Abstract
Context: “Aroeira” [Myracrodruon urundeuva Allemão (Anacardiaceae)] is a tree whose leaves have been studied for therapeutic purposes in medicine and dentistry.
Objective: The study chemically identifies the leaf extract of aroeira and determines its effect on human gingival fibroblasts.
Materials and methods: An 80% methanol leave extract was obtained by maceration and chemically identified through flow-injection analysis–electrospray ionization–ion trap–tandem mass spectrometry (FIA–ESI–IT–MSn). Cytotoxicity of the aroeira’s methanol extract was evaluated in lineage of fibroblasts. Adherent cells were treated with different concentrations of aroeira’s methanol extract in the medium: 0.1, 1, 10, 100 and 1000 μg/mL. Control cells were cultivated in the medium only. Analyses were done at 24, 48, 72 and 96 h of culture by neutral red assay; and at 24, 48 and 96 h by crystal violet assay.
Results: FIA–ESI–IT–MS analysis determined the presence of compounds, for the first time in the species: quercetin-O-glucuronide and quercetin-O-deoxyhexose-O-glucose in the extract. On one hand, neutral red and crystal violet assay showed a reduction (to 50% up until 100%) of cellular viability of groups of 100 and 1000 μg/mL compared with control at 96 h (p < 0.05). On the other hand, lower concentrations (0.1; 1 and 10 μg/mL) of the extract were similar to that of the control at 96 h (p < 0.05), in general.
Conclusions: In view of the results, we can conclude that the extract of aroeira presents tannins and flavonoids. Furthermore, the extract is capable of modulating the viability of human gingival fibroblasts according to its concentration.
Introduction
Medicinal plants are recognized as being the base for popular medicine in Brazil, a belief derived, during the colonization period, from a mixture of African, European and indigenous Brazilian cultural influences (Cartaxo et al. Citation2010). Phytotherapeutic drugs are a pharmaceutic preparation of medicinal herbs (extracts, tinctures, ointments and capsules) obtained from one or more plants used in the treatment of many diseases (Cartaxo et al. Citation2010).
One of the Brazilian Cerrado species with many interesting activities reported in popular medicine and has attracted attention in diverse fields is Myracrodruon urundeuva Allemão (Anacardiácea). The Myracrodruon genus was described by Freire Allemão and Freire Allemão (Citation1862) based on M. urundeuva.
According to Souza (Citation2012), analyzing the stability of the hydroalcoholic extract of the leaves of the aroeira can verify an easy decomposition due to the common characteristics of the gallotannins present that are responsible for its classification as hydrolyzable tannins where depsidic connections are easily broken by solvolysis (Souza Citation2012). Furthermore, the phytochemical analysis of 70% ethanol extract of the leaves and bark showed constituents such as gallic acid, methyl gallate, ethyl gallate, chlorogenic acid and protocatechuic acid (Souza Citation2012).
The cellular mechanisms and details regarding the aroeira extract’s effect on cells and tissue, as well as secure concentration/dosage, are little known. The M. urundeuva bark extract showed cytotoxic activity in MDA-MB-435, HCT-8 and SF-295, three carcinogenic lineages (Mahmoud et al. Citation2011). The M. urundeuva seed’s ethanol extract also demonstrated cytotoxic activity toward in vitro human tumour cells, being two times more active against the HL-60 leucaemic lineage than against SF-295 glioblastoma cells and 180 sarcoma (Ferreira et al. Citation2011).
Some adverse effects of the extracts of aroeira have been described, although the aroeira generally provides positive results. The bark extract of Schinus terebinthifolius (“aroeira-da-praia”) and M. urundeuva (“aroeira-do-sertão”) induced deficient bone formation in rats’ puppies. A high dosage of S. terebinthifolius decreased significantly the number of haemocytes, haemoglobin and blood red cells. In the case of M. urundeuva extract, there is a significant decrease in haemocyte values, despite an insignificant decrease in the haemoglobin and erythrocyte numbers (Carlini et al. Citation2013).
Heretofore, few studies have appeared in the literature characterizing aroeira extract, such as its cytotoxic effect in oral cells. Therefore, the aim of the study was to characterize the methanol extract of M. urundeuva and evaluate its cytotoxic effect in human gingival fibroblasts at different concentrations.
Materials and methods
Plant material and extraction
Fresh leaves of M. urundeuva were collected in 2010 at Horto Florestal in Bauru, São Paulo, Brazil. Voucher specimens were prepared, identified by A. L. Dokkedal, and deposited at the Herbarium Rioclarense (HRCB) of the Universidade Estadual Paulista “Júlio de Mesquita Filho,” IB-Rio Claro, Brazil, under code number HRCB59831.
Fresh leaves were dried at 40 °C for 48 h. The separated powdered leaves (ca. 604 g) were extracted with MeOH/H2O (8:8 v/v) via maceration at room temperature. The filtrate was concentrated to dryness under a reduced pressure of 40 °C in order to provide the hydroalcoholic extract (304 g).
Chemical profiling and identification
The chemical profile of the 80% MeOH extract from M. urundeuva leaves was achieved via flow injection analysis (FIA) by using mass spectrometer (MS) Accela (Thermo Scientific, San Jose, CA), LCQ fleet with ion trap analyzer (IT) and electrospray ionization (ESI) in a negative mode. The 80% MeOH extract was dissolved in MeOH/H2O (8:2 v/v) and injected at a final concentration of 1 mg/mL. This solution was infused in the ESI source by FIA by using a syringe pump; the flow rate was 33 μL/min. The capillary voltage was −20 V, the spray voltage was 4 kV, and the tube lens offset was −55 V. The capillary temperature was 275 °C. Nitrogen was used both as a drying gas at a flow rate of 60 (arbitrary units) and as a nebulizing gas. The nebulizer temperature was set at 280 °C, and a potential of −4 V was used on the capillary. Negative ion mass spectra were recorded in the range of m/z 200–2000. The first event was a full-scan spectrum to acquire data on the deprotonated compounds within the scan range, and a 1-s scan event was an experiment performed using a data-dependent scan on a deprotonated molecule [M–H]−. The constituents present in the 80% MeOH extract were identified by a comparison of their MS/MSn data with authentic compounds and data from the literature (Da Silva et al. Citation2011).
Cell culture
The cells used in this study were human gingival fibroblasts (FGH lineage) that were obtained by primary culture and stored in liquid nitrogen. This research was approved by the Ethical Committee on Human Research of the Bauru School of Dentistry, University of São Paulo (#086/2011). The cells were cultured in a conventional Dulbecco’s Modified Eagle’s Medium (DMEM), supplemented by a 10% foetal bovine serum (FBS) and a 1% antibiotic–antimycotic solution (penicillin 10,000 UI, streptomycin 0.050 g/L, amphotericin B) (Cultilab™, Campinas, Brazil) in a humidified air–5% carbon dioxide (CO2) atmosphere. Cells from the sixth passage were used for the experiment in a concentration of 2 × 10³ cells/well in 96-well plates (TPP®). The experimental groups were in sextuplicate. After 24 h of incubation, the medium was substituted by a conditioned medium with different concentrations of aroeira’s methanol extracts: 0 (control group); 0.1; 1; 10; 100 and 1000 μg/mL. The cell viability was analyzed at 24, 48, 72, and 96 h after the addition of conditioned medium by colorimetric assays.
Cellular viability analysis
Neutral red
Started from a stock solution, 0.4% neutral red was obtained from a 50 μg/mL neutral red solution in DMEM. An obtained solution remained overnight in a 37 °C oven to allow for the precipitation of crystals that were then filtered in a millipore filter (0.22 μm). The cells were treated with this solution (50 μg/mL) and the incubation plate for 3 h at 37 °C to stain lysosomes of the viable cells. The cells were washed with PBS-Ca+2, and the stain was extracted in a 50% ethanol solution and 1% acetic acid, adding 200 μL of this solution per well (Volpato et al. Citation2011). Next, the optical density was determined at 540 nm (FluoStar OPTIMA, microplate reader, BMG LABTECH, Ortenberg, Germany).
Crystal violet
Cells were plated in a 2 × 103 cells/well proportion in a 96-well plate for 24, 48, and 96 h. Then, the viable cells were washed with PBS and fixed in absolute glacial ethanol and acetic acid (3:1, vol/vol) for 10 min at ambient temperature. The cells were stained with a 0.1% crystal violet (weight/vol) for 10 min at ambient temperature. The stain excess was removed for decantation and washed twice with distilled water. The stain was extracted in a 10% acetic acid (vol/vol), and optical density was available to 550 nm (FluoStar OPTIMA, microplate reader, BMG LABTECH, Ortenberg, Germany).
Statistical analysis
The statistic was realized in variance analysis by one criterion, followed by analysis with Tukey’s test (p < 0.05). For all the analyses, p values <0.05 were considered to be statistically significant. All the statistics tests were applied by Statistical Program 11.0.s (SPSS Inc., Chicago, IL).
Results
Chemical profiling and identification
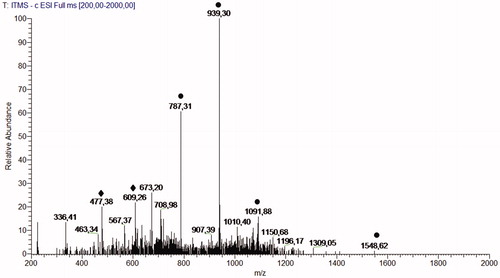
The full-scan spectra obtained via FIA–ESI–IT–MS of the 80% MeOH leaves extract of M. urundeuva () highlighted the presence of precursor ions related to hydrolyzable tannins and flavonoids glycosides. Second-generation product ion spectra of precursor ions at m/z 1547 [M-H]−, m/z 1091 [M-H]−, m/z 939 [M-H]− and m/z 787 [M-H]− confirm the presence of a series of galloylglucose compounds after neutral losses of 152 DA. Second-generation spectra of the precursor ion at m/z 477 [M-H]− generate an ion at m/z 301 [M–176–H]−, confirming the presence of quercetin-O-glucuronide (Kajdžanoska et al. Citation2010), and the precursor ion at m/z 609 [M-H]− generates an ion at m/z 301 [M–308–H]−, confirming the presence of quercetin-O-deoxyhexose-O-glucose, compounds described for the first time in this species.
Analysis of neutral red in periods of 24, 48, 72 and 96 h
The cellular viability results that were analyzed by the neutral red assay are given in . In the 24 h period, viability was similar at all the tested concentrations except 1000 μg/mL, which presented decreased cellular viability in relation to the control (p < 0.05). After 48 h, all the tested concentrations, including the control group, were similar; only the 1000 μg/mL concentration showed decreased cellular viability (p < 0.05). After 72 h of observation, a cellular viability reduction in the 1000 μg/mL and 100 μg/mL concentrations was compared with the other concentration amounts and the control (p < 0.05). The 96 h period results were similar to those for 72 h ().
Table 1. Optical density values (mean ± standard deviation) in neutral red assay in all the concentrations and experimental periods.
Analysis of crystal violet in periods of 24, 48 and 96 h
In terms of the cellular viability results (optical density value) that are available, the crystal violet assay can be observed in . In a 24 h period, there were no cellular viability differences between the tested concentrations (p > 0.05), but a decrease in 1000 μg/mL concentration was noted. After 48 h, the results remained similar; however, although there was no statistical difference, the optical density values increased according to the dilution increase. After 96 h, the 1000 μg/mL concentration did not have a reading, and 100 μg/mL showed a significantly cellular viability compared with the other concentrations and the control (p < 0.05) ().
Table 2. Optical density values (mean ± standard deviation) crystal violet assay in all the concentrations and experimental periods.
Discussion
The present study results showed that methanol extract of aroeira (Myracrodruon urundeuva) presented, in its chemical composition, galloylglucose and the glycosides of flavonoids, especially quercetin-O-glucuronide and quercetin-O-deoxyhexose-O-glucose. The cytotoxicity assay in human gingival fibroblasts showed a cellular viability reduction standard when the extract groups were in the higher concentrations (100 and 1000 μg/mL).
Studies on the Anacardiaceae chemical constituent report the presence of flavonoids, in addition to biflavonoids, terpene, xanthone, chalcone, and, mainly, total lipids and phenolic derivatives (Gebara et al. Citation2011; Nawwar et al. Citation2011; Souza Citation2012). This information coincides with our results which classified the flavonoids, gallic acid and quercetin derivatives in the extract.
Souza’s work (2012) used the HPLC-PAD chromatograph method to identify the following chemical constituents: gallic acid, methyl gallate, ethyl gallate, chlorogenic acid and protocatechuic acid. The flavonoid can be present in both a state free from glycoside and a state associated with glycoside (Di Carlo et al. Citation1999). This way, our results agree with Souza (Citation2012), who once obtained suggestive results that associated glucose with flavonoids.
In the present study, it was possible to note that both colorimetric assays demonstrated similar results to cellular viability: reduction happened over time and with greater concentration extracts. Once the other concentrations were similar to the control group, we suggested the use of a concentration of at least 0.1 μg/mL to remove toxicity risk in a future studies.
Although some authors (Pell Citation2004) showed the absence of significant toxic effects of aroeira extracts, others described some toxicity, mainly when the ethanol extracts were used (Machado et al. Citation2012). On one hand, aroeira’s ethanol extracts induced more oedema in mice and maintained histological signs of inflammation for prolonged periods. On the other hand, the aqueous extracts are biocompatible (Machado et al. Citation2012). In the study by Machado et al. (Citation2012), the ethanol extract concentration was 80 mg/mL. The present study showed that, with higher dilutions of the extract (0.1, 1.0 and 10 μg/mL), there is no cytotoxic effect, even with a methanol extract.
Queires et al. (Citation2006) showed that crude aroeira extracts [Schinus terebinthifolius Raddi (Anacardiaceae)] could promote cellular proliferation inhibition of the cell linage from human prostate carcinoma. Their work revealed that polyphenol fraction promoted a termination of the cellular cycle (G0/G1) and induced apoptosis. These results were similar to our study, where there was a reduction of cellular viability when fibroblasts were treated with extract of aroeira with high concentration. Another interesting point, discussed by the same authors, is the suggestion of a mechanism of action of autophagy induction in cells by polyphenols of aroeira because a change was observed in the activity of the lysosomal acid phosphatase. These data converge to a result found in our study: viability, measured by the neutral red test on cells treated with the extract. We know that the neutral red test is based on the staining of the lysosomes in viable cells. Neutral red’s 24 h results for the higher concentrations presented high values, but, in the last periods, these values decreased. This could be evidence that there was a lysosomal acid phosphatase activation to begin the death-by-autophagy process in the treated cells with a higher concentration of the aroeira extract, following the same mechanism proposed by Queires et al. (Citation2006).
Some phenolic compounds like gallic acid and its derivatives have a selective cytotoxicity against a variety of tumour cells (human and mice) while preserving normal cells (Pellegrina et al. Citation2005; Ferreira et al. Citation2011). In the work of Sakao et al. (Citation2009), the authors suggest that the apoptotic effects of flavonoids on tumour cells were mainly exerted by hydroxyl groups of the quercetin, suggesting an action model: The hydroxyl groups of quercetin contribute to the generation of intracellular superoxide, inhibiting cell proliferation and inducing apoptosis in abnormal cells (HL-60). These published reports converge in the sense that some plant extracts of components (gallic acid, quercetin, etc.) can promote the control of cell proliferation and induction of death by apoptosis, data similar to those found in our study to higher concentration of the extract.
Conclusion
The aroeira methanol extract presents compounds such as tannin and heterosides of flavonoids. The aroeira’s extract is able to modulate human gingival fibroblast viability according to its concentration by inhibiting the viability in high concentrations until there is no interference with cellular viability at low concentrations.
Acknowledgements
Special thanks to Prof. Heitor Marques Honório (FOB–USP) for the support in the statistical analyses and to coordinator Prof. Vinícius Carvalho Porto of Research Integrated Center 1 from FOB-USP.
Disclosure statement
The authors report that they have no conflicts of interest. Appreciation is also expressed to Fapesp (Processes: #2010/02026-9, #2010/14039-8 and #2011/22243-7) and CAPES (research grant to the first author) for their financial support.
References
- Carlini EA, Duarte-Almeida JM, Jaboch R. 2013. Assessment of the toxicity of the Brazilian pepper trees Schinus terebinthifolius Raddi (aroeira-da praia) e Myracrodruon urundeuva Allemão (aroeira-do-sertão). Phytoter Res. 27:692–698.
- Cartaxo SL, Souza MMA, Albuquerque UP. 2010. Medicinal plants with bioprospecting potential used in semi-arid northeastern Brazil. J Ethnopharmacol 131:326–42.
- Da Silva VC, Napolitano A, Eletto D, Rodrigues CM, Pizza C, Vilegas W. 2011. Characterization of gallotannins from Astronium species by flow injection analysis-electrospray ionization-ion trap-tandem mass spectrometry and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Eur J Mass Spectrom. 17:365–375.
- Di Carlo G, Mascolo N, Izzo AA, Capasso F. 1999. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 65:337–353.
- Ferreira Júnior WS, Ladio AH, Albuquerque UP. 2011. Resilience and adaptation in the use of medicinal plants with suspected anti-inflammatory activity in the Brazilian Northeast. J Ethnopharmacol. 138:238–252.
- Freire Allemão F, Freire Allemão M. 1862. Ordinis therebinthacearum. Trab Comm Sc Expl Séc Bot. 1:3–6.
- Gebara SS, de Oliveira Ferreira W, Ré-Poppi N, Simionatto E, Carasek E. 2011. Volatile compounds of leaves and fruits of Mangifera indica var. coquinho (Anacardiaceae) obtained using solid phase microextraction and hydrodistillation. Food Chem. 127:689–693.
- Kajdžanoska M, Gjamovski V, Stefova M. 2010. HPLC-DAD-ESI-MSn identification of phenolic compounds in cultivated strawberries from Macedonia. Macedonian J Chem Chem Eng. 29:181–194.
- Machado AC, Dezan Junior E, Gomes-Filho JE, Cintra LT, Ruviére DB, Zoccal R, Damante CA, Jardim Junior EG. 2012. Evaluation of tissue reaction to Aroeira (Myracrodruon urundeuva) extracts: a histologic and edemogenic study. J Appl Oral Sci. 20:414–418.
- Mahmoud TS, Marques MR, Pessoa CO, Leticia VC, Lotufo LVC, Magalhães HIF, Moraes MO, Lima DP, Tininis AG, Oliveira JE. 2011. In vitro cytotoxic activity of Brazilian Middle West plant extracts. Braz J Pharmacogn. 21:456–464.
- Nawwar M, Hussein S, Ayoub N, Hashim A, El-Sharawy R, Lindequist U, Harms M, Wende K. 2011. Constitutive phenolics of Harpephyllum caffrum (Anacardiaceae) and their biological effects on human keratinocytes. Fitoterapia. 82:1265–1271.
- Pell SK. 2004. Molecular systematic of the cashew family (Anacardiaceae). [207 f. Dissertation (Doctor of Philosophy)]. Louisiana: Department of Biological Science, Faculty of the Louisiana State University and Agricultural and Mechanical College.
- Pellegrina CD, Padovani G, Mainente F, Zoccatelli G, Bissoli G, Mosconi S, Veneri G, Peruffo A, Andrighetto G, Rizzi C, Chignola R. 2005. Anti-tumour potential of a gallic acid-containing phenolic fraction from Oenothera biennis. Cancer Lett. 226:17–25.
- Queires LC, Fauvel-Lafètve F, Terry S, De la Taille A, Kouyoumdjian JC, Chopin DK, Vacherot F, Rodrigues LE, Crépin M. 2006. Polyphenols purified from the Brazilian aroeira plant (Schinus terebinthifolius, Raddi) induce apoptotic and autophagic cell death of DU145 cells. Anticancer Res. 26:379–387.
- Sakao K, Fuhii M, Hou DX. 2009. Clarification of the role of quercetin hydroxyl groups in superoxide generation and cell apoptosis by chemical modification. Biosci Biotechnol Biochem. 73:2048–2053.
- Souza LP. 2012. Padronização de extratos vegetais: Astronium urundeuva (Anacardiácea). [Dissertação]. Araraquara, Brazil: Mestrado-Área de Concentração em Quimica- Universidade Estadual Paulista.
- Volpato LE, de Oliveira RC, Espinosa MM, Bagnato VS, Machado MA. 2011. Viability of fibroblasts cultured under nutritional stress irradiated with red laser, infrared laser, and red light-emitting diode. J Biomed Opt. 16:1–7.