Abstract
Context: Obesity has become a major health concern, and it places both personal and economic burdens on the world’s population. Traditional Chinese medicinal herbs are rich source of lead compounds and are possible drug candidates, which may be used to treat this condition.
Objective: This study screened potent pancreatic lipase inhibitors found in traditional Chinese medicinal herbs for ability to treat obesity.
Materials and methods: A porcine pancreatic lipase inhibition assay was established, and the inhibitory activity of 35 traditional Chinese medicinal herbs was evaluated at a concentration of 200 μg/mL. Two elutions of herbal extracts with strong lipase inhibitory activity were further fractionated by preparative high-performance liquid chromatography into 22 sub-fractions each, and these sub-fractions were tested for anti-lipase activity. Sub-fractions, which exhibited strong lipase inhibitory activity, were continuously fractionated into individual compounds. Two active compounds with potent anti-lipase activity were finally isolated and identified from two traditional Chinese medicinal herbs, respectively.
Results: Among 35 traditional Chinese medicinal herbs, the 95% ethanol elutions of Panax notoginseng (Burk.) F.H. Chen (Araliaceae) and Magnolia officinalis Rehd. et Wils (Magnoliaceae) showed strong anti-lipase activity. Two compounds, including 20(S)-ginsenoside Rg3 and honokiol were identified using bioactivity-guided isolation with IC50 = 33.7 and 59.4 μg/mL, respectively.
Conclusion: 20(S)-ginsenoside Rg3 and honokiol might be suitable candidates for the treatment of obesity.
Introduction
Obesity has become a serious public health problem worldwide (Stevens et al. Citation2012). Obesity not only causes diseases and other painful conditions, such as hyperlipidemia, hypertension, arteriosclerosis, non-insulin-dependent diabetes mellitus, and coronary heart disease (Jung Citation1997; Kopelman Citation2000) but also places an enormous economic burden on individuals and society. In 2005, about 190 billion had been expensed for obesity-related healthcare in the U.S. alone (Lehnert et al. Citation2013). Medical costs have increased dramatically and may continue to grow (Wang et al. Citation2008; Mora et al. Citation2015). In addition to these direct expenses, obesity also causes loss of productivity (Trogdon et al. Citation2008). The research and development of new anti-obesity drugs has recently drawn attention from both the pharmaceutical industry and academic research centers.
Obesity involves the accumulation of excessive amounts of body fat (Hill et al. Citation2000). The subjects were fed high-fat diets, and triacylglycerols from those fats degraded by lipase and absorbed by intestinal cells (Shi & Burn Citation2004). In the digestion process, pancreatic lipase is a key lipid-digesting enzyme (Hadvary et al. Citation1988). Inhibiting pancreatic lipase is one method to prevent and treat obesity (Birari & Bhutani Citation2007). For instance, one pancreatic lipase inhibitor, orlistat, was approved by U.S. Food and Drug Administration in 1999 to treat obesity (Leung et al. Citation2003). Although the drug has a significant anti-obesity effect, it also causes non-negligible gastrointestinal side effects (Cheung et al. Citation2013). It is important to find and develop more pancreatic lipase inhibitors that are safe and effective and do not share orlistat’s drawbacks.
Natural products from traditional medicinal plants and microbial sources are recognized as an important source of novel drug leads (Molinari Citation2009). About 50% of approved drugs are derived from natural products (Newman & Cragg Citation2007). In recent years, natural products have been found to exhibit promising activity toward the inhibition of pancreatic lipase (Birari & Bhutani Citation2007; de la Garza et al. Citation2011). This has drawn the interest of a large number of researchers (Kim & Kang Citation2005; Sharma et al. Citation2005; Slanc et al. Citation2009; Zheng et al. Citation2010; Ramirez et al. Citation2012; Roh & Jung Citation2012). Their efforts are likely to render the identification and development of potent pancreatic lipase inhibitors from traditional Chinese medicinal herbs, a viable alternative strategy for safe and effective anti-obesity drug discovery (de la Garza et al. Citation2011).
In this study, 35 traditional Chinese medicinal herbs that showed promising inhibitory activity against obesity, hyperlipidemia, or both under molecular docking analysis and literature mining were selected for discovery of new lead compounds and pancreatic lipase inhibitors through bioassay-guided isolation.
Materials and methods
Plant materials and chemicals
All herbs were supplied by a Kangji drugstore from May to June 2013 and identified by Dr. Zhenzhong Wang (Jiangsu Kanion Pharmaceutical Co. Ltd.). A collection of voucher specimens is available for confirmation in the research center of Jiangsu Kanion Pharmaceutical Co. Ltd. (Lianyungang, P.R. China). Porcine pancreatic lipase (PPL) type VI-S and p-nitrophenylpalmitate (PNP) were purchased from Sigma Chemical (Shanghai, China). 20(S)-Ginsenoside Rg3 and honokiol were obtained from National Institutes for Food and Drug Control (Beijing, China). High-performance liquid chromatography-grade (HPLC) acetonitrile was provided by Tedia (Anhui, China). All other chemicals and solvents were of analytical grade.
Preparation of samples
All herbs were air-dried and ground into fine powder. The powders (50 g) were extracted with distilled water (10 times volume per weight) under reflux twice, 2 h each time. After filtering, the water extract was adsorbed onto HP-20 macroporous resin (100 mL) (Diaion HP-20, Mitsubishi Chemical Ind. Ltd., Tokyo, Japan). Then, the HP-20 column was washed with H2O (400 mL), 20% ethanol (400 mL), 40% ethanol (400 mL), 95% ethanol (400 mL) in sequence. Then, the solutions were dried on a rotary vacuum evaporator at 50 °C. Samples were stored under dry conditions for further studies.
The 95% ethanol elution of Magnolia officinalis Rehd. et Wils (Magnoliaceae) and Panax notoginseng (Burk.) F.H. Chen (Araliaceae) were fractionated by preparative HPLC (Agilent 1260 equipped with multiple wavelength detector and a Fuji-C-18 column). The mobile phase consisted of water (A) and acetonitrile (B) at a flow rate of 30 mL/min: 20–30% B at 0–20 min; 30–45% B at 20–40 min; 45–90% B at 40–55 min; 90–100% B at 55–60 min; 100% B at 60–70 min. The detection wavelength was set at 210 nm. The sub-fractions were collected every 3 min and concentrated by distillation under reduced pressure. Then the sub-fractions were lyophilized and stored at room temperature.
PPL inhibition assay
Lipase activity was measured using PNP as substrate (Winkler & Stuckmann Citation1979; Lee et al. Citation1993). PPL stock solution (1.2 mg/mL) was prepared in 50 mM Tris-HCl buffer pH 8.0 (combined with 0.2% sodium deoxycholate and 0.1% gum arabic). This mixture was aliquoted and stored at −70 °C. PNP was dissolved in isopropanol at a stock solution of 25 mM and stored at 4 °C. To determine inhibitory activity against lipase, the samples and compounds were pre-incubated with the enzyme (5 μg/mL) for 10 min in Tris-HCl buffer at 37 °C. The reaction was then started by adding 0.1 mL PNP (0.5 mM). The final volume was 0.2 mL. After incubation at 37 °C for 60 min, the absorbance at 405 nm was measured using a micro-plate reader (Molecular Devices, Sunnyvale, CA).
The inhibitory rate was calculated using the following formula (Zheng et al. Citation2010):
Here, A is the activity in the absence of the inhibitor; a is the negative control in the absence of inhibitor; B is the activity in the presence of the inhibitor; and b is the negative control in the presence of the inhibitor.
HPLC (LC-DAD/Q-TOF/MS) analysis
Agilent 1290 HPLC (Santa Clara, CA) equipped with diode array detector and quality Time-of-Flight 6538 mass spectrometer was used. Chromatographic separation was performed on a Phenomenex Luna C18 column (Torrance, CA) for the 19th sub-fraction of Magnolia officinalis (MO-19) and the 17th sub-fraction of Panax notoginseng (PN-17). The mobile phase was composed of water (A) and acetonitrile (B). The gradient elution was performed as follows: 0–25 min, 30–35% B; 25–55 min, 35–50% B; 55–80 min, 50–90% B at a flow rate of 1 mL/min for MO-19; 0–30 min, 35–50% B; 30–35 min, 50% B; and 35–50 min, 50–90% B at a flow rate of 1 mL/min for PN-17. The detection wavelengths were set at 230 and 210 nm for MO-19 and PN-17, respectively. For MS detection, the parameters were set as follows: column oven temperature, 30 °C; collision gas, ultra-high-purity helium; nebulizing gas, high-purity nitrogen; capillary voltage, 4000 V; drying gas flow rate, 10 L/min; gas temperature, 350 °C; pressure of nebulizer, 50 psi; skimmer voltage, 65 V; and fragmentor voltage, 100 V. The mass range was set to m/z 100–3200 Da. Each sample was analyzed in positive modes to determine its structure.
Statistical analysis
All results are expressed as the means ± standard deviation (n = 3). The significance of differences from the control was determined by Duncan’s test and Kruskal–Wallis test, and p < 0.05 was considered significant. The IC50 values were calculated using GraphPad Prism (La Jolla, CA).
Results
Anti-lipase activity of herbal elutions
In the current study, 35 traditional Chinese herbal medicines were extracted and eluted with 20%, 40% and 95% ethanol. Then the pancreatic lipase inhibitory activities of the elutions were tested at a concentration of 200 μg/mL. The 95% ethanol elution of most herbal extracts exhibited stronger anti-lipase activity than the 20% and 40% ethanol elutions (data not shown). Among the thirty-five 95% ethanol elutions, the inhibitory rates of five herbal elutions were greater than 20%, including Panax notoginseng (58.1%), Magnolia officinalis (44.2%), Arnebia euchroma (Royle) Johnst (Boraginaceae) (28.3%), Prunus persica (L.) Batsch (Rosaceae) (28.7%) and Paeonia suffruticosa Andr (Ranunculaceae) (20.4%). The other elutions exhibited weak inhibitory activities against lipase, ranging from 1.1% to 18.6% ().
Table 1. PPL inhibitory activities of various Chinese medicinal herbs.
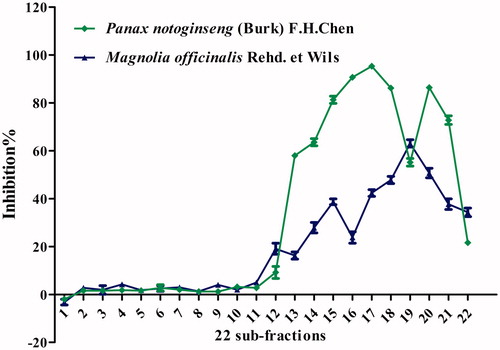
Anti-lipase activity of sub-fractions from Panax notoginseng and Magnolia officinalis
Both Panax notoginseng and Magnolia officinalis exhibited strong anti-lipase activity. Twenty-two sub-fractions from the 95% ethanol elution of Panax notoginseng and Magnolia officinalis were then prepared using preparative HPLC. The inhibitory activities of the sub-fractions were investigated on pancreatic lipase. As shown in , PN-17 of Panax notoginseng exhibited the strongest anti-lipase activity of these 22 sub-fractions, and MO-19 of Magnolia officinalis showed the most potent anti-lipase activity out of the 22 sub-fractions.
Identification of active compounds
PN-17 and MO-19 were separated by LC-DAD/Q-TOF/MS ( ). The retention time of the reference substances were compared, the three peaks of PN-17 were attributed to ginsenoside Rh4, 20(S)-ginsenoside Rg3 and 20(R)-ginsenoside Rg3 (Yao et al. Citation2011; Kim et al. Citation2013), and the only peak of MO-19 was attributed to honokiol (Lee et al. Citation2011). Their identities were then confirmed by mass spectrometry (). The result of the analysis of the compound activities further suggested that the second peak had the strongest anti-lipase effect of the three peaks of PN-17 (). The dose-response curve of 20(S)-ginsenoside Rg3 and honokiol revealed the two compounds to be strong pancreatic lipase inhibitors ().
Figure 2. PPL inhibitory activity of 20(S)-ginsenoside Rg3. (a) Chromatograms of (A) reference substance 20(S)-ginsenoside Rg3, (B) mixture sub-fraction PN-17 including (1) ginsenoside Rh4, (2) 20(S)-ginsenoside Rg3, (3) 20(R)-ginsenoside Rg3 at 210 nm. (b) PPL inhibitory activity of three compound components of PN-17. The final concentration of three compound components was 50 μg/mL. (c) Concentration dependent inhibition of PPL by 20(S)-ginsenoside Rg3. The inhibition rate was plotted against the log of the cumulative doses of 20(S)-ginsenoside Rg3 (Mean ± SD, n = 3).
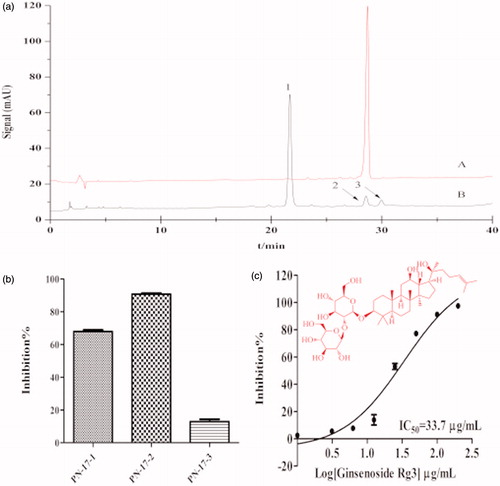
Figure 3. PPL inhibitory activity of honokiol. (a) Chromatograms of (A) reference substance honokiol, (B) sub-fraction MO-19 at 230 nm. (b) The structure of honokiol. (c) Concentration dependent inhibition of PPL by honokiol. The inhibition rate was plotted against the log of the cumulative doses of honokiol (Mean ± SD, n = 3).
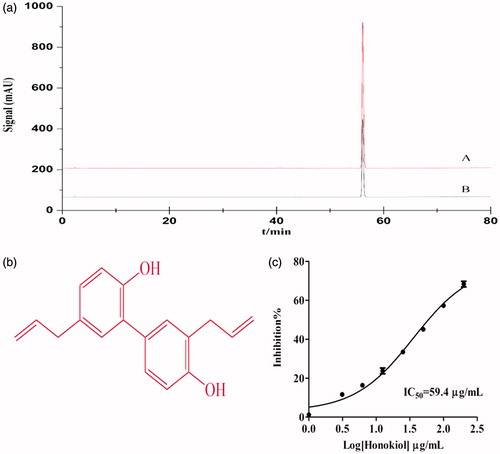
Table 2. ESI mass analysis of PN-17-2 and MO-19.
Discussion
Natural products are vastly diverse in chemical structure and biological activity. They have been an important source of lead compounds for drug discovery (Xie et al. Citation2012; Tsai et al. Citation2013; Deng et al. Citation2014; Zhou et al. Citation2014). The purpose of this study was to screen potential pancreatic lipase inhibitors derived from traditional Chinese medicinal herbs for utility in anti-obesity treatment. In this study, 35 herbs were extracted and eluted with 20%, 40% and 95% ethanol. Then their anti-lipase activity was assessed in vitro. The 95% ethanol elutions of Panax notoginseng and Magnolia officinalis exhibited strong anti-lipase activity, so these were further fractionated into 22 sub-fractions each. PN-17 and MO-19 were found to have the most potent anti-lipase effects. Then 20(S)-ginsenoside Rg3 and honokiol were found in PN-17 and MO-19, respectively.
Magnolia officinalis has been reported to contain several bioactive components, including magnolol, honokiol, 4-O-methylhonokiol, and obovatol (Lee et al. Citation2011). A recent study indicated that Magnolia officinalis extract and its active component, 4-O-methylhonokiol, could protect mice from high-fat diet-induced obesity by improving lipid metabolism (Zhang et al. Citation2014). The results of the current study showed that it is reasonable that honokiol, a lipase inhibitor, may be the anti-obesity component of Magnolia officinalis.
Reports have shown that saponins from Platycodi radix, Acanthopanax senticosus and Panax japonicus can inhibit pancreatic lipase and ameliorate the body weight gain induced by high-fat diets in mice (Han et al. Citation2002; Han et al. Citation2005; Li et al. Citation2007). A previous study suggested that 20(S)-ginsenoside Rg3 can inhibit lipid accumulation during 3T3-L1 adipocyte differentiation through AMPK and PPAR-γ signaling pathways (Hwang et al. Citation2009). The current study showed 20(S)-ginsenoside Rg3 to be the active component of Panax notoginseng against pancreatic lipase. Taken together, 20(S)-ginsenoside Rg3 might be a suitable lead compound for anti-obesity treatment and that it acts by reducing lipid absorption and accumulation (Cicero et al. Citation2003; Kim & Park Citation2003).
Two major, valuable compounds, 20(S)-ginsenoside Rg3 and honokiol, were found to have anti-lipase effects by bioassay-guided isolation. However, in this study, other compounds with strong anti-lipase activity may have been present and gone undetected. One limitation of our study is that some potent compounds in herbal elution may have been present in concentrations too low to be isolated and identified. To address this problem, it may be necessary to combine the compound library of herbal medicine and high-throughput screening.
Here, thirty-five traditional Chinese medicinal herbs were screened for anti-lipase activity. This is the first report to show 20(S)-ginsenoside Rg3 isolated from Panax notoginseng and honokiol isolated Magnolia officinalis to have anti-lipase activity. The findings of this and previous studies suggest that 20(S)-ginsenoside Rg3 and honokiol might be compounds with potent anti-obesity properties. Further investigations are in progress to evaluate the anti-obesity activity of honokiol and 20(S)-ginsenoside Rg3 in animal models.
Funding information
The authors would like to thank the National Science and Technology Major Project-Key New Drug Creation and Manufacturing Program (2013ZX09402203) for financial support.
Disclosure statement
The authors have no conflicts of interest to report. The authors claim responsibility for the content and composition of the paper.
References
- Birari RB, Bhutani KK. 2007. Pancreatic lipase inhibitors from natural sources: unexplored potential. Drug Discov Today. 12:879–889.
- Cheung BM, Cheung TT, Samaranayake NR. 2013. Safety of antiobesity drugs. Ther Adv Drug Saf. 4:171–181.
- Cicero AF, Vitale G, Savino G, Arletti R. 2003. Panax notoginseng (Burk.) effects on fibrinogen and lipid plasma level in rats fed on a high-fat diet. Phytother Res. 17:174–178.
- de la Garza AL, Milagro FI, Boque N, Campión J, Martínez JA. 2011. Natural inhibitors of pancreatic lipase as new players in obesity treatment. Planta Med. 77:773–785.
- Deng X, Jiang M, Zhao X, Liang J. 2014. Efficacy and safety of traditional Chinese medicine for the treatment of acquired immunodeficiency syndrome: a systematic review. J Tradit Chin Med. 34:1–9.
- Hadvary P, Lengsfeld H, Wolfer H. 1988. Inhibition of pancreatic lipase in vitro by the covalent inhibitor tetrahydrolipstatin. Biochem J. 256:357–361.
- Han LK, Zheng YN, Xu BJ, Okuda H, Kimura Y. 2002. Saponins from Platycodi radix ameliorate high fat diet-induced obesity in mice. J Nutr. 132:2241–2245.
- Han LK, Zheng YN, Yoshikawa M, Okuda H, Kimura Y. 2005. Anti-obesity effects of chikusetsusaponins isolated from Panax japonicus rhizomes. BMC Complement Altern Med. 5:9.
- Hill JO, Melanson EL, Wyatt HT. 2000. Dietary fat intake and regulation of energy balance: implications for obesity. J Nutr. 130:284S–288S.
- Hwang JT, Lee MS, Kim HJ, Sung MJ, Kim HY, Kim MS, Kwon DY. 2009. Antiobesity effect of ginsenoside Rg3 involves the AMPK and PPAR-gamma signal pathways. Phytother Res. 23:262–266.
- Jung RT. 1997. Obesity as a disease. Br Med Bull. 53:307–321.
- Kim HY, Kang MH. 2005. Screening of Korean medicinal plants for lipase inhibitory activity. Phytother Res. 19:359–361.
- Kim IW, Sun WS, Yun BS, Kim NR, Min D, Kim SK. 2013. Characterizing a full spectrum of physico-chemical properties of (20S)- and (20R)-ginsenoside Rg3 to be proposed as standard reference materials. J Ginseng Res. 37:124–134.
- Kim SH, Park KS. 2003. Effects of Panax ginseng extract on lipid metabolism in humans. Pharmacol Res. 48:511–513.
- Kopelman PG. 2000. Obesity as a medical problem. Nature. 404:635–643.
- Lee YJ, Lee YM, Lee CK, Jung JK, Han SB, Hong JT. 2011. Therapeutic applications of compounds in the Magnolia family. Pharmacol Ther. 130:157–176.
- Lee YP, Chung GH, Rhee JS. 1993. Purification and characterization of Pseudomonas fluorescens SIK W1 lipase expressed in Escherichia coli. Biochim Biophys Acta. 1169:156–164.
- Lehnert T, Sonntag D, Konnopka A, Riedel-Heller S, König HH. 2013. Economic costs of overweight and obesity. Best Pract Res Clin Endocrinol Metab. 27:105–115.
- Leung WY, Thomas GN, Chan JC, Tomlinson B. 2003. Weight management and current options in pharmacotherapy: orlistat and sibutramine. Clin Ther. 25:58–80.
- Li F, Li W, Fu H, Zhang Q, Koike K. 2007. Pancreatic lipase-inhibiting triterpenoid saponins from fruits of Acanthopanax senticosus. Chem Pharm Bull (Tokyo). 55:1087–1089.
- Molinari G. 2009. Natural products in drug discovery: present status and perspectives. Adv Exp Med Biol. 655:13–27.
- Mora T, Gil J, Sicras-Mainar A. 2015. The influence of obesity and overweight on medical costs: a panel data perspective. Eur J Health Econ. 16:161–173.
- Newman DJ, Cragg GM. 2007. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 70:461–477.
- Ramirez G, Zavala M, Perez J, Zamilpa A. 2012. In vitro screening of medicinal plants used in Mexico as antidiabetics with glucosidase and lipase inhibitory activities. Evid Based Complement Alternat Med. 2012:701261.
- Roh C, Jung U. 2012. Screening of crude plant extracts with anti-obesity activity. Int J Mol Sci. 13:1710–1719.
- Sharma N, Sharma VK, Seo SY. 2005. Screening of some medicinal plants for anti-lipase activity. J Ethnopharmacol. 97:453–456.
- Shi Y, Burn P. 2004. Lipid metabolic enzymes: emerging drug targets for the treatment of obesity. Nat Rev Drug Discov. 3:695–710.
- Slanc P, Doljak B, Kreft S, Lunder M, Janes D, Strukelj B. 2009. Screening of selected food and medicinal plant extracts for pancreatic lipase inhibition. Phytother Res. 23:874–877.
- Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM, Bahalim AN, McIntire RK, Gutierrez HR, Cowan M, et al. 2012. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 10:22
- Trogdon JG, Finkelstein EA, Hylands T, Dellea PS, Kamal-Bahl SJ. 2008. Indirect costs of obesity: a review of the current literature. Obes Rev. 9:489–500.
- Tsai WH, Yang CC, Li PC, Chen WC, Chien CT. 2013. Therapeutic potential of traditional chinese medicine on inflammatory diseases. J Tradit Complement Med. 3:142–151.
- Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. 2008. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring). 16:2323–2330.
- Winkler UK, Stuckmann M. 1979. Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J Bacteriol. 138:663–670.
- Xie W, Zhao Y, Du L. 2012. Emerging approaches of traditional Chinese medicine formulas for the treatment of hyperlipidemia. J Ethnopharmacol. 140:345–367.
- Yao H, Shi P, Shao Q, Fan X. 2011. Chemical fingerprinting and quantitative analysis of a Panax notoginseng preparation using HPLC-UV and HPLC-MS. Chin Med. 6:9.
- Zhang Z, Chen J, Jiang X, Wang J, Yan X, Zheng Y, Conklin DJ, Kim KS, Kim KH, Tan Y, et al. 2014. The magnolia bioactive constituent 4-O-methylhonokiol protects against high-fat diet-induced obesity and systemic insulin resistance in mice. Oxid Med Cell Longev. 2014:965954.
- Zheng CD, Duan YQ, Gao JM, Ruan ZG. 2010. Screening for anti-lipase properties of 37 traditional Chinese medicinal herbs. J Chin Med Assoc. 73:319–324.
- Zhou J, Zhou T, Jiang M, Wang X, Liu Q, Zhan Z, Zhang X. 2014. Research progress on synergistic anti-tumor mechanisms of compounds in traditional Chinese medicine. J Tradit Chin Med. 34:100–105.