Abstract
Context: Cotinus coggygria Scop. (Anacardiaceae) leaves that were used as wound healing in traditional Balkan and Anatolian folk medicine, could be potentially effective in treating diabetic wounds.
Objective: This study investigates biochemical and histological effects of ethanol extract of C. coggygria (CCE) on excision wound model in diabetic rats.
Materials and methods: This study was conducted on diabetic Wistar albino rats, which were injected by a single dose (50 mg/kg i.p.) streptozotocin. Afterward an excision wound model was created in all animals; diabetic control rats were applied topically simple ointment and diabetic treatment rats were applied topically 5% (w/w) ointment with CC, once a day during the experimental period. Malondialdehyde, glutathione and hydroxyproline levels in wound tissues were investigated at the end of 3rd, 7th, and 14th days. Histopathological examination was also performed.
Results: Hydroxyproline content was significantly increased in the CCE treated group versus control after the 3rd and 7th days (15.33 versus 11.83; 19.67 versus 15.67 mg/g, p < 0.05; respectively). A statistically significant elevation in glutathione at the end of 3rd, 7th, and 14th days (5.13 versus 1.58, p < 0.05; 4.72 versus 1.88, p < 0.05; 3.83 versus 1.88 μmol/g, p < 0.05, respectively) and a statistically significant decrease in malondialdehyde level at the end of 7th day (4.49 versus 1.48 nmol/g, p < 0.05) were determined in the treated group versus control group. These results were also supported by histological analyses.
Discussion and conclusion: These findings indicate that CCE accelerated the cutaneous wound healing process in diabetic wounds, in confirmation of its traditional use.
Introduction
In patients with diabetes mellitus, wound healing process is mostly a therapeutic challenge, lasting for weeks in spite of enough and suitable care (Kumar & Gupta Citation2009). Prolonged inflammation, impaired neovascularization, reduced synthesis of collagen, elevated levels of proteases, and disordered macrophage function are abnormalities associated with wound healing process in diabetes mellitus (Gupta et al. Citation2008). Thus, wound healing process could be slowed down in the presence of diabetes. Although the exact mechanism of this process has not been elucidated yet, most of plants used in traditional medicine are reported as effective in accelerating the wound healing (Kumar & Gupta Citation2009).
The genus Cotinus L. (Anacardiaceae) is represented by one species in Turkey, Cotinus coggygria Scop. (Davis Citation1967). The leaves of this species have been used in Balkan and Anatolian folk medicine as antipyretic, antiseptic, antihemorrhagic, treatment of diarrhoea and wound healing (Sayar et al. Citation1995; Duran Citation1998; Baytop Citation1999; Matić et al. Citation2015).
A previous study reported that ethanol extract prepared from leaves of C. coggygria have significant effects on the wound-healing process in rats (Aksoy et al. Citation2016). The present study determines wound healing activity of the ethanol extract of C. coggygria leaves, used for wound treatment in diabetic rats as traditional medicine.
Materials and methods
Plant material and extraction
Cotinus coggygria Scop. (Anacardiaceae) leaves were identified and collected from the Hamidiye village of Kırklareli province of Turkey, in June 2002 by Sukran Kultur. Voucher specimens have been deposited in the herbarium of the Faculty of Pharmacy, Istanbul University (ISTE 80926). Cotinus coggygria leaves were dried in the shade at room temperature. Powdered samples (100 g) were extracted with ethanol (96% extra pure) in a Soxhlet apparatus, filtered and dried under vacuum and stored under refrigeration for further analysis.
Quantitative determination of the total phenolic and flavonoid content of CCE
Total phenolic compound content was determined according to Gao et al. (Citation2000). Gallic acid was used as a standard and the total phenolic content, and expressed as mg GAE/g plant extract. Total flavonoid compound content was determined according to Zhang et al. (Citation2013). Catechin was used as a standard and total flavonoid content was expressed as mg CE/g plant extract.
Study design
Wistar albino rats weighing 150–200 g were supplied from Marmara University, Animal Center (DEHAMER). All rats were fed with standard laboratory pellets and tap water ad libitum during the present experiment. The protocol of the present study was approved by the Animal Care and Use Committee of the Marmara University (Protocol number: 19.2013.mar). Each rat was housed in a plastic cage individually after wound was made. All animals were randomly assigned to the control and treatment groups, each of which consisted of six rats. In the present study, in order to see the wound healing process by time, biochemical and histological changes were examined at three different times as the 3rd, 7th, and 14th days.
Induction of diabetes in rats
Streptozotocin is injected to all rats as a single dose (50 mg/kg i.p. in 0.1 M buffered citrate solution, pH 4.5). After 48 h, blood glucose level of rats were measured by glucometer and the rats with blood glucose level above 250 mg/dL were considered to be diabetic and selected for the experimental study (Apikoglu-Rabus et al. Citation2010).
Wound creation
Ketamine 10 mg/kg was injected intraperitoneally to all rats and dorsal hairs of rats were shaved, removed with a chemical depilation agent and cleaned with 70% ethanol before wound creation. Two parallel full thickness skin excisions were created with punch biopsy instrument with 6 mm diameter on the dorsal thoracic area of the rat as described previously (Apikoglu-Rabus et al. Citation2010).
Wound management
The 5% (w/w) ointment formulation was prepared by mixing 5 g of the ethanol extract of C. coggygria and 95 g simple ointment base B.P. (Arunachalam & Parimelazhagan Citation2013). Simple ointment and 5% (w/w) ointment formulation with C. coggygria ethanol extract were applied in aliquots of 0.5–1 g to each wound of rats in the control and the treatment groups; respectively, throughout the experiment periods. At the end of the study, all the animals were sacrificed by ketamine anaesthesia on the 3rd, 7th, and 14th days and the tissue samples were collected for biochemical and histological examinations.
Biochemical analysis
First, to obtain 10% tissue homogenate, tissue samples obtained from each group were homogenized in ice-cold with 150 mM potassium chloride. Malondialdehyde (MDA) levels were assayed to determine lipid peroxidation products as described previously (Beuge & Aust Citation1978). All MDA levels were expressed as nmol/g. Glutathione (GSH) levels were obtained with spectrophotometric method by using Ellman solution (Beutler Citation1975). GSH levels were presented as μmol/g. Hydroxyproline content was also assessed according to previously described method (Reddy & Enwemeka Citation1996) and these results were presented as mg/g tissue.
Histological examination
For histological examination, wounded skin specimens were collected at the end of 3rd, 7th and 14th day from both the groups. The specimens were fixed in 10% neutral buffered formalin solution. After fixation, tissue samples were dehydrated in graded ethanol series and cleared in toluene. Parafin-embedded samples were cut (5 μm thick) by rotary microtome, skin sections were stained by Hematoxylin and Eosin (H&E) stain. Sections were evaluated for histopathological changes and photographed under a light microscope (Olympus BX51, Tokyo, Japan) by an experienced histologist who was unaware of the experimental groups. Histological features (epidermal and dermal regeneration, neutrophil infiltration, oedema, degree of angiogenesis and fibrosis) were evaluated.
Statistical analysis
Komolgorov–Smirnov test has been performed to clarify distribution of data. All the results were presented as median and 25–75 percentiles. Significance of difference in biochemical parameters between the control and treatment groups was tested using Mann–Whitney U test. p value was expressed as p < 0.05.
Results
The total phenolic and flavonoid contents of the extract were 380.2 ± 6.38 mg/g gallic acid equivalent and 68.4 ± 1.56 mg/g (+)-catechin equivalent, respectively. The concentration of hydroxyproline in the granulation tissue of the rats treated with CCE was significantly greater than that of the control after the 3rd day and 7th day (15.33 ± 2.94 versus 11.83 ± 2.14 and 19.67 ± 1.75 versus 15.67 ± 2.73 mg/g; p < 0.05; respectively) (). In the CCE treatment group, glutathione levels demonstrated a statistically significant elevation relative to levels in control group at the end of 3rd, 7th, and 14th day (5.13 ± 2.13 versus 1.58 ± 0.58, p < 0.05; 4.72 ± 1.68 versus 1.88 ± 0.98, p < 0.05; 3.83 ± 1.00 versus 1.88 ± 0.79 μmol/g, p < 0.05, respectively) (). A statistically significant decrease in malondialdehyde levels were observed in the CCE treatment group when compared with the control group at the end of 7th day (1.48 ± 0.64 versus 4.49 ± 2.10 nmol/g, p < 0.05) ().
Table 1. Comparison of biochemical parameters between control and treatment groups at different days in wound tissue.
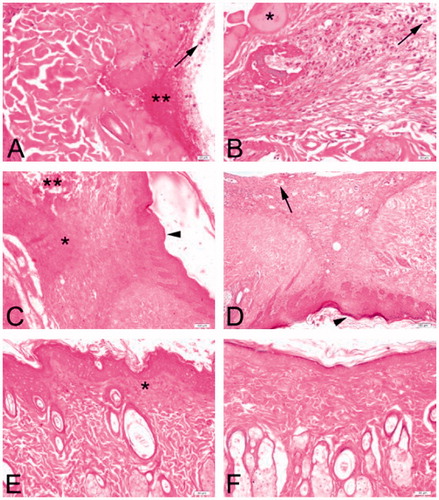
Light microscopic evaluation of H&E stained skin sections in both the groups demonstrated exudate with high neutrophil infiltration at day 3 (). Increased oedema and haemorrhage in some areas compared to treated group was evident in the control group. Epidermis and dermis organization were totally disturbed. On day 7, decreased fibrosis and oedema were observed in the treated group compared to the controls. Epithelial regeneration and mild neutrophil infiltration were apparent in both the groups. However, it was not possible to identify skin appendages. Wounds (treated or control) displayed granulation tissue. On day 14, the wounds of both the groups were almost completely epithelialized. In the treated group, dense angiogenesis in the dermis was seen on day 14 compared to controls. Some amount of granulation tissue in the underlying dermis was noted with several fibroblasts in the controls, however, the epidermis and dermis were observed healed better with easily identified skin appendages in the treated group.
Figure 1. Representative skin wound sections from both experimental groups on days 3 (A, B), 7 (C, D) and 14 (E, F). Large amount of inflammatory infiltrate consisting predominantly of neutrophils (arrows) in control (A) and treated (B) groups on day 3. Marked accumulation of exudate (*) and hemorrhage (**) can be observed. Diffuse granulation tissue (*) and completely disrupted areas in the dermis (**) in the controls (C) and dilated neovessels (arrow) in the treated group (D) are observed at day 7. Epithelial regeneration (arrowheads) is apparent. Almost complete epithelization of wound sites on day 14 are observed. Ongoing granulation tissue in the subepidermal region (*) is noted in the controls (E) compared to almost totally healed wound site in the treated rats (F). Hematoxylin and Eosin (H&E) stain, Bars: 20 μm (A, B); 100 μm (C, D); 50 μm (E, F).
Discussion
The present study firstly investigated the effect of CCE on wound healing in streptozotocin-induced diabetic rats.
Delayed wound healing is one of the major problems in diabetes. Impaired blood flow, cellular infiltration, inadequate granulation tissue formation, decreased collagen and fibronectin synthesis, elevated reactive oxidative species (ROS), deficiency of insulin and growth hormone, and an altered patterns of apoptosis leads to delayed wound healing in diabetes (Gautam et al. Citation2015).
Plant extracts are considered as a cure in acceleration of wound healing, reducing ROS, microorganisms, and inflammation, and stimulating the proliferation fibroblasts, producing the collagen (Pinto et al. Citation2015).
Wound healing process depends on an increase in concentration of collagen, which is a significant constituent of the extracellular matrix (ECM) on wound site. Hydroxyproline is an integral part of collagen and the content of collagen at the tissue is determined by measuring hydroxyproline (Ponrasu & Suguna Citation2012).
Prasad et al. (Citation2010) have suggested that the hydroalcoholic fraction of Withania coagulans Dunal (Solanaceae) increased collagen content in the granulation tissues in diabetic rats, reaching maximum on day 8. In agreement with this report, in the present study the hydroxyproline content in granulation tissue of rats significantly increased in the treatment group when compared with the control group, reaching maximum on day 7. According to previous studies about phytochemical investigation of C. coggygria, it is seen that flavonoids (fisetin, fustin, sulphuretin, myricetin, quercetin); anthocyanins (leucodelphinidin, leucocyanidin, delphinidin 3-galactoside, cyanidin 3-galactoside, petunidin 3-glucoside, delphinidin 7-glucoside, cyanidin 3-glucoside-7-rhamnoside); essential oils (limonene, (Z)-β-ocimene, (E)-β-ocimene); gallic tannins (gallic acid), methyl gallate, pentagalloyl glucose, biaurone are chemical constituents of C. coggygria (Hegnauer Citation1964; Tanchev & Timberlake Citation1969; Westernburg et al. Citation2000; Demirci et al. Citation2003; Kultur & Bitiş Citation2007; Novaković et al. Citation2007; Dulger et al. Citation2009). The results of the present study indicated that CCE contained high amounts of phenolic compounds and flavonoids. When compared hydroxyproline content in control and treated wound tissues clearly revealed that CEE enhanced the production of new collagen, probably due to the presence of phenolic compounds as flavonoids, tannins in CCE.
The imbalance between ROS and endogenous antioxidants causes the progress of oxidative stress. It brings about tissue injury or cell death because of necrosis and apoptosis, respectively. The contribution of oxidative stress has been proven in the different complications of diabetes mellitus, including delayed wound healing (Gautam et al. Citation2015). Increased oxidative stress leads to degradation of unsaturated fatty acids, expressed as the increased level of lipid peroxidation (LPO), in cell lipid membranes of endothelial cells and fibroblasts (Gautam et al. Citation2015). Oxidative injury and lipid peroxidation can be determined by MDA measurement. LPO is considered to be an important cause tissue damage led by free radicals (Agbafor et al. Citation2014). GSH are well-known antioxidants that act as free radical sweeper. In diabetic condition, reduction in levels of antioxidant enzymes existed at wound area could not decrease oxidative damage causing weak granular tissue formation and therefore healing delays (Gautam et al. Citation2015).
Agbafor et al. (Citation2014) have demonstrated that aqueous and ethanol extracts of fresh leaves of Zapoteca portoricensis (Jacq) HM Hernández (Fabaceae) significantly (p < 0.05) increased GSH levels, while they decreased MDA levels significantly (p < 0.05) in the treated groups relative to the control. Pavlov et al. (Citation2013) described the effect of aqueous infusion from C. coggygria leaves on indomethacin-induced gastric mucosal damage in Wistar rats and its possible effect on the gastric oxidative status. The infusion of C. coggygria leaves reduced the indomethacin-induced elevation of gastric malondialdehyde (MDA). Similarly, in the present study, a statistically significant elevation in glutathione (GSH) at the end of 3rd, 7th, and 14th days and a statistically significant decrease in malondialdehyde (MDA) level at the end of 7th day were determined in the CCE treatment group when compared with the control group.
Phenols could provide wound healing by scavenging free radicals emerged during the inflammatory phase. Tannins stimulate wound healing by chelation of free radicals, encouraging contraction of the wound, elevating the formation of capillary vessels and fibroblasts, urging keratinocytes proliferation (Prasad et al. Citation2010). Flavonoids are generally known for their antioxidant and anti-inflammatory activities and their capability to bring down lipid peroxidation (Gautam et al. Citation2015).
CCE which was reported to exhibit high antioxidant activity and total phenolic content in a previous study (Nıćıforovıć et al. Citation2010) may accelerate wound healing by reducing oxidative damage to the cell at wound site. The wound healing effects of CCE in the diabetic rats could be due to the presence of the phenolic constituents as flavonoids, phenolic acids, and tannins found in CCE.
In rats, neutrophils appear in the wound lesion 4–6 h after injury and increase in number at first day (Taoka et al. Citation1997; Carlson et al. Citation1998). Inflammation does not persist unless the wound gets chronic (Trengove et al. Citation1999). Normally, inflammatory phase cease before granulation phase starts (Singer & Clark Citation1999). The granulation phase involves recruitment of fibroblasts, angiogenesis and reepithelialization (Jackson et al. Citation2005). In case of a condition where the angiogenesis is disturbed, nutrients are prevented from accessing the newly formed tissue (Jackson et al. Citation2005). Also, if angiogenesis fails, fibroblast migration is arrested and wound healing fails to proceed (Stadelmann et al. Citation1998). Remodelling and contraction of the granulation tissue result in an organized network of collagen and elastin fibers leading to the formation of scar tissue (Jackson et al. Citation2005). However, abundant granulation tissue and excessive fibrosis leads to scar formation and loss of function (Stadelmann et al. Citation1998). Perfusion is decreased in an oedematous infected wound, leading to decreased nutrition of the wound margins (Wackenfors et al. Citation2005). According the results of histopathological examination, it was clearly demonstrated that CCE has a significant wound healing activity on excision wound model in diabetic rats
Conclusion
CCE could be considered as an effective agent in wound healing in diabetics. This result is concordance with the traditional use of this plant.
Funding information
This study was supported by Marmara University Scientific Research Projects Committee under Grant (file no. SAG-A-030114-0004).
Disclosure statement
The authors have no conflicts of interest to declare.
References
- Agbafor KN, Akubugwo EI, Ukpabi CF, Elom SO, Ugwuja E, Nwachukwu N. 2014. Wound healing and antioxidant properties of leaf extracts of Zapoteca portoricensis. Int J Bioassays. 3:2066–2069.
- Aksoy H, Sancar M, Sen A, Okuyan B, Bitis L, Uras F, Akakin D, Cevik O, Kultur S, İzzettin FV. 2016. The effect of topical ethanol extract of Cotinus coggygria Scop. on cutaneous wound healing in rats. Nat Prod Res. 30:452–455.
- Apikoglu-Rabus S, Izzettin FV, Turan P, Ercan F. 2010. Effect of topical insulin on cutaneous wound healing in rats with or without acute diabetes. Clin Exp Dermatol. 35:180–185.
- Arunachalam K, Parimelazhagan T. 2013. Anti-inflammatory, wound healing and in-vivo antioxidant properties of the leaves of Ficus amplissima Smith. J Ethnopharmacol. 145:139–145.
- Baytop T. 1999. Therapy with medicinal plants in Turkey past and present. İstanbul, Turkey: Tıp Basımevi (In Turkish).
- Beuge JA, Aust SD. 1978. Microsomal lipid peroxidation. Meth Enzymol. 52:302–311.
- Beutler E. 1975. Glutathione in red blood cell metabolism. In: Beutler E, editor. A manual of biochemical methods. 2nd ed. New York: Grune & Stratton. p. 112–114.
- Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L. 1998. Acute inflammatory response in spinal cord following impact injury. Exp Neurol. 151:77–88.
- Davis PH. 1967. Flora of Turkey and the East Aegean Islands. Edinburgh: Edinburgh University Press.
- Demirci B, Demirci F, Baser KHC. 2003. Composition of the essential oil of Cotinus coggygria Scop. from Turkey. Flavour Fragr J. 18:43–44.
- Dulger B, Hacioglu N, Bilen S. 2009. Antimicrobial activity of Cotinus coggygria from Turkey. Asian J Chem. 21:4139–4140.
- Duran A. 1998. Akseki (Antalya) ilçesindeki bazı bitkilerin yerel adları ve etnobotanik özellikleri. Ot Sistematik Botanik Dergisi. 5:77–92. (In Turkish).
- Gao X, Ohlander M, Jeppsson N, Björk L, Trajkovski V. 2000. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J Agric Food Chem. 48:1485–1490.
- Gautam MK, Gangwar M, Singh SK, Goel RK. 2015. Effects of Azardirachta indica on vascular endothelial growth factor and cytokines in diabetic deep wound. Planta Med. 81:713–721.
- Gupta A, Upadhyay NK, Sawhney RC, Kumar R. 2008. A poly-herbal formulation accelerates normal and impaired diabetic wound healing. Wound Repair Regen. 16:784–790.
- Hegnauer R. 1964. Chemotaxonomie der Pflanzen. Basel und Stuttgart: Birkhauser Verlag.
- Jackson CJ, Xue M, Thompson P, Davey RA, Whitmont K, Smith S, Buisson-Legendre N, Sztynda T, Furphy LJ, Cooper A, et al. 2005. Activated protein C prevents inflammation yet stimulates angiogenesis to promote cutaneous wound healing. Wound Repair Regen. 13:284–294.
- Kultur S, Bitis L. 2007. Anatomical and preliminary chemical studies on the leaves of Cotinus coggygria Scop. (Anacardiaceae). J Fac Pharm Istanbul. 39:65–71.
- Kumar N, Gupta AK. 2009. Wound-healing activity of Onosma hispidum (Ratanjot) in normal and diabetic rats. J Herbs Spices Med Plants. 15:342–351.
- Matić S, Stanić S, Mihailović M, Bogojević D. 2015. Cotinus coggygria Scop.: an overview of it chemical constituents, pharmacological and toxicological potential. Saudi J Biol Sci. doi: 10.1016/j.sjbs.2015.05.012. [In press]. Available from: http://www.sciencedirect.com/science/article/pii/S1319562X15001138.
- Novaković M, Vučković I, Janaćković P, Soković M, Filipović A, Tešević V, Milosavljević S. 2007. Chemical composition, antibacterial and antifungal activity of the essential oils of Cotinus coggygria from Serbia. J Serb Chem Soc. 72:1045–1051.
- Nıćıforovıć N, Mıhaılovıć V, Maskovıć P, Solujić S, Stojković A, Pavlović Muratspahić D. 2010. Antioxidant activity of selected plant species; potential new sources of natural antioxidants. Food Chem Toxicol. 48:3125–3130.
- Pavlov DV, Ivanova DG, Eftimov M, Tzaneva MA, Nashar MA, Kobakova I, Valcheva-Kuzmanova SV. 2013. Effect of aqueous infusion from Cotinus coggygria leaves on indomethacin-induced gastric mucosal damage and oxidative stress in rats. Scr Sci Med. 45:32–38.
- Pinto SC, Bueno FG, Panizzon GP, Morais G, Dos Santos PV, Baesso ML, Leite-Mello EV, de Mello JC. 2015. Stryphnodendron adstringens: clarifying wound healing in streptozotocin-induced diabetic rats. Planta Med. 81:1090–1096.
- Ponrasu T, Suguna L. 2012. Efficacy of Annona squamosa on wound healing in streptozotocin-induced diabetic rats. Int Wound J. 9:613–623.
- Prasad SK, Kumar R, Patel DK, Hemalatha S. 2010. Wound healing activity of Withania coagulans in streptozotocin-induced diabetic rats. Pharm Biol. 48:1397–1404.
- Reddy GK, Enwemeka CS. 1996. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 29:225–229.
- Sayar A, Guvensen A, Ozdemir F, Öztürk M. 1995. Muğla (Türkiye) İlindeki Bazı Türlerin Etnobotanik Özellikleri. Ot Sistematik Botanik Dergisi. 2:151–160. (In Turkish).
- Sınger AJ, Clark RA. 1999. Cutaneous wound healing. N Engl J Med. 341:738–746.
- Stadelmann WK, Digenis AG, Tobin GR. 1998. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg. 176:26S–38S.
- Tanchev SS, Timberlake CF. 1969. Anthocyanins in leaves of Cotinus coggygria. Pyhtochemistry. 8:2367–2369.
- Taoka Y, Okajima K, Uchiba M, Murakami K, Kushimoto S, Johno M, Naruo M, Okabe H, Takatsuki K. 1997. Role of neutrophils in spinal cord injury in the rat. Neuroscience. 79:1177–1182.
- Trengove NJ, Stacey MC, Macauley S, Bennett N, Gibson J, Burslem F, Murphy G, Schultz G. 1999. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 7:442–452.
- Wackenfors A, Gustafsson R, Sjögren J, Algotsson L, Ingemansson R, Malmsjö M. 2005. Blood flow responses in the peristernal thoracic wall during vacuum-assisted closure therapy. Ann Thorac Surg. 79:1724–1730.
- Westernburg HE, Lee KJ, Lee SK, Fong HH, van Breemen RB, Pezzuto JM, Kinghorn AD. 2000. Activity-guided isolation of antioxidative constituents of Cotinus coggygria. J Nat Prod. 63:1696–1698.
- Zhang R, Zeng Q, Deng Y, Zhang M, Wei Z, Zhang Y, Tang X. 2013. Phenolic profiles and antioxidant activity of litchi pulp of different cultivars cultivated in Southern China. Food Chem. 136:1169–1176.
