Abstract
Context: Solidago virgaurea L. (Asteraceae) is traditionally used as an anti-inflammatory for the treatment of various symptoms including cystitis. However, little is known concerning the constituents responsible for this activity and the mechanism of their action.
Objective: To assess the anti-inflammatory activity of the phenolic-rich fraction of S. virgaurea aerial parts in rats, isolate and assess the activity of the major compounds present.
Materials and methods: An HPLC method was developed for the analysis of the phenolic-rich fraction (EtFr). The in vivo anti-inflammatory activity of the EtFr and four isolated compounds (at 25 and 50 mg/kg) were assessed in adult male rats using the carrageenan-induced rat paw oedema model. The levels of the pro-inflammatory cytokines (TNF-α and IL-1β) were measured using ELISA.
Results: 3,5-O-Dicaffeoylquinic acid (1), 3,4-O-dicaffeoylquinic acid (2), 3,4,5-O-tricaffeoylquinic acid (3) and 4,5-O-dicaffeoylquinic acid (4) were isolated from EtFr. Compound 3 (50 mg/kg) showed a highly significant activity in inhibiting the oedema volume after 3 h (88% of the activity of indomethacin at 10 mg/kg). The EtFr and the isolated compounds largely inhibited the excessive production of the inflammatory mediators TNF-α and IL-1β.
Discussion and conclusion: This is the first report of 3,4,5-tri-O-caffeoylquinic acid (3) in Solidago species. The tricaffeoylquinic acid (3) showed a significantly higher activity than the other three dicaffeoylquinic acids (1, 2, 4) and indomethacin in reduction of TNF-α and IL-1β concentrations (8.44 ± 0.62 and 5.83 ± 0.57 pg/mL compared to 12.60 ± 1.30 and 52.91 ± 5.20 pg/mL induced by indomethacin, respectively).
Introduction
Overproduction of pro-inflammatory cytokines in macrophages is responsible for many inflammatory diseases, including rheumatoid arthritis, atherosclerosis, cystitis and hepatitis (Srinivasan et al. Citation2011). Therefore, inhibiting their production may serve to prevent or suppress a variety of inflammatory diseases.
The genus Solidago comprises about 130 species of herbaceous perennial flowering plants, growing in most parts of the world, that is, Asia, America and Europe. They grow wild or are cultivated, usually for decorative purposes (Li et al. Citation2009). Numerous secondary metabolites such as flavonoids, triterpenoids, saponins, phenolic acids, glucosides, polysaccharides, diterpenes and essential oils were reported for the genus Solidago (Thiem et al. Citation2001).
Solidago virgaurea L. (Asteraceae), commonly known as goldenrod, has been traditionally used as an anti-inflammatory herbal medicine for the treatment of various symptoms including cystitis and as a mouth rinse to treat inflammation of the mouth and throat (Radusiene et al. Citation2015). Most commonly used in phytotherapy are the apical shoots of goldenrod which have been applied in the Middle Ages in the diseases of the urinary tract, nephrolithiasis and prostate (Thiem et al. Citation2001). According to the European Medicines Agency (EMEA Citation2008), goldenrod has been traditionally used in the form of oral comminuted dried herb, tincture (ethanol–water, 45%), or as a dried extract (5–7:1, ethanol–water, 30–60%).
Several compounds such as essential oils, terpenes, sterols, saponins, hydroxybenzoates and phenolic compounds were isolated from S. virgaurea (Bader et al. Citation1992, Citation1995; Thiem et al. Citation2001; Choi et al. Citation2004, Citation2005; Kalemba & Thiem Citation2004; Starks et al. Citation2010). Numerous biological activities of S. virgaurea such as cytotoxic, antimicrobial, antimutagenic, antifungal, analgesic, anti-inflammatory and diuretic were attributed to these active constituents (Metzner et al. Citation1984; Chodera et al. Citation1985, Citation1986, Citation1988, Citation1991; Schilcher & Rau Citation1988; Okpanyi et al. Citation1989; El Ghazaly et al. Citation1992; Strehl et al. Citation1995; Budzianowski Citation1999; Bader et al. Citation2000; Sampson et al. Citation2000; Choi et al. Citation2004; Starks et al. Citation2010; Kolodziej et al. Citation2011).
The diuretic activity was reported to the flavonoids present in S. virgaurea and to the phenolic glycoside leiocarposide (Chodera et al. Citation1985, Citation1986, Citation1988; Schilcher & Rau Citation1988; Budzianowski Citation1999). The anti-inflammatory activity was carried out on the hydroalcoholic extract and also attributed to leiocarposide (Metzner et al. Citation1984; Okpanyi et al. Citation1989; El Ghazaly et al. Citation1992; Strehl et al. Citation1995).
The present study was carried out in order to study the anti-inflammatory activity of the ethyl acetate/n-butanol fraction, rich in phenolic compounds, of S. virgaurea aerial parts in an animal model and to determine the major constituents present in this fraction which may be responsible for this activity. The levels of the pro-inflammatory cytokines, interleukin (IL-1β) and tumour necrosis factor (TNF-α) were assessed in the inflammatory exudate.
Materials and methods
Chemicals
Inflammatory-grade carrageenan was purchased from FMC (Rockland, ME). All the extracts, isolated compounds and standard were dissolved in 0.5% carboxymethylcellulose before being injected into the animals. Indomethacin was purchased from Sigma-Aldrich (Taufkirchen, Germany). TNF-α and IL-1β were quantified using enzyme-linked immunosorbent assay kits (Boster Biological Technology Co., Inc., Valley Ave Pleasanton, CA). All other chemicals were of the highest available commercial grade.
Fractionation of the extract
S. virgaurea extract (20 g; dried aerial parts; ethanol–water, 30:70; dry extract, 5–7:1) was bought from Finzelberg (Vestenbergsgreuth, Germany). It was suspended in 500 mL water and partitioned successively with hexane, chloroform and ethyl acetate/n-butanol mixture (2:1) (500 mL × 2, each). The successive fractions were evaporated (60 °C) under reduced pressure yielding 0.2, 0.7 and 2.9 g, respectively.
HPLC fingerprint of the phenolics rich fraction
An analytical HPLC method was developed for the analysis of the ethyl acetate/n-butanol fraction (EtFr) and to show the major compounds present. An Agilent Technologies 1100 series HPLC equipped with an Agilent 1200 series G1322A quaternary pump and degasser, and a G1314A variable wavelength detector were used (Agilent, Santa Clara, CA). Isolated compounds and the mother fraction (EtFr) were injected into a Lichrospher 100 RP-18, 5 μm, 250 × 4 mm column (Merck, Darmstadt, Germany) equipped with a 5 μm, 10 × 4 mm guard column and maintained at 25 °C. The mobile phases used were acetonitrile (solvent A) and 0.3% O-phosphoric acid in water (solvent B). Gradient elution was carried out at a flow rate of 1.0 mL/min as follows: 10% solvent A into solvent B for 25 min until the concentration of solvent A in B reached 75%. The injection volume was 20 μL and UV detection was performed at 325 nm.
Isolation of compounds from the bioactive ethyl acetate/n-butanol fraction
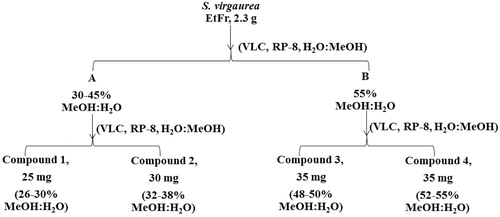
EtFr (2.3 g) was dissolved in 3% methanol in water and applied on a vacuum liquid chromatography (VLC) column loaded with silica gel RP8 (55 mm × 40 mm, 23 g). Elution was carried out by water followed by increasing percentages of methanol in water. Successive VLC columns were prepared to isolate compounds (1–4) according to the scheme in . Fractionation and isolation were monitored by HPLC. A Varian Mercury-VX-300 NMR instrument (Palo Alto, CA) was used for 1H NMR (300 MHz) and 13C NMR (75 MHz) measurements. The NMR spectra were recorded in DMSO-d6, and chemical shifts were given in δ (ppm) relative to TMS as an internal standard.
In vivo anti-inflammatory activity of the phenolic rich fraction and the major isolated compounds
Adult male Sprague-Dawley rats (age, 8 weeks; weight, 180–200 g) were used (Pharmacology and Toxicology Department, Faculty of Pharmacy, Ain Shams University). They were housed at a temperature of 23 ± 2 °C, relative humidity (60%), alternating 12 h light–dark cycle and with free access to water and standard food pellets. All experimental procedures were conducted in accordance with internationally accepted principles for laboratory animal use and care, and were approved by the Ethics Committee (No. 9-031) in accordance with recommendations for the proper care and use of laboratory animals (NIH Publication No. 80-23; revised 1978).
Rats were acclimatized in our animal facility for at least 1 week prior to the experiment. They were then randomly divided into 13 equal groups (1–13), six animals per group. Animals were fasted, with free access to water, 12 h before the experiment. Groups 1 and 2 were given the vehicle (0.5% carboxymethylcellulose) using an intragastric tube. Animals in group 3 received indomethacin (10 mg/kg) as a standard anti-inflammatory drug, whereas remaining groups were orally treated with EtFr and the isolated compounds (1–4) at two dose levels (25 and 50 mg/kg). The two doses were chosen guided by a previous study carried out for the evaluation of the anti-inflammatory activity of Solidago chilensis extracts (Goulart et al. Citation2007). The dosing volume was kept constant (10 mL/kg) for all the orally treated groups. Thirty minutes after oral treatment, group 1 received 0.05 mL of saline, whereas groups 2–13 received 0.05 mL of carrageenan (1% solution in saline) subcutaneously on the plantar surface of the right hind paw. The rats were sacrificed 3 h after the induction of inflammation. The right hind paw volume was measured immediately after carrageenan injection by water displacement using a plethysmometer (model 7140, Ugo Basile, Comerio, Italy). The paw volume was re-measured 1, 2 and 3 h after carrageenan injection (Winter et al. Citation1962; Matsumoto et al. Citation2015).
The mean response for increase in the paw oedema after acute inflammation was calculated
The percentage of inhibition in the mean of the treated group in comparison with the control non-treated group was estimated and calculated according to the following equation:
Measurement of TNF-α and IL-1β levels in the rat paw
Right hind paws were removed. A volume of 0.1 mL of saline containing 10 μM indomethacin was injected to aid removal of the eicosanoid-containing fluid and to stop further production of TNF-α and IL-1β. Paws were incised with a scalpel and suspended off the bottom of polypropylene tubes with Eppendorf pipette tips to facilitate drainage of the inflammatory exudates. For the purpose of the removal of the inflammatory exudates, paws were centrifuged at 1800g for 15 min. TNF-α and IL-1β were quantified in the collected exudates using enzyme-linked immunosorbent assay kits (Boster Biological Technology Co., Inc.) according to the manufacturer’s instructions. Both assays were based on the sandwich technique in which specific antibodies to TNF-α or IL-1β were pre-coated on to a 96-well plate. The specific detection antibodies were biotinylated. The test samples and biotinylated detection antibodies were added sequentially followed by washing. Avidin–biotin–peroxidase complex was added and unbound conjugates were washed. A substrate solution was added to the wells to determine the bound enzyme activity. The colour development was stopped, and the absorbance was read at 450 nm using an ELISA microplate reader (ChroMate-4300, Palm City, FL). The intensity of the colour was directly proportional to the concentration of TNF-α or IL-1β in the sample.
Statistical analysis
Data were expressed as the means ± SEM. The differences between groups were tested by one-way analyses of variance followed by the Tukey post hoc test. All statistical analyses were performed using Graph Pad Instat software version 3 (ISI software, Marina del Rey, CA). The probability of p < 0.05 was considered statistically significant.
Results and discussion
HPLC chromatographic profiling and isolation of major compounds
Several previous reports stated that the phenolic constituents of S. virgaurea were responsible for the anti-inflammatory activity of the aerial parts (Radusiene et al. Citation2015). An HPLC method was developed for the analysis of the phenolic rich bioactive EtFr and an HPLC chromatographic profile was established (). Four major compounds (1–4) were monitored at Rt 10.2, 10.6. 16.2 and 17.0 min, respectively. The four compounds were purified using successive VLC columns as mentioned in . They were identified based on their spectral data analyses ( and ) as 3,5-O-dicaffeoylquinic acid (1), 3,4-O-dicaffeoylquinic acid (2), 3,4,5-O-tricaffeoylquinic acid (3) and 4,5-O-dicaffeoylquinic acid (4) ().
Figure 2. HPLC chromatographic profile of the bioactive ethyl acetate/n-butanol fraction (EtFr), showing major compounds (1–4) at Rt 10.2, 10.6. 16.2 and 17.0 min, respectively.
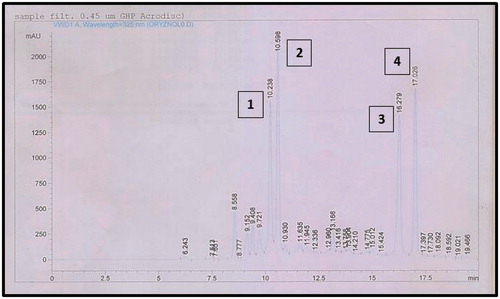
Figure 3. The four compounds isolated from S. virgaurea; 3,5-di-O-caffeoylquinic acid (1), 3,4-di-O-caffeoylquinic acid (2), 3,4,5-tri-O-caffeoylquinic acid (3) and 4,5-di-O-caffeoylquinic acid (4).
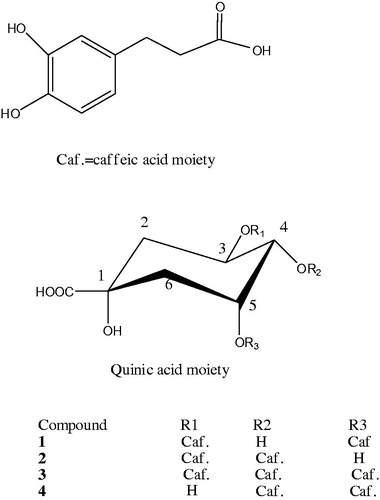
Table 1. 1H NMR chemical shifts (δ in ppm) for compounds (1–4) (DMSO, 300 MHZ, J in Hz).
Table 2. 13C NMR chemical shifts (δ in ppm) for compounds (1–4) (DMSO, 75 MHz).
Compounds 1–4 were obtained as yellowish white powder, 1H and 13C NMR spectral data indicated the presence of two trans-caffeoyl groups in compounds 1, 2 and 4. 13C NMR spectral data of compounds 1–4 showed two methylene carbons, four oxygenated carbons and a carbonyl carbon typical for a quinic acid (Lee et al. Citation2004). 1H and 13C NMR spectral data of compounds 1, 2 and 4 were typical of dicaffeoylquinic acid derivatives (Clifford Citation1986). The position of the two caffeic acid groups was established in 1H NMR by the downfield shift of H-3 and H-5 (δ 5.47 and 5.44, respectively) in compound 1, the downfield shift of H-3 and H-4 (δ 5.35 and 4.94, respectively) in compound 2, and the downfield shift of H-4 and H-5 (δ 4.95 and 5.27, respectively) in compound 4. The position of the two caffeic acid groups was confirmed from the 13C NMR spectral data from the downfield shift of C-3 and C-5 (δ 72.5 and 71.9, respectively) in compound 1, the downfield shift of C-3 and C-4 (δ 72.1 and 75.1, respectively) in compound 2, and the downfield shift of C-4 and C-5 (δ 75.7 and 71.9, respectively) in compound 4. The assignments of the protons and carbons were established by the aid of correlations in the COSY, HSQC and HMBC. From the aforementioned data compounds 1, 2 and 4 were identified as 3,5-di-O-caffeoylquinic acid, 3,4-di-O-caffeoylquinic acid and 4,5-di-O-dicaffeoylquinic acid, respectively ( and ).
1H and 13C NMR spectral data of compound 3 were typical of tricaffeoylquinic acid derivatives (Soliman et al. Citation2008). The position of the three caffeic acid groups was established in 1H NMR by the downfield shift of H-3, H-4 and H-5 (δ 5.42, 4.97 and 5.20, respectively). The position of the three caffeic acid groups was confirmed from the 13C NMR spectral data from the downfield shift of C-3, C-4 and C-5 (δ 72.1, 75.7 and 71.9, respectively). This compound was identified as 3,4,5-tri-O-caffeoylquinic acid ( and ).
This is the first report of 3,4,5-tri-O-caffeoylquinic acid (3) in Solidago species. 3,5-Di-O-caffeoylquinic acid (1) was previously isolated from S. virgaurea (Thiem et al. Citation2001; Choi et al. Citation2004), while 3,4-di-O-caffeoylquinic acid (2) and 4,5-di-O-caffeoylquinic acid (4) were previously reported in other Solidago species (Bradette-Hebert et al. Citation2008).
In vivo anti-inflammatory activity
The carrageenan-induced rat paw oedema model is a reproducible experimental model used for evaluating the activities of anti-inflammatory drugs. Carrageenan stimulates the release of inflammatory and pro-inflammatory mediators including interleukin (IL-1β) and tumour necrosis factor (TNF-α) (Matsumoto et al. Citation2015). Those pro-inflammatory cytokines are crucial in inflammation as they are involved in its initiation and amplification. An injection of carrageenan into the hind paw induced progressive oedema that reached its maximum within 3 h (Matsumoto et al. Citation2015). The intraplantar injection of carrageenan into adult male rats resulted in a severe inflammation and significant increase in the mean volume of the right hind paw compared to that of the untreated paws after 2 and 3 h of injection (). Pretreatment with EtFr and the major isolated compounds (1–4) at dose levels of 25 and 50 mg/kg showed a significant inhibition in the carrageenan-induced oedema after 3 h of carrageenan injection (). However, the tricaffeoylquinic acid (3), at a dose level of 50 mg/kg, showed a highly significant activity which was about 70% and 88% of the activity of the standard drug indomethacin (10 mg/kg) after 2 and 3 h of injection, respectively, while the EtFr and the other three dicaffeoylquinic acids (1, 2 and 4) exhibited an average oedema inhibition of 58% of that of indomethacin at 50 mg/kg after 3 h ().
Table 3. The effect of the bioactive fraction and isolated compounds (1–4) on rat paw volume in carrageenan-induced rat paw oedema model.
Injection of carrageenan into the rat hind paw induced a significant increase in the hind paw TNF-α and IL-1β concentrations, 3 h after injection, compared to those found after saline injection. Pretreatment with tricaffeoylquinic acid (3) caused a significant reduction in the high concentration of TNF-α generated by carrageenan to 12.81 ± 1.2 and 8.44 ± 0.62 pg/mL at 25 and 50 mg/kg, respectively, compared to 12.60 ± 1.30 pg/mL induced by indomethacin (). Compound 3 showed a highly significant activity in reducing the carrageenan increased concentration of the pro-inflammatory interleukin IL-1β at the two dose levels to 20.36 ± 1.69 and 5.83 ± 0.57 pg/mL, respectively. It was far more active in decreasing the concentration of IL-1β in the inflammatory exudate than indomethacin (), while the treatments with EtFr and the three dicaffeoylquinic acids (1, 2 and 4) displayed moderate reduction in TNF-α concentration and significant reduction in the concentration of IL-1β ( and ).
Table 4. Effect of oral administration of the bioactive fraction and isolated compounds (1–4) on TNF-α level in carrageenan-induced rat paw oedema model.
Table 5. Effect of oral administration of the bioactive fraction and isolated compounds (1–4) on IL-1β concentration in carrageenan-induced rat paw oedema model.
It has been reported that several quinic acid derivatives from Pimpinella brachycarpa (Apiaceae) exerted anti-neuroinflammatory activity in lipopolysaccharide-induced microglia (Lee et al. Citation2013), and caffeoyl glucosides from Nandina domestica (Berberidaceae)inhibited LPS-induced endothelial inflammatory responses (Kulkarni et al., Citation2015). In addition, an extract rich in flavonoids and caffeoylquinic acids from Gnaphalium affine (Asteraceae), traditionally used for the treatment of rheumatoid arthritis, significantly reduced the paw volume in carrageenan induced rat paw oedema and the levels of TNF-α and IL-1β (Huang et al. Citation2015).
Anticancer activities against several cell lines were reported for 3,5-di-O-caffeoyl quinate isolated from the aerial parts of S. virgaurea (Choi et al. Citation2004). The anti-inflammatory activity of S. virgaurea was attributed to leiocarposide in previous reports (Metzner et al. Citation1984; Okpanyi et al. Citation1989). However, this is the first report for the anti-inflammatory activity of caffeoylquinic acid derivatives from S. virgaurea, and the isolation of 3,4,5-tri-O-caffeoylquinic acid (3) possessing a significantly high in vivo anti-inflammatory activity compared to indomethacin. Indomethacin is a cycloxygenase inhibitor (Kumari et al. Citation2012), thus compounds (1–4) might possess the same mechanism of action. Furthermore our findings provide evidence that compounds (1–4) exert the anti-inflammatory activity by inhibiting the most prominent pro-inflammatory cytokines TNF-α and IL-1β.
Conclusion
The ethyl acetate/n-butanol fraction of S. vigaurea, rich in phenolic compounds, showed significant in vivo anti-inflammatory activity comparable to that of indomethacin and reduced the levels of the pro-inflammatory mediators TNF-α and IL-1β. Our study correlated the anti-inflammatory activity of S. virgaurea to four major caffeoylquinic acids, where the tricaffeoylquinic acid (3) showed significantly higher activities than the three dicaffeoylquinic acids (1, 2 and 4).
Disclosure statement
The authors declare no conflict of interest.
References
- Bader G, Seibold M, Tintelnot K, Hiller K. 2000. Cytotoxicity of triterpenoid saponins. Part 2: Relationships between the structures of glycosides of polygalacic acid and their activities against pathogenic Candida species. Pharmazie. 55:72–74.
- Bader G, Wray V, Hiller K. 1992. Virgaureasaponin 3, a 3,28-bisdesmosidic triterpenoid saponin from Solidago virgaurea . Phytochemistry. 31:621–623.
- Bader G, Wray V, Hiller K. 1995. The main saponins from the aerial parts and the roots of Solidago virgaurea subsp. virgaurea. Planta Med. 61:158–161.
- Bradette-Hebert ME, Legault J, Lavoie S, Pichette A. 2008. A new labdane diterpene from the flowers of Solidago canadensis. Chem Pharm Bull. 56:82–84.
- Budzianowski J. 1999. The urological effect of leiocarposides. Drogen report. 12:20–21.
- Chodera A, Dabrowska K, Bobkiewicz-Kozlowska T, Tkaczyk J, Skrzypczak L, Budzianowski J. 1988. Effect of leiocarposide on experimental urinary calculi in rats. Acta Pol Pharm. 45:181–186.
- Chodera A, Dabrowska K, Seńczuk M, Wasik-Olejnik A, Skrzypczak L, Budzianowski J, Ellnain-Wojtaszek M. 1985. Diuretic effect of the glycoside from a plant of the Solidago L. genus. Acta Pol Pharm. 42:199–204.
- Chodera A, Dabrowska K, Skrzypczak L, Budzianowski J. 1986. Further studies on the diuretic effect of leiocarposide. Acta Pol Pharm. 43:499–503.
- Chodera A, Dabrowska K, Sloderbach A, Skrzypczak L, Budzianowski J. 1991. Effect of flavonoid fractions of Solidago virgaurea L on diuresis and levels of electrolytes. Acta Pol Pharm. 48:35–37.
- Choi SZ, Choi SU, Bae SY, Pyo S, Lee KR. 2005. Immunobiological [correction of Immunobioloical] activity of a new benzyl benzoate from the aerial parts of Solidago virga-aurea var. gigantea. Arch Pharm Res. 28:49–54.
- Choi SZ, Choi SU, Lee KR. 2004. Phytochemical constituents of the aerial parts from Solidago virga-aurea var. gigantea. Arch Pharm Res. 27:164–168.
- Clifford MN. 1986. Coffee bean dicaffeoylquinic acids. Phytochemistry. 25:1767–1769.
- El Ghazaly M, Khayyal MT, Okpanyi SN, Arens-Corell M. 1992. Study of the anti-inflammatory activity of Populus tremula, Solidago virgaurea and Fraxinus excelsior. Arzneimittelforschung. 42:333–336.
- [EMEA] European Medicines Agency. 2008. Ref. EMEA/HMPC/285758/2007, September 4, 2008.
- Goulart S, Moritz MIG, Lang KL, Liz R, Schenkel EP, Fröde TS. 2007. Anti-inflammatory evaluation of Solidago chilensis Meyen in a murine model of pleurisy. J Ethnopharmacol. 113:346–353.
- Huang D, Chen Y, Chen W, Liu Y, Yao F, Xue D, Sun L. 2015. Anti-inflammatory effects of the extract of Gnaphalium affine D. Don in vivo and in vitro. J Ethnopharmacol. 176:356–364.
- Kalemba D, Thiem B. 2004. Constituents of the essential oils of four micropropagated Solidago species. Flavour Frag J. 19:40–43.
- Kolodziej B, Kowalski R, Kędzia B. 2011. Antibacterial and antimutagenic activity of extracts above ground parts of three Solidago species: Solidago virgaurea L., Solidago canadensis L. and Solidago gigantea Ait. J Med Plants Res. 5:6770–6779.
- Kulkarni RR, Lee W, Jang TS, Lee J, Kwak S, Park MS, Lee HS, Bae JS, Na M. 2015. Caffeoyl glucosides from Nandina domestica inhibit LPS-induced endothelial inflammatory responses. Bioorg Med Chem Lett. 25:5367–5371.
- Kumari STK, Lincy MP, Muthukumarasamy S, Mohan VR. 2012. Anti-inflammatory activity of Sarcostemma secamone (L) Bennet whole plant against carrageenan induced paw edema. Biosci Discov. 3:288–291.
- Lee SO, Choi SZ, Choi SU, Ryu SY, Lee KR. 2004. Phytochemical constituents of the aerial parts from Aster hispidus. Nat Prod Sci. 10:335–340.
- Lee SY, Moon E, Kim SY, Ryu SY, Lee KR. 2013. Quinic acid derivatives from Pimpinella brachycarpa exert anti-neuroinflammatory activity in lipopolysaccharide-induced microglia. Bioorg Med Chem Lett. 23:2140–2144.
- Li YK, Zhao QJ, Hu J, Zou Z, He XY, Yuan HB, Shi XY. 2009. Two new quinolione alkaloid mannopyranoside from Solidago canadensis. Helvetica Chim Acta. 92:928–931.
- Matsumoto K, Obara S, Kuroda Y, Kizu J. 2015. Anti-inflammatory effects of linezolid on carrageenan-induced paw edema in rats. J Infect Chemother. 21:889–891.
- Metzner J, Hirschelmann R, Hiller K. 1984. [Antiphlogistic and analgesic effects of leiocarposide, a phenolic bisglucoside of Solidago virgaurea L]. Pharmazie. 39:869–870.
- Okpanyi SN, Schirpke-von Paczensky R, Dickson D. 1989. Anti-inflammatory, analgesic and antipyretic effect of various plant extracts and their combinations in an animal model. Arzneimittelforschung. 39:698–703.
- Radusiene J, Marskab M, Ivanauskasb L, Jakstasb V, Karpaviciene B. 2015. Assessment of phenolic compound accumulation in two widespread goldenrods. Ind Crop Prod. 63:158–166.
- Sampson JH, Phillipson JD, Bowery NG, O’Neill MJ, Houston JG, Lewis JA. 2000. Ethnomedicinally selected plants as sources of potential analgesic compounds: indication of in vitro biological activity in receptor binding assays. Phytother Res. 14:24–29.
- Schilcher H, Rau H. 1988. Nachweis der aquaretischen Wirkung von Birkenblatter- und Goldrutenkrautauszugen im Tierversuch. Urologe B. 28:274–280.
- Soliman FM, Shehata AH, Khaleel AE, Ezzat SM, Sleem AA. 2008. Caffeoyl derivatives and flavonoids from three Compositae species. Phcog Mag. 4:1–11.
- Starks CM, Williams RB, Goering MG, Johnson MO, Norman VL, Hu JF, Garo E, Hough GW, Rice SM, Eldridge GR. 2010. Antibacterial clerodane diterpenes from goldenrod (Solidago virgaurea). Phytochemistry. 71:104–109.
- Strehl E, Schneider W, Elstner EF. 1995. Inhibition of dihydrofolate reductase activity by alcoholic extracts from Fraxinus excelsior, Populus tremula and Solidago virgaurea. Arzneimittelforschung. 45:172–173.
- Srinivasan K, Muruganandan S, Lal J, Chandra S, Tandan SK, Ravi Prakash V. 2011. Evaluation of anti-inflammatory activity of Pongamia pinnata leaves in rats. J Ethnopharmacol. 78:151–157.
- Thiem B, Wesolowska M, Skrzypczak L, Budzianowski J. 2001. Phenolic compounds in two Solidago L. species from in vitro culture. Acta Pol Pharm Drug Res. 58:277–281.
- Winter CA, Risley EA, Nuss GW. 1962. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 111:544–547.