Abstract
Context: Crataegus monogyna Jacq L. (Lind.) (Rosaceae) is used in folk medicine as a sedative, aerial parts being rich in polyphenols with antioxidant ability.
Objectives: To study the variation in polyphenolic composition and antioxidant ability of spontaneous samples of C. monogyna in order to assess the relationship among these variables.
Materials and methods: Aerial parts of C. monogyna were collected from nine different locations in central Spain and extracted with methanol after drying. Total polyphenols were determined by the Folin-Ciocalteu method using gallic acid (GA) as standard. Sixteen polyphenolic compounds (11 flavonoids and 5 phenolic acids) were identified and quantified by reversed-phase HPLC in one single analysis. The antioxidant ability was evaluated by the oxygen radical absorbance capacity (ORAC) and the free radical scavenging activity (DPPH) methods. Linear correlation analysis was used to explore the relationships between the studied variables.
Results: Total polyphenol content ranged between 117.729 ± 0.011 and 204.286 ± 0.015 mg GAE/g extract, depending on the geographic origin. No relationship was found between total polyphenols and antioxidant ability by the ORAC or DPPH methods. Chromatographic analysis yielded lower amounts of polyphenols (23.3–143.26 mg/kg), as only flavonoids and phenolic acids were quantified. All the samples exhibited antioxidant activity between 1.32 ± 0.08 and 2.76 ± 0.007 μmol Trolox equivalents/mg and IC50 from 0.82 ± 0.10 to 3.76 ± 0.67 μg/mL.
Conclusion: A statistically significant relationship between flavonoids and phenolic acids content and the antioxidant potential obtained by the ORAC method for C. monogyna samples was proven.
Introduction
Crataegus monogyna Jacq. (Lindm.) (Rosaceae) is a shrub that naturally grows in Western and Centre Europe, the Middle East, Caucasus, Anatolia and North-Western Africa. In the Iberian Peninsule, it is widely distributed along the whole territory, Majorca and Minorca islands. Hawthorn leaf and flower are used in folk medicine since Ancient times for the treatment of nervous cardiac function disorders, to regulate blood pressure, palpitations, irritability and exhaustion. Crataegus is mentioned for the first time in the first century by Dioscorides as a ‘cardiotonic’ remedy (Kaul Citation1998). The use of hawthorn in therapeutics is also documented in America since the nineteenth century to treat different heart diseases, angina pectoris, to support the effect of Digitalis, or in cases of irregular heartbeat when Digitalis was not tolerated. Its use as a tonic preparation for the heart and to regulate circulation was mentioned. It was also used to treat climacteric inconveniences, arteriosclerosis and cardiac neuroses of dyspeptics (Madaus Citation1976).
Nowadays, traditional use in the European Union is recognized for C. monogyna for two different therapeutic indications: to relieve symptoms of temporary nervous cardiac complaints (e.g. palpitations, perceived extra heart beat due to mild anxiety) and for the relief of mild symptoms of mental stress and to aid sleep.
Main chemical constituents of the leaves and flowers are flavonoids (flavones and flavonols) mainly in form of glycosides (e.g. vitexin, vitexin-2″-rhamnoside, isovitexin, hyperoside, quercetin), flavan compounds [e.g., (+)-catechin, (−)-epicatechin, oligo- and polymeric procyanidins], triterpenic acids (e.g., crataegolic acid, urolic acid, oleanic acid), amines (e.g. phenethylamine, acetylcoline, ethylamine) and organic acids (e.g. caffeic acid, chlorogenic acid). Among them, flavone-C-glycosides and oligomeric procyanidines may represent the main effective constituents (Chang et al. Citation2002; Bradley Citation2006; Edwards et al. Citation2012; Sendker et al. Citation2013).
Pharmacological studies with C. monogyna proved a positive inotropic effect, an increase in coronary blood flow, an antiarrhythmic effect, a cardioprotective effect on the ischaemic-reperfused heart, preventing development of cardiac hypertrophy induced by primary or secondary hypertension, prolongation of the effective refractory period and an increase in myocardial action, these activities mediated by flavonoids and related to the inhibition of the Na+/K+ adenosine triphosphatase (Na+/K+ pump); catecholamine-like cAMP-dependent effects; inhibition of the phosphodiesterase, among others. Hawthorn has been found to inhibit the Na+/K+ adenosine triphosphatase, which in turn indirectly hampers the activity of the Na+/Ca2+ antiport leading to increased intracellular calcium levels and the positive inotropic effect (Momekov & Benbassat Citation2013).
Oxidative reactions are physiological processes aimed to release different substances which are needed in cellular metabolism. These reactions involve the transfer of electrons and may generate compounds known as reactive oxygen species (ROS), among which there are the free radicals (FRs) and oxygen derived molecules with high reactivity. Oxidative stress is defined as the imbalance between the production of FRs and the ability of a biological system to quickly detoxify the reactive intermediates and repair the damage caused at protein, lipid and DNA levels (Darvesh et al. Citation2010).
Antioxidant compounds are defined as those which at low concentration are capable of preventing oxidative damage mediated by FRs. This activity can be achieved by direct uptake of ROS, modulation of enzyme activity or chelating metal ions (Fe3+, Cu+), among others (Leopoldini et al. Citation2004). Some antioxidants show a protein structure, while others are smaller molecules. During the last years, several studies shown that phenolic compounds such as flavonoids exert broad pharmacological effects such as antiproliferative, antitumour, anti-inflammatory, apoptosis-inducing and antioxidant activity, which provide important health benefits related to metabolic syndrome, cancer, brain health and immune system (Gómez-Serranillos & Palomino Citation2012).
In this study, aerial parts of nine samples from spontaneous population of C. monogyna were collected in central Spain, in order to study their phenolic composition variability and antioxidant activity with the aim of assessing the influence of the geographic origin in the polyphenol profile and content and the possible relationship with their antioxidant ability.
Materials and methods
Plant material
Aerial parts of nine samples of C. monogyna spontaneously growing in Spain were harvested during flowering in May 2011 (). Samples were identified by the Department of Aromatic and Medicinal Plants Research, National Institute of Agricultural and Food Technology (INIA). A voucher specimen was deposited for internal control at the INIA (Madrid, Spain). Samples were dried in an oven at 35 °C, grind down and sieved through a 2-mm mesh, and kept protected from light and moisture until use.
Table 1. Geographic location and extraction yield of the wild samples of C. monogyna in Spain.
Extraction process
Each sample (60 mg) was extracted with 20 mL methanol for 1 h, under shaking. The suspension was then filtered through one filter paper; 10 mL methanol was added to the sample and filtered again over the first methanol extract. The extract was left for overnight to dry. Dried samples of each extract were weighed to determine the yield of soluble constituents and stored at 5 °C and protected from light until use.
Reagents
Standards of rutin, quercetin, luteolin, kaempferol, myricetin, hesperidin, isovitexin, hesperetin, quercitrin, apigenin, apigenin-7-glucoside, arbutin and 3,5-dihydroxy-benzoic, isovanillic, gentisic, chlorogenic and ursolic acids of HPLC grade were purchased from Extrasynthese (Genay, France).
Fluorescein (3′,6′-dihydroxyspiro [isobenzofuran-1[3H],9′[9H]-xanthen]-3-one), AAPH (2,2′-azobis (2-amidinopropane)dihydrochloride), Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), 2,2-diphenyl-1-picrylhydrazyl (DPPH), Folin-Ciocalteu’s phenol reagent were from Sigma-Aldrich (Barcelona, Spain).
Polyphenol quantification, antioxidant capacity and oxygen scavenging activity
Total polyphenol content was determined by the spectrophotometric method of Folin-Ciocalteu (Montreau Citation1972) using gallic acid (GA) as standard with small modifications. A UVIKON 930 Kontron instruments spectrophotometer (Bedfordshire, UK) was used. Briefly, 0.5 mL solution of each sample (concentration 1 mg/mL) was mixed with 0.5 mL Folin-Ciocalteu reagent; after 3 min, a saturated sodium carbonate reagent (75 g/L) was added to the solution.
The mixture was then incubated for 1 h at room temperature protected from light, and the absorbance was measured at 760 nm. A standard curve was obtained for GA and results were expressed as mg of gallic acid equivalents (GAE)/mg dry weight (DW).
The antioxidant capacity was evaluated in the C. monogyna methanol extracts by the oxygen radical absorbance capacity (ORAC) method (Dávalos et al. Citation2004). Briefly, sample of Trolox was mixed with fluorescein in a 96-multiwell plate and the AAPH added. AAPH was used to generate peroxyl radicals that oxidize fluorescein, causing a decrease in fluorescence (excitation wavelength 485 nm and emission wavelength 528 nm), which is measured every 4 s for 90 min at 37 °C in a multiwell plate reader (FLUOstar OPTIMA fluorimeter, BMG LABTECH; Offenburg, Germany). Results calculate the relationship of the areas under the curve between blank and samples and are expressed as micromoles of Trolox equivalents per gram.
The oxygen scavenging activity of the samples was determined by the DPPH assay according to Sharma and Bhat (Citation2009) in a multiwell plate reader (FLUOstar OPTIMA fluorimeter, BMG LABTECH). Briefly, a stock solution of DPPH of 23 mg/10 mL MeOH was kept at 5 °C until use; then increasing concentrations of each sample were added and absorbance was recorded at 715 nm. The FR-scavenging activity of each solution was then calculated as the percentage of inhibition and results are expressed as IC50 value, which is defined as the concentration of extract (μg/mL) required to scavenge 50% DPPH radicals. The lower the IC50 value is the higher antioxidant activity. Assays were done in triplicate.
HPLC analysis of C. monogyna extract
Analysis was conducted within Agilent 1100 series liquid chromatograph. A Spherisorb C18 column (250 mm ×4.6 mm, 5 μm) was used with a mobile phase consisting in A: methanol, B: water/acetic acid (0.05%) in gradient elution. A linear gradient was employed with a final solvent composition of 95% A in 45 min. The initial solvent conditions were then restored over a 5-min ramp and the column was allowed to re-equilibrate for an additional 7 min. Flow rate was set at 1 mL/min. Phenolic compounds in hawthorn samples were identified according to their retention time and their purity checked throughout their absorption spectra at four λ: 254, 280, 350 and 590 nm. Injection volume was 10 μL and every sample was analyzed in triplicate.
Statistical analysis
Each experiment was carried out in triplicate. Data are expressed as mean ± SD values of at least three experiments. Linear correlation analysis was used to explore the relationships between the studied variables. Correlation coefficients (R) and p values were evaluated to judge the fit of the correlation; two-sided p< 0.05 and p < 0.01 values of correlations were considered significant and highly significant, respectively. The statistical analyses were conducted using the statistical package SPSS version 22.0 for Windows (SPSS, Chicago, IL).
Results
Extraction yield and polyphenol content
The different extraction yield for each sample is shown in .
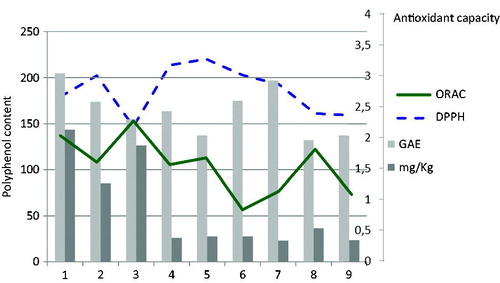
The total polyphenolic content of C. monogyna samples, as determined by the Folin-Ciocalteu method, was between 117.729 ± 0.011 and 204.286 ± 0.015 mg/g of methanol extract ().
Table 2. Total phenolic content, antioxidant activity (ORAC) and oxygen scavenging activity (DPPH) of C. monogyna Jacq. samples.
HPLC analysis
Sixteen polyphenolic components were identified in C. monogyna methanol extract in one single analysis (). The polyphenolic profile showed the major flavonoids and phenolic acids in every sample ().
Figure 1. HPLC chromatogram of C. monogyna sample number 9 (λ = 254, 280, 350 and 590 nm). Peaks: 1: 3,5: dihydroxybenzoic acid; 2: rutin; 3: quercetin; 4; kaempferol; 5: isovanillic acid; 6: hesperidin; 7: hesperetin; 8: quercitrin; 9: apigenin; 10: gentisic acid; 11: chlorogenic acid; 12: arbutin (see Table 3 for further details).
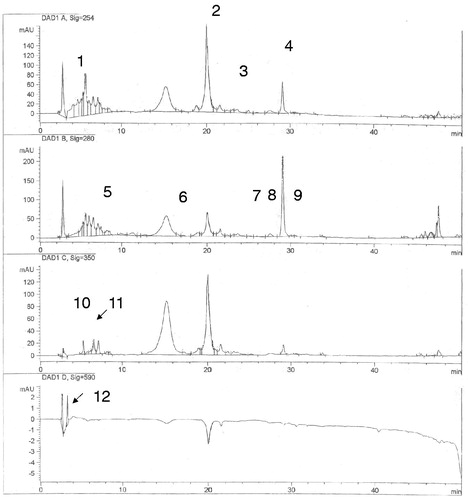
Table 3. Polyphenol composition of samples from C. monogyna methanolic extract.
Sample number 1 showed the highest amount of flavonoids (134.242 mg/kg dry plant), followed by sample number 3 (122.267 mg/kg), while sample number 4 had the lowest amount (9.26 mg/kg), showing a wide range of flavonoids content among the studied samples.
With respect to phenolic acids, samples 4 and 5 showed the highest amount (16.973 and 14.853 mg/kg dry plant, respectively), both samples collected within the province of Guadalajara. Sample number 3 content in phenolic acids was the lowest (3.97 mg/kg).
Kaempferol was the most abundant flavonol (111 mg/kg in sample number 3), followed by the flavone apigenin and the flavonol quercitrin (superior to 74 and 12 mg/kg, respectively, in sample number 1). The most abundant phenolic acid was ursolic acid (superior to 11 mg/kg in sample number 7) followed by isovanillic acid (superior to 9 mg/kg in sample number 4).
Statistical analysis
Pearson’s tests were run to explore linear correlations between experimental variables. The following significant correlations in C. monogyna were found: the sum of flavonoids plus phenolic acid content (HPLC) was positively correlated with the total antioxidant activity by ORAC method (R = 0.740; p< 0.05; ). Also, a positive correlation was found between kaempferol and total antioxidant activity by ORAC method (R = 0.708; p < 0.05; ).
Table 4. Correlation coefficient R between assays and isolated compounds (*p < 0.05; **p < 0.01).
Discussion
In our study, great differences can be found between the phenolic content and composition of the samples, regardless of their geographic origin. The predominant flavonoids were kaempferol (superior to 111 mg/kg dry plant in sample 3), apigenin and quercitrin (more than 74 and 12 mg/kg, respectively, in sample 1), while the most abundant phenolic acids were ursolic (greater than 11 mg/kg in sample 6 from Peñalver, Guadalajara) and isovanillic acid (9 mg/kg in sample 4 from Ambite, Guadalajara). The flavonoids, kaempferol, quercitrin, rutin, hesperetin and arbutin, were detected in every sample.
Our results agree with previous publications describing flavonoids such as O-glycosides derivatives of quercetin, kaempferol, apigenin and luteolin, together with C-glycosides as vitexin, orientin and apigenin derivatives. Most proanthocyanidins present in the drug were oligomeric (dimers, trimmers, tetramers, etc.) (Sendker et al. Citation2013; Simirgiotis Citation2013).
Phenolic compounds are known to exert strong antioxidant activity due to its redox properties, which act as chelating agents, and hydrogen donors; metal ion chelating agents or reducing agents. The antioxidant ability of isolated flavonoids has been related to several pharmacological effects as antiproliferative, antitumoural, antimicrobial, antithrombotic or anti-inflammatory, these activities being involved in heart disease, diabetes, osteoporosis and neurodegenerative disorders (Naasani et al. Citation2003; O’Leary et al. Citation2004; Hussain et al. Citation2005; Leifert & Abeywardena Citation2008). Also, proanthocyanidins and phenolic acids may be involved in the observed beneficial actions (Folcará & Vanaciocha Citation2000; Bruneton Citation2001; Simirgiotis Citation2013).
The antioxidant activity of hawthorn preparations has been described (Cui et al. Citation2006; Kiselova et al. Citation2006). In vivo studies were conducted with some flavonoids from C. pinnalfidia on brain ischemic insults in Mongolian gerbil stroke model. The flavonoids were given via drinking water (0.5 mg/mL and 2.5 mg/mL). Results showed that pretreatment of the animals with the flavonoids over 15 days decreased ROS production, thiobarbituric acid reactive substances content, and nitrite/nitrate concentration in brain homogenate, increased the brain homogenate-associated antioxidant level in a dose-dependent manner. Flavonoid pretreatment increased the amount of biologically available NO by scavenging of superoxide anion produced during reperfusion. At same time, in the process of ischemia/reperfusion brain damage, the content of nitrite/nitrate (the end product of NO) increased, and of NO decreased. Oral pretreatment with flavonoids decreased the nitrite/nitrate content in the brain homogenate and increased the biologically available NO concentration in a dose-dependent manner. iNOS was implied in delayed neuron death after brain ischemic damage and it was found that pretreatment with flavonoids could decrease the protein level of tumour necrosis factor-alpha and nuclear factor-kappa B, and increase the mRNA level of NOS estimated by western blotting and RT-PCR. More neurons survived and fewer cells suffered apoptosis in the hippocampal CA1 region of flavonoid treated animal brain. It was discussed by the authors that oral administration of this antioxidant increases the antioxidant level in the brain and protects the brain against delayed cell death caused by ischemia/reperfusion injury (Zhang et al. Citation2004).
Our total phenolic content measured in all samples from Spain was 4-(sample 8) to 7-(sample 1) times higher than one sample from Chile (Simirgiotis Citation2013). In our study, C. monogyna was endorsed with a medium-high antioxidant capacity according the ORAC assay (), with only slight differences among samples, this indicating no great influence of the geographical origin on the activity. The ORAC values ranged from 1.32 μmol TE to 2.79 μmol TE/mg of vegetal material. Samples 1 and 3 showed the highest antioxidant capacity (2.60 and 2.79 μmol TE/mg, respectively), these values corresponding to samples with the highest amount in polyphenols, according the HPLC analysis (). Also, sample number 3 showed the lowest IC50 value of 2.67 ± 0.14, this indicating that the same C. monogyna sample was the most active as an antioxidant and radical scavenger (). Moreover, every assayed sample exhibited a comparable antioxidant activity with that of the standard ascorbic acid.
When analyzing the influence of isolated compounds (), only the flavonol kaempferol seemed to influence the antioxidant activity according the ORAC method (R = 0.708, p < 0.05), but not DPPH values. Our results agree with those previously obtained by other authors who related kaempferol, quercitrin, rutin, hesperetin and arbutin to C. monogyna activity (Folcará & Vanaciocha Citation2000; Zhang et al. Citation2004).
On the basis of the correlation matrix (), each coefficient was considered in order to establish the correlations between different couples of assays. No statistically significant relationship was found between total flavonoids content (according the Folin-Ciocalteu analysis) and the antioxidant ability. The highly significant correlation between flavonoids plus phenolic acid content (as obtained by HPLC analysis) and total antioxidant ability by the ORAC method (R = 0.740, p < 0.05) indicated that flavonoids/phenolic acids are responsible of the antioxidant capacity. No statistically significant correlation was found between both assays performed for the antioxidant capacity, ORAC and DPPH (R = −0.346).
Conclusions
This study demonstrates that C. monogyna is rich in polyphenols and that geographic origin of the wild plant influences its amount and composition. Also, the antioxidant and radical scavenging activities of the methanol extract of hawthorn are shown, which are related to polyphenols, mainly flavonoids and phenolic acids and suggest that the combination of flavonoids and phenolic acids may synergistically enhance the antioxidant activity of C. monogyna.
Moreover, these results demonstrate that the total phenolic amount is not a valuable parameter to estimate the antioxidant potential of any sample, as a considerable proportion of these polyphenols are not endorsed with antioxidant ability (i.e. simple polyphenols or tannins).
Further experiments are needed to find the mechanism of action of C. monogyna against an oxidative stress chemically induced in cell culture and its possible beneficial effect on cell integrity, enzymatic and nonenzymatic antioxidant defences.
Disclosure statement
The authors report no declarations of interest.
References
- Bradley P. 2006. British Herbal Compendium – a handbook of scientific information on widely used plant drugs. Vol. 2. Bournemouth: British Herbal Medicine Association.
- Bruneton J. 2001. Farmacognosia, Fitoquímica. Plantas medicinales. Zaragoza: Acribia Editors.
- Chang Q, Zuo Z, Harrison F, Chow MSS. 2002. Hawthorn. J Clin Pharmacol. 42:605–612.
- Cui T, Nakamura K, Tian S, Kayahara H, Tian Y. 2006. Polyphenolic content and physiological activities of Chinese Hawthorn extracts. Biosci Biotechnol Biochem. 70:2948–2956.
- Darvesh AS, Carroll RT, Bishayee A, Geldenhuys WJ, Van der Schyf CJ. 2010. Oxidative stress and Alzheimer’s disease: dietary polyphenols as potential therapeutic agents. Expert Rev Neurother. 10:729–745.
- Dávalos A, Gomez-Cordoves C, Bartolome B. 2004. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J Agric Food Chem. 52:48–54.
- Edwards JE, Brown PN, Talent N, Dickinson TA, Shipley PR. 2012. A review of the chemistry of the genus Crataegus. Phytochemistry. 79:5–26.
- Folcará SC, Vanaciocha BV. 2000. Interés terapéutico de la sumidad de espino blanco (Crataegi folium cum flore). Rev Fitoter. 1:85–93.
- Gómez-Serranillos MP, Palomino OM. 2012. Quercetin: natural sources and health benefits. In: Chikamatsu T, Hida Y, editors. Quercetin: dietary sources, functions and health benefits. Hauppauge (NY): Nova Science Publishers.
- Hussain T, Gupta S, Adhami VM, Mukhtar H. 2005. Green tea constituent epigallocatechin-3-gallate selectively inhibits COX-2 without affecting COX-1 expression in human prostate carcinoma cells. Int J Cancer. 113:660–669.
- Kaul R. 1998. Der Weißdorn. Botanik, Inhaltsstoffe, Qualitätskontrolle, Pharmakologie, Toxikologie und Klinik. Stuttgart: Wissenschaftliche Verlagsgesellschaft mbH.
- Kiselova Y, Ivanova D, Chervenkov T, Gerova D, Galunska B, Yankova T. 2006. Correlation between the in vitro antioxidant activity and polyphenol content of aqueous extracts from Bulgarian herbs. Phytother Res. 20:961–965.
- Leifert WR, Abeywardena MY. 2008. Grape seed and red wine polyphenol extracts inhibit cellular cholesterol uptake, cell proliferation, and 5-lipoxygenase activity. Nutr Res. 28:842–850.
- Leopoldini M, Marino T, Russo N, Toscano M. 2004. Antioxidant properties of phenolic compounds: H-atom versus electron transfer. Mechanism. J Phys Chem A. 108:4916–4922.
- Madaus G. 1976. Lehrbuch der biologischen Heilmittel. Vol. 2. Hildesheim (NY): Georg Olms Verlag.
- Momekov G, Benbassat N. 2013. Pharmacological properties of Hawthorn leaf and flower as a cardiovascular agent. Pharmacia. 60:24–36.
- Montreau FR. 1972. Sur le dosage des composés phénoliques totaux dans les vins par la méthode Folin-Ciocalteau. Connaiss Vigne Vin. 24:397–404.
- Naasani I, Oh-hashi F, Oh-hara T, Feng WY, Johnston J, Chan K, Tsuruo T. 2003. Blocking telomerase by dietary polyphenols is a major mechanism for limiting the growth of human cancer cells in vitro and in vivo. Cancer Res. 63:824–830.
- O’Leary KA, de Pascual-Teresa S, Needs PW, Bao YP, O'Brien N, Williamson G. 2004. Effect of flavonoids and vitamin E on cyclooxygenase-2(COX-2) transcription. Mutat Res. 551:245–254.
- Sendker J, Petereit F, Lautenschläger M, Hellenbrand N, Hensel A. 2013. Phenylpropanoid-substituted procyanidins and tentatively identified procyanidin glycosides from hawthorn (Crataegus spp.). Planta Med. 79:45–51.
- Sharma OP, Bhat TK. 2009. DPPH antioxidant assay revisited. Food Chem. 113:1202–1205.
- Simirgiotis MJ. 2013. Antioxidant capacity and HPLC-DAD-MS profiling of Chilean Peumo (Cryptocarya alba) fruits and comparison with German Peumo (Crataegus monogyna) from Southern Chile. Molecules. 18:2061–2080.
- Zhang DL, Zhang YT, Yin JJ, Zhao BL. 2004. Oral administration of Crataegus flavonoids protects against ischemia/reperfusion brain damage in gerbils. J Neurochem. 90:211–229.