α-humulene have been reported to be cytotoxic against several human cancer cell lines, such as Hela, MCF-7, MDA-MB-468, UACC-257, DLD-1, A549 and HT-29 (Cole et al. Citation2007; Legault & Pichette Citation2007; Silva et al. Citation2008; Su & Ho Citation2013). Owolabi et al. (Citation2013) reported in vitro cytotoxicity activity of (E)-β-caryophellene, caryophyllene oxide and α-humulene obtained from A. muricana leaf oil against MCF-7 cells with LC50 values of 38.8, 23.0 and 22.1 μg/mL. Also, the role of (E)-β-caryophellene has been demonstrated in flora defence against pathogens and repellent activities against leaf-cutting ants and termites (Hubbell et al. Citation1983; Messer et al. Citation1990; Huang et al. Citation2012). The fresh leaves oils (LC50 = 0.01 μg/mL) showed more potency than the standardized pesticidal extracts obtained from small twigs of pawpaw tree (LC50 = 0.04 μg/mL) reported by McLaughlin and Rogers (Citation1998). Given these reported activities, the oils could be employed as safe, effective, economical and environmentally friendly pesticides for home garden, ornamental and green-house.
Antioxidants are defined as compounds that can delay, inhibit or prevent the oxidation of oxidizable materials by scavenging free radicals and diminishing oxidative stress. Oxidative stress is associated with the development of chronic degenerative diseases, such as coronary heart disease, cancer and aging (Moure et al. Citation2001). The results in this study also confirmed that some volatile oils are good natural antioxidants (Foti & Ingold Citation2003; Bozin et al. Citation2006; Proestos et al. Citation2013).
Conclusion
It is evident from the data obtained that there is variation in S. mombin volatile oils (of fresh and dried leaves) composition, which could be responsible for difference in their bioactivities. The oils (particularly the fresh leaf oil) could be a good source of natural pesticides and antitumour agents in the area of drug discovery and formulation. Also, the oils show good antioxidant potential. This may be useful in the food industry as well as medicine in preventing lipid peroxidation and oxidative stress.
Acknowledgements
We gratefully acknowledge Dr. I. A. Oladosu (Chemistry Department, University of Ibadan, Nigeria) for his technical assistance. We also thank Prof. S. A. Lawani and Mrs. Oladimeji, Y. Yusrah for proof-reading and type-setting, respectively. Third World Academy of Science (TWAS) and International Centre for Chemical and Biological Sciences are thanked for Postgraduate Fellowship support to A. O. Oladimeji.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Ajiboye TO, Raji HO, Muritala HF, Ojewuyi OB, Yakubu MT. 2013. Anthocyanin extract of Lannea microcarpa fruits stall oxidative rout associated with aflatoxin B1 hepatocarcinogenesis. Food Biosci. 4:58–67.
- Anderson JE, Chang CJ, McLaughlin JL. 1988. Bioactive components of Allamanda schottii. J Nat Prod. 51:307–308.
- Ayoka AO, Akomolafe RO, Akinsomisoye OS, Ukponmwan OE. 2008. Medicinal and economic value of Spondias mombin. Afr J Biomed Res. 11:129–136.
- Baratta MT, Dorman HJD, Deans SG, Figueiredo AC, Barroso JG, Ruberto G. 1998. Antimicrobial and antioxidant properties of some commercial essential oils. Flav Frag J. 13:235–244.
- Bozin B, Mimica-Dukic N, Simin N, Anackov G. 2006. Characterization of the volatile composition of essential oils of some lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J Agric Food Chem. 54:1822–1828.
- Cole RA, Haber WA, Setzer WN. 2007. Chemical composition of essential oils of seven species of Eugenia from Monteverde, Costa Rica. Biochem Syst Ecol. 35:877–886.
- Ekundayo O, Laakso I, Hiltunen R. 1988. Composition of ginger (Zingiber officinale Roscoe) volatile oils from Nigeria. Flav Frag J. 3:85–90.
- Foti MC, Ingold KU. 2003. Unexpected superoxide dismutase antioxidant activity of ferric chloride in acetonitrile. J Org Chem. 68:9162–9165.
- Huang M, Sanchez-moreiras AM, Abel C, Sohrabi R, Lee S, Gershenzon J, Tholl D. 2012. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytologist. 193:997–1008.
- Hubbell SP, Wiemer DF, Adejare A. 1983. An antifungal terpenoid defends a neotropical tree (Hymenaea) against attack by fungus-growing ants (Atta). Oecologia. 60:321–327.
- Legault J, Pichette A. 2007. Potentiating effect of beta-caryophyllene on anticancer activity of alpha-humulene, isocaryophyllene and paclitaxel. J Pharmacy Pharmacol. 59:1643–1647.
- McLaughlin JL, Rogers LL. 1998. The use of biological assays to evaluate botanicals. Drug Inf J. 32:513–524.
- Messer A, McCormick K, Sunjaya H, Tumbel F, Meinwald J. 1990. Defensive role of tropical tree resins: antitermitic sesquiterpenes from Southeast Asian Dipterocarpaceae. J Chem Ecol. 16:3333–3352.
- Moronkola DO, Adeleke AK, Ekundayo O. 2003. Constituents of the Spondias mombin Linn and the comparison between its fruit and leaf essential oils. J Essent Oil Bear Plants. 6:148–152.
- Moure A, Cruz JM, Franco D, Manuel DJ, Sineiro J, Domínguez H, Núñez MJ, Carlos PJ. 2001. Natural antioxidants from residual sources. Food Chem. 72:145–171.
- Mukazayire MJ, Tomani JC, Stevigny C, Chalchat JC, Conforti F, Menichini F, Duez P. 2011. Essential oils of four Rwandese hepatoprotective herbs: gas chromatography–mass spectrometry analysis and antioxidant activities. Food Chem. 129:753–760.
- Ogunwande IA, Eresanya O, Avoseh NO, Oyegoke T, Ogunmoye AO, Flamini G. 2012. Chemical composition of essential oils from Nigeria plants. Der Chem Sin. 3:279–286.
- Owolabi MS, Ogundajo AL, Dosoky NS, Setzer WN. 2013. The cytotoxic activity of Annona muricata leaf oil from Badagary, Nigeria. Am J Essent Oil Nat Prod. 1:1–3.
- Oyaizu M. 1986. Studies on products of browning reaction: antioxidative activities of product of browning reaction prepared from glucosamine. Jpn J Nutr. 44:307–315.
- Proestos C, Lytoudi K, Mavromelanidou O, Zoumpoulakis P, Sinanoglou V. 2013. Antioxidant capacity of selected plant extracts and their essential oils. Antioxidants (Basel). 2:11–22.
- Sari M, Biondi DM, Kaabeche M, Mandalari G, D'Arrigo M, Bisignano G, Saija A, Daquino C, Ruberto G. 2006. Chemical composition, antimicrobial and antioxidant activities of the essential oil of several populations of Algerian Origanum glandulosum Desf. Flav Frag J. 21:890–898.
- Silva SL, Chaar JS, Figueiredo PS, Yano T. 2008. Cytotoxic evaluation of essential oil from Casearia sylvestris Sw on human cancer cells and erythrocytes. Acta Amaz. 38:107–112.
- Smirnoff N, Cumbes QJ. 1989. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 28:1057–1060.
- Smith J, Tucker D, Alter D, Watson K, Jones G. 2010. Phytochemistry intraspecific variation in essential oil composition of Eremophila longifolia F. Muell. (Myoporaceae): evidence for three chemotypes. Phytochemistry. 71:1521–1527.
- Su YC, Ho CL. 2013. Composition and in-vitro cytotoxic activities of the leaf essential oil of Beilschmiedia erythrophloia from Taiwan. Nat Prod Commun. 8:143–144.
- Tawaha KA. 2006. Cytotoxicity evaluation of Jordanian wild plants using brine shrimp lethality test. Jordanian J Appl Sci. 8:12–7.