Abstract
Context: Among the 4-azasteroids, finasteride is biologically the most important compound having preventive effect against male pattern baldness (MPH) and benign prostatic hyperplasia commonly called enlargement of prostate gland.
Objective: The microbial transformation of finasteride by fungus Aspergillus niger (ATCC 10549) has been investigated to obtain biologically more potent derivatives.
Materials and methods: Fermentation of finasteride was performed with filamentous fungus Aspergillus niger (ATCC 10549). This transformation resulted in the production of two transformed products, which were purified through column chromatography. In vitro lipoxygenase inhibitory potential was determined by incubating 20 mL of the enzyme with 10 mL of test sample (100 μM) in 0.1 mM (pH 7.0) phosphate buffer for 5 min at 258 °C followed by addition of 10 μL of substrate (linolenic acid) to reaction mixture and measuring the formation of complex spectrophotometrically.
Results: Structure elucidation of biotransformed metabolites was ascertained through extensive 1D and 2D spectroscopic techniques. This study established the fact that A. niger promoted stereospecific dihydroxylation at C-11 and C-15 of finasteride. The resulting biotransformed metabolites were characterized as 11α-hydroxyfinasteride and 15β-hydroxyfinasteride, respectively. Finasteride along with transformed metabolites were analyzed for their in vitro lipoxygenase (LOX) inhibition assay. Among the tested compounds 15β-hydroxyfinasteride showed good activity with IC50 value 112.56 ± 2.23 μM while inhibitory effect in case of 11α-hydroxyfinasteride was low with IC50 value 186.05 ± 1.34 μM. Standard compound baicalein revealed IC50 value being 22.0 ± 0.05 μM.
Conclusion: The present investigation highlighted the fact that potentially active compound can be produced through the technology of biotransformation.
Introduction
Microbial transformation is a quick and key method for changes in the skeleton of drugs and isolated organic compounds of complicated structures (Riva Citation2001; Dai et al. Citation2005; Dong et al. Citation2010). Due to high degree of selectivity in the reaction and environmental benignancy, the technology of biotransformation has greater advantages over simple organic synthesis (Li et al. Citation2007; Zhang et al. Citation2010). Most importantly, through microbial transformation certain reactions can be carried out which are chemically impossible to conduct. For example, the introduction of functional group such as hydroxylation of an inactivated organic compound is chemically inert while the same transformation can be easily achieved through microbial intervention (Li et al. Citation2007; Zhang et al. Citation2007; Wang et al. Citation2011).
Finasteride is an important compound which is recommended in clinical trial for the prevention of male pattern baldness (MPH) and benign prostatic hyperplasia commonly called enlargement of prostate gland.
The major substrates for lipoxygenase enzyme are archidonic, linoleic and other polyunsaturated fatty acids which are converted into metabolites having biologically important role in inflammatory and immune responses (Catalano & Procopio Citation2005). A variety of LOX enzymes have been reported to exist, among them 5-LOX and platelet type 12-LOX are generally considered as pro-carcinogenic, 15-LOX-2 suppresses carcinogenesis while 15-LOX-1 remain s controversial (Pidgeon et al. Citation2007). The activity of these enzymes is correlated to the inflammatory and allergic reactions because of the formation of leukotriens (LTs) (Pontiki & Hadjipavlou-Litina Citation2008), which is usually observed in conditions of asthma, psoriasis, allergic rhinitis, rheumatoid arthritis and colitis ulcerosa (Schneider & Bucar Citation2005). Thus, by inhibiting the lipoxygenase pathway the production of LTs can be prevented (Kamatou Citation2006). Lipoxygenase inhibitors may lead to the design of biologically and pharmacologically targeted therapeutic strategies inhibiting LOX isoforms and/or their biologically active metabolites which may be useful in cancer treatment (Pidgeon et al. Citation2007).
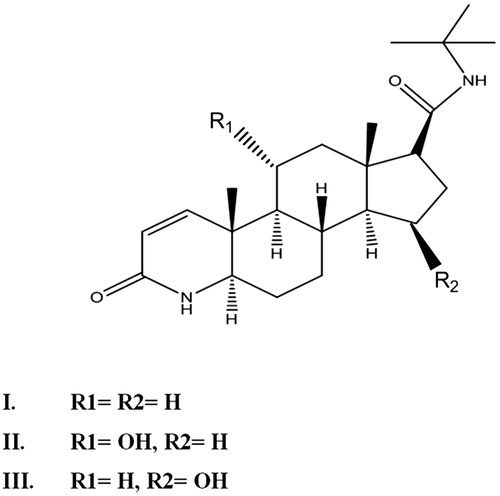
In the present study, an attempt was made to biotransform finasteride (1) with Aspergillus niger cultures. The main objective of present research was to derivatize finasteride through fungal culture and to evaluate the lipoxygenase inhibitory potential of biotransformed metabolites (Scheme 1).
Materials and methods
General experimental procedures
IR spectra were recorded on a Bruker FT-IR model IFS-88 spectrometer. 1H (400 MHz) and 13C NMR (400 MHz) spectra were obtained on a AVANCE AV- 400 NMR spectrometer, using TMS or solvent peaks as reference standard. MS spectra were obtained on a JEOL MS Route spectrometer. Incubation of microorganisms and biotransformation was performed on an Incubator Shaker JS-FS-2500 (Johnsam Co., Inchon, Korea).
Reagents and materials
All solvents used in this study were analytical grade; silica gel for column chromatography was product of E. Merck, Darmstadt, Germany.
Microorganism and chemicals
Aspergillus niger (ATCC 10549) was obtained from Pathology Department, Agriculture University, Peshawar and was maintained on SDA and kept at 4 °C in refrigerator. Finasteride was obtained from Ferozsons pharmaceutical, Nowshera, Pakistan. The purity of finasteride for the biotransformation was higher than 98%, as determined by HPLC method.
Culture medium
The broth medium used for A.niger was comprised of glucose (5.0 g), KH2PO4 (2.5 g), peptone (2.5 g), yeast extracts (2.5 g), NaCl (2.5 g) and glycerol (2.5 mL) in 500 mL of distilled water.
Procedure for biotransformation
Stage II fermentation protocol (Rosazza & Smith Citation1979) was used for conducting all types of biotransformation reactions. Stage I culture was prepared by transferring spores from freshly prepared two days old culture aseptically into 250 mL flask having sterile broth medium (100 mL), which was then incubated on shaking table for 48 h at 30 °C. Stage II culture was prepared by aseptic transfer of the contents of seed flask (stage I culture) into 30 flasks each having 100 mL of pre-autoclaved broth media. All these flasks were incubated on shaking table for a period of 48 h at 30 °C.
Substrate (300 mg) was dissolved in EtOH (15 mL) and solution was evenly distributed among thirty flasks having well developed stage II culture. All these flasks were incubated on shaker at 180 rpm and 30 °C for a period of 12 days. Two kinds of controls were run in all experiments i.e. substrate control (having only substrate without biomass) for checking stability of substrate and culture control (having biomass without substrate) for checking metabolites endogenously produced by the fungi.
Isolation and characterization of biotransformed products
Fungal mycelia were removed from the broth media through filtration and were thoroughly washed with ethyl acetate (1 L). Extraction of organic portion from filtrate was performed with ethyl acetate (5 L). Whole organic part was dried with anhydrous Na2SO4 and was finally concentrated under reduced pressure with the help of rotary evaporator. In a similar way the controls were also harvested. Biotransformation of substrate was confirmed with the help of thin layer chromatography. The biotransformed products were purified through column chromatography using methanol- chloroform (7:93) as solvent system. The biotransformed products were characterized through detailed spectroscopic techniques i.e., 1H NMR, 13C NMR and 2D-NMR.
11α-Hydroxyfinasteride II
White amorphous solid; IR νmax: 3292, 2970, 3404, 1700, 1600, 1373 cm−1. 1H NMR (CD3OD, 500 MHz) and 13C NMR (CD3OD, 125 MHz) were illustrated in ; HR EIMS m/z 389.5725 [M + H]+ (Calculated for C23H37N2O3, 389.2759).
Table 1. 13C- and 1H NMR chemical shift assignments of compounds II and III.
15β-Hydroxyfinasteride III
White amorphous solid; IR νmax: 3292, 2970, 3404, 1700, 1600, 1373 cm−1. The 1H NMR (CD3OD, 500 MHz) and 13C NMR (CD3OD, 125 MHz) see ; HR-EIMS m/z 389.5725 [M + H]+ (Calculated for C23H37N2O3, 389.2759).
Biological potential
Lipoxygenase (LOX) inhibition assay
In vitro lipoxygenase inhibitory effect of finasteride (I) and its biotransformed metabolites were performed spectrophotometrically with a slight modification in the reported procedure (Tappel Citation1962). All chemicals used in this bioassay were of analytical grade. The test samples and controls were dissolved in 50% ethanol. Linolenic acid and lipoxygenase (1.13.11.12) Type I-B (soybean) were obtained from Sigma (St. Loius, MO) and were used without further purification. The inhibition assay was performed by the incubation of 20 mL of lipoxygenase solution with 10 mL of test sample solution in 160 mL of 0.1 mM (pH 7.0) of sodium phosphate buffer at 258 °C for 5 min. After completion of the incubation time the reaction was started with 10 μL of substrate solution (linoleic acid solution). The formation of (9Z, 11E)-13S)-13 hydroperoxyoctadeca-9,11-dienoate was observed spectrophotometrically for 10 min. the experiments were conducted in triplicate. Baicalein the standard inhibitor of lipoxygenase was used as positive control. IC50 was calculated through EZ-Fit Enzyme Kinetics program (Perrella Scientific Inc., Amherst, MA).
Results and discussion
Screening scale fermentation
The ability of A. niger to transform finasteride (I) was initially confirmed by screening scale fermentation. TLC results showed the presence of new spots relative to positive and negative controls thus confirming the ability of A. niger to transform the substrate (I) at specific sites on the main frame work. In order to pool up the measurable quantity of biotransformed metabolites, the same experiment was repeated several times on the substrate (I). This attempt was proved successful to scale up and standardize these biotransformed metabolites for performing spectroscopic studies as well as biological screening.
Structure elucidation of biotransformed products
Incubation of finasteride (I) with A. niger produced two biotransformed products as shown in . These biotransformed products were characterized through detailed 1D and 2D spectroscopic techniques, respectively.
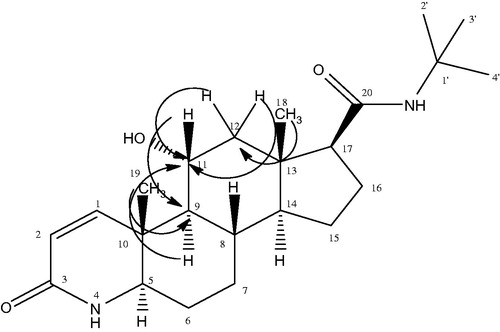
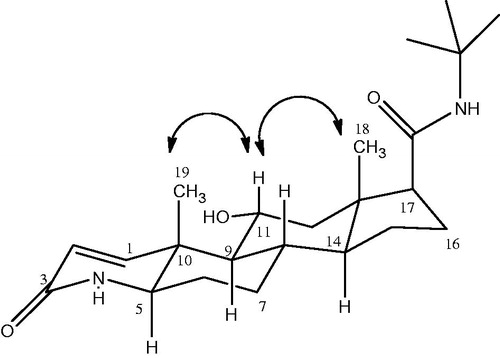
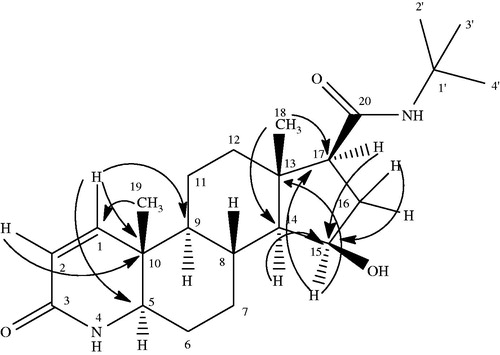
Metabolite II (11α-hydroxyfinasteride) was obtained as a white amorphous solid. Structure elucidation was performed by developing the molecular formula C23H37N2O3 via HR EIMS which showed molecular ion peak at m/z 389.2848 [M + H]+ (Calcd. for C23H37N2O3 + H+ = 389.2759). The molecular weight was 16 amu greater than that of substrate molecule indicating the introduction of an oxygen atom in the substrate molecule. IR spectrum showed the presence of amide, hydroxyl and carbonyl functionalities, respectively. A downfield signal in the 1H NMR spectrum resonating at δ 4.0 (dt, J = 10.8 Hz, J = 4.2 Hz) was assigned to the methine proton bearing the oxygen atom thus suggesting the hydroxylation of substrate. Similarly in the 13C NMR spectrum there appeared a new downfield methine signal resonating at δ 70.0 (C-11) which further supported the hydroxylation of the starting material. A downfield shift in the 13C values of C-9 (δ 54.5) and C-12 (δ 50.0) inferred the attachment of new oxygen to C-11. 1H NMR and 13C NMR data of compound (II) is shown in . The stereochemistry of OH-11 was α and equatorial which was found from the multiplicity of H-11 signal (δ 4.0, dt, J11ax, 9ax = J11ax, 12ax = 10.8 Hz, J11ax, 12eq = 4.2 Hz) and NOESY correlation of H-11β (δ 4.0) with CH3-19β (δ 1.06) and CH3-18β (0.70) as depicted in . The HMBC correlation further confirmed the presence of OH on C-11 as illustrated in . The H-11(δ 4.0) showed HMBC correlation with C-9 (δ 54.5), and C-11 (δ 70.0) similarly H-9 (δ 1.19), H-12 (δ 1.41, 2.05) showed correlation with C-11 (δ 70.0). In addition, COSY correlation of H-11(δ 4.0) with H-9 (δ 1.19) and H-12 (δ 1.41, 2.05) further supported the hydroxylation on C-11 as depicted in . Based upon the above mentioned spectroscopic evidences the metabolite II was characterized as 11α-hydroxyfinasteride.
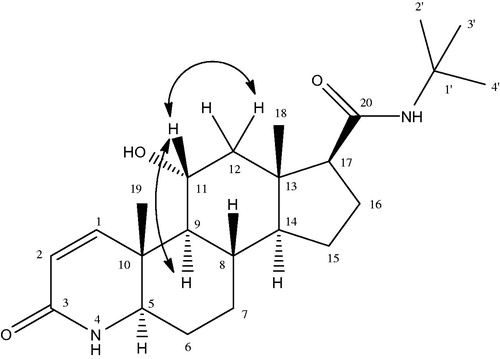
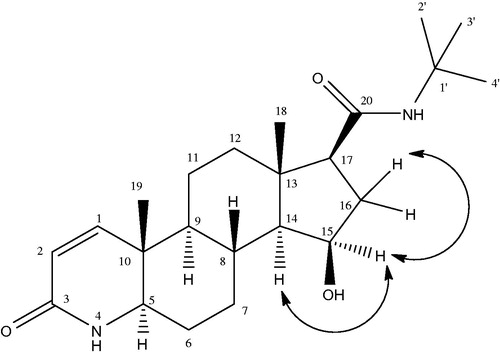
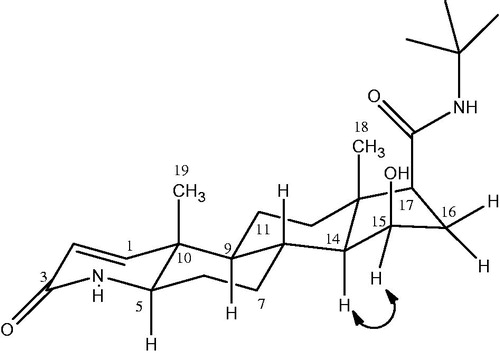
Metabolite III (15β-hydroxyfinasteride) was obtained as colorless amorphous powder. The molecular formula of compound III was developed as C23H37N2O3 on the basis of HR EIMS showing molecular ion peak at m/z 389.2848 [M + H] +) (Calcd. for C23H37N2O3 + H+ = 389.2759). This showed that the molecular weight of the metabolite III was 16 amu greater than that of the parent compound I thus indicating the monohydroxylation of the substrate. A downfield signal in the 1H NMR spectrum was allotted to C-15 methine proton which appeared as a broad triplet at δ 4.24 (J = 6.0 Hz), indicating the presence of OH group on this carbon. The presence of OH group on C-15 (δ 71) was further confirmed from the downfield in the 13C chemical shift values of C-14 (δ 61) and C-16 (δ 37.6). The detailed 1H NMR and 13C NMR chemical shift values are illustrated in . The position of the OH group at C-15 was further confirmed from HMBC and COSY experiments and , respectively. According to the HMBC assignment, H-15 (δ 4.24) showed correlation with H-17 (δ 2.14) and C-15 (δ 71) showed correlation with H-14 (δ 0.99) and H-16 (δ 1.09, 2.13), respectively. Similarly in the COSY spectrum, the C-15 methine proton resonating at δ 4.24 showed cross peak with C-14 methine proton (δ 0.99) and C-16 methylene protons (δ 1.09, 2.13), respectively. On the basis of NOESY experiment the OH group at C-15 was allotted β orientation as depicted in . As in the NOESY spectrum, H-15 (δ 4.24) showed correlation with α-oriented C-17 methine protons (δ 2.14) so the OH-15 was given a β-orientation. Compound III was given the name 15β-hydroxyfinasteride according to the spectral data.
Biological potential
Lipoxygenase (LOX) inhibition assay
Lipooxygenase is a target of choice for controlling inflammation (Shu et al. Citation2006). Lipoxygenase (EC 1.13.11.12) is a non-heme iron containing enzymes. Lipoxygenases are capable of catalyzing many reactions involved in xenobiotic metabolism (Kulkami Citation2001). Catalysis of the fatty acid metabolism and their metabolites by the lipoxygenases are the most important reactions which lead to the initiation of inflammatory responses in the body. From mechanistic point of view, peripheral side of action; cyclooxygenase inhibition was supported by lipoxygenase for more profound effects. ()
Table 2. In vitro quantitative inhibition of lipoxygenase by compounds I, II and III.
In order to discover new LOX inhibitors, finasteride I and its biotransformed metabolites II and III were screened for their ability to inhibit lipoxygenase enzyme the main cause of inflammation. The starting compound I was found inactive against lipoxygenase enzyme at tested dose of 100 μM. Interestingly the biotransformed metabolites were found to possess the inhibitory effect at tested dose of 100 μM, however the inhibitory effect was weak compared to the standard drug baicalein. The inhibitory potential of 11α-hydroxyfinasteride (II) was 50% with IC50 186.05 ± 1.34 μM, similarly in case of 15β-hydroxyfinasteride (III) the inhibitory potential was 58% with IC50 112.56 ± 2.23 μM.
Lipoxygenase also has significant role in the development of cancerous cells, metastasis, invasiveness, cell survival and induction of tumor necrosis factor (Sulaiman et al. Citation2010). Large number of COX-2 or 5-LOX inhibitors has been developed as drugs to treat inflammation (Viji & Helen Citation2008). Thus by inhibiting the lipoxygenase enzyme the risk of cancer development can be reduced to a greater extent. It is clear from the results of the present investigations that the biological potential of a compound can be improved through microbial technology.
Funding information
The authors are greatly indebted to the Higher Education Commission of Pakistan for financial support under its indigenous PhD fellowship scheme.
Disclosure statement
The authors of this article have no declaration of interest.
References
- Catalano A, Procopio A. 2005. New aspects on the role of lipoxygenases in cancer progression. Histol Histopathol. 20:969–975.
- Dong T, Wu GW, Wang XN. 2010. Microbiological transformation of diosgenin by resting cells of filamentous fungus, Cunninghamella echinulata CGMCC 3.2716. J Mol Catal B: Enzym. 67:251–256.
- Dai J, Yang LL, Sakai J. 2005. Biotransformation of chinen-siolide B and the cytotoxic activities of the transformed products. J Mol Catal B: Enzym. 33:87–91.
- Kulkami AP. 2001. Lipoxygenase a versatile biocatalyst for biotransformation of endobiotics and xenobiotics. Cell Mol Life Sci. 58:1805–1825.
- Kamatou GPP. 2006. Indigenous Salvia species- an investigation of their pharmacological activities and phytochemistry. Phd thesis. [Johannesburg (South Africa)]: University of the Witwatersrand.
- Li J, Dai J, Chen X, Zhu P. 2007. Microbial transformation of cephalomannine by Luteibacter sp. J Nat Prod. 70:1846–1849.
- Pidgeon GP, et al. 2007. Lipoxygenase metabolism: roles in tumor progression and survival. Cancer Metast Rev. 26:504–524.
- Pontiki E, Hadjipavlou-Litina D. 2008. Lipoxygenase inhibitors: a comparative QSAR study review and evaluation of new QSARs. Med Res Rev. 28:39–117.
- Riva S. 2001. Biocatalytic modification of natural products. Curr Opin Chem Biol. 5:106–111.
- Rosazza JP, Smith RV. 1979. Microbial models of mammalian metabolism: microbial reduction and oxidation of pentoxifylline. Adv Appl Microbiol. 25:169–208.
- Shu XS, Gao ZH, Yang XL. 2006. Anti-inflamatory and antinociceptive activities of Smilax china L. aqueous extract. J Ethnopharmacol. 103:327–332.
- Sulaiman MR, Perimal EK, Akhtar MN. 2010. Anti-inflammatory effect of zerumbone on acute and chronic inflammation models in mice. Fitoterapia. 81:855–858.
- Schneider I, Bucar F. 2005. Lipoxygenase inhibitors from natural plant sources. Part 1: medicinal plants with inhibitory activity on Arachidonate 5-lipoxygenase and 5-lipoxygenase/cyclooxygenase. Phytother Res. 19:81–102.
- Tappel AL. 1962. Methods in Enzymology. New York (NY): Academic Press.
- Viji V, Helen A. 2008. Inhibition of lipoxygenases and cyclooxygenase-2 enzymes by extracts isolated from Bacopa monniera (L.) Wettst. J Ethnopharmacol. 118:305–311.
- Wang Y, Chen L, Zhao F. 2011. Microbial transformation of neoandrographolide by Mucor pinosus (AS 3.2450). J Mol Catal B: Enzym. 68:83–88.
- Zhang X, Zou JH, Dai DJ. 2010. Microbialtransformationof(-)-huperzine A. Tetrahedron Let. 51:3840–3842.
- Zhang H, Kang N, Qiu F. 2007. Four novel metabolites from microbial transformation of curcumol by Cunninghamella blakesleana. Chem Pharm Bull. 55:451–454.