Abstract
Context: There is paucity of information in literature on the natural products in cocoyam [Colocasia esculenta Linn (Araceae)] that confer it with biological properties.
Objectives: This study investigated the antioxidant properties of C. esculenta and also reported for the first time the natural products in C. esculenta that justify its biological properties.
Materials and methods: The antioxidant activity of the methanol extract (50–250 μg/mL) of C. esculenta was determined using the 2,2-diphenyl-1-picryl hydrazyl (DPPH) radical and reducing power assays. Characterization of the natural products in C. esculenta was done using the gas chromatographic–mass spectrometric (GC–MS) technique. The experiment lasted for 3 months.
Results: GC–MS analysis of methanol/chloroform extract of the flour of C. esculenta indicated the presence of eight compounds, namely hexadecanoic acid methyl ester (0.43%), octadecanoic acid (20.91%), 9,12-octadecadienoyl chloride (0.77%), 11-octadecenoic acid methyl ester (2.12%), 9-octadecenoic acid (64.37%), 3-hexadecyloxycarbonyl-5-(2-hydroxylethyl)-4-methylimidazolium(1.36%), hexanedioic acid, bis(2-ethylhexyl)ester (1.36%) and 3,5-di-t-butyl phenol (3.27%). The total phenolic content of C. esculenta was 15.15 ± 0.35 mg Gallic Acid Equivalence/g and it was significantly higher (p < 0.05) than the total flavonoid (8.50 ± 0.42 mg Quercetin Equivalence/g) and condensed tannin (4.40 ± 0.14 mg Catechin Equivalence/g) contents, respectively. C. esculenta possessed strong antioxidant capacity though it was lower than that of standard quercetin.
Discussion and conclusion: Results showed that C. esculenta possesses strong antioxidant activity and also contains some important bioactive compounds that justify its medicinal properties as used in ethno-medicine.
Introduction
Food provides not only essential nutrients needed for life, but also other bioactive compounds for health promotion and disease prevention. Previous epidemiologic studies have consistently shown that diet plays a crucial role in the prevention of chronic diseases (Willett Citation1995). Consumption of fruits, vegetables and grains has been associated with reduced risks of cardiovascular diseases, cancer, diabetes, Alzheimer disease, cataracts and age-related functional decline (Willett Citation1994).
Phytochemicals, the natural products occurring in fruits, vegetables, grains and other plant foods have been linked with reduction in the risks of major chronic diseases (Rui Citation2003). It is estimated that more than 5000 phytochemicals have been identified, but a large percentage still remain unknown and need to be identified before their health benefits could be fully understood (Rui Citation2003).
These phytocompounds have been found useful in pharmaceutical industries where they are used in drug development. Moreover, the development of a drug begins with identification of active principles, detailed biological assays and dosage formulations followed by clinical studies to establish the safety, efficacy and pharmacokinetic profile of the drug (Karikalan & Rajangam Citation2014).
Cocoyam [Colocasia esculenta Linn (Araceae)] is a tropical perennial starchy plant that is native to Asia and the Pacific, and widely distributed in the tropical latitudes (Kaensombath & Lindberg Citation2012).
Carbohydrate is the major component of C. esculenta tuber, while other constituents include proteins, vitamin C, thiamin, riboflavin, niacin, dietary fibre and minerals (Niba Citation2003).
In ethno-medicine, C. esculenta tuber is used in the management of diabetes mellitus, treatment of ringworm, cough, sore throat, wounds (Chatterjee & Pakrashi Citation2001) and it has also been reported to possess antihelminthic and anticancer properties (Kumawat et al. Citation2010).
These biological properties of C. esculenta were speculated to arise from the natural products in it such as alkaloids, flavonoids, tannins, phytates, minerals and so on (Sefa-Dedeh & Emmanuel Citation2004; Adegunwa et al. Citation2011; Olajide et al. Citation2011). However, detailed identification of the actual natural products/bioactive constituents in C. esculenta tuber is yet to be undertaken as the methods employed in these studies were quantitative rather than qualitative. This indicates that as of the time of this study, there were limited publications identifying the exact natural products in C. esculenta that could be responsible for its pharmacological actions.
Antioxidants were defined as substances that delay, prevent or remove oxidative damage to a target molecule (Halliwell & Gutteridge Citation1998). The physiological role of these compounds, as this definition suggests, is to prevent damage to cellular components arising as a consequence of chemical reactions involving free radicals (Procházková et al. Citation2011).
A number of medicinal plants have been found to possess antioxidant properties and thus important health benefits due to the natural products such as flavonoids, phenols, tannins and so on that confer these plants with redox and metal chelating properties. However, there is scarcity of information in literature on the antioxidant properties of this species of C. esculenta that is used in the South Eastern parts of Nigeria as a soup thickener.
In pursuit of the natural products in C. esculenta that confer it with biological properties, the present study therefore sought to determine the polyphenol contents (total phenols, flavonoids and condensed tannins), antioxidant activity and phytochemical screening (using the GC–MS technique) of the tuber of C. esculenta.
Materials and methods
Chemicals
Standard quercetin, gallic acid, catechin, 2,2-diphenyl-1-picryl hydrazyl (DPPH) radical, sodium dihydrogen orthophosphate, disodium hydrogen orthophosphate and potassium ferricyanide used were products of Sigma and Aldrich Chemical Company (England, UK). Every other chemical used was bought in Nigeria and was also of analytical grade.
Plant materials
Fresh samples of C. esculenta tubers were obtained in 2015 at harvest from National Root Crops Research Institute (NRCRI), Umudike, Nigeria. They were identified by Dr. G. O. Chukwu, the erstwhile Coordinator, Cocoyam Programme, NRCRI, Umudike as well as by Mr. Ibe, a Taxonomist in Michael Okpara University of Agriculture, Umudike, Nigeria (MOUAU). A voucher specimen is in the herbarium of MOUAU. The samples were washed with distilled water, sliced, dried and pulverized.
Plant extraction
The C. esculenta flour (100 g) was soaked in 500 mL of methanol for 24 h. The solution was filtered (Whatman No. 41 filter paper) and the filtrate was extracted for 3 h in a Soxhlet apparatus. On completion of the extraction, it was cooled and concentrated with a rotary evaporator. The extract was divided into two portions: A portion was subjected to total phenol, flavonoid, condensed tannins and antioxidant assays, while the second portion (reconstituted in water instead of methanol) was transferred to a separatory funnel, re-extracted with chloroform, filtered and concentrated with a rotary evaporator (Mooza et al. Citation2014 with modifications). It was then diluted with methanol (1/10, v/v), filtered and a given quantity (1 μL) was analysed for the phytochemical constituents using the GC–MS technique.
GC–MS analysis
GC–MS analysis of compounds in the crude extract was carried as described by Igwe and Okwu (Citation2013). The components of the extract were identified by matching the peaks with Computer 100 Wiley Ms Libraries and confirmed by comparing the mass spectra of the peaks with those from the database of the National Institute’s Standard and Technology.
Polyphenol assays
The total phenol content of the extract was determined using the method of Singleton et al. (Citation1999). A measured quantity (0.5 mL) of appropriate dilution of extract was added to 2.5 mL 10% Folin-Cioalteu’s reagent (v/v), followed by 2.0 mL 7.5% sodium carbonate. The mixture was incubated at 45 °C for 40 min, and the absorbance was measured at 765 nm with a spectrophotometer (JENWAY 6305). Gallic acid was used as the standard.
Assay of total flavonoids
The total flavonoid content of the extract was determined using the method of Meda et al. (Citation2005). A given quantity (0.5 mL) of appropriate dilution of extract was mixed with 0.5 mL methanol, 50 μL of 10% AlCl3 (in ethanol), 50 μL of 1 mol/L potassium acetate and 1.4 mL water. The reaction mixture was incubated at room temperature for 30 min. Thereafter, the absorbance of the reaction mixture was measured at 415 nm with a spectrophotometer (JENWAY 6305). The total flavonoid content was calculated using quercetin as the standard.
Assay of total condensed tannins
The total condensed tannins contents of the extract were determined following the method of Sun et al. (Citation1998). Briefly, to 50 μL of the appropriately diluted extract, 3 mL of 4% vanillin solution in methanol and 1.5 mL of concentrated HCl were added. The mixture was allowed to stand for 15 min, and the absorbance was read at 500 nm against methanol as the blank. The total condensed tannin content was calculated using catechin as the standard.
Antioxidant assays
DPPH radical scavenging assay
A modified version of the method of Blois (Citation1985) was used to determine the DPPH radical scavenging activity of the extract. Briefly, the extract (5 g) was dissolved in 100 mL of methanol. The mixture was filtered through Whatman No. 1 filter paper with a vacuum pump. Then, 10, 20, 30, 40, and 50 μL aliquots of the extract were further diluted with methanol to give final concentrations of 50, 100, 150, 200, and 250 μg/mL, respectively. Finally, 0.1 mL of 0.3 mM DPPH (in methanol) was added to the reaction mixtures and the whole setup was well shaken and left in the dark for 30 min. After the incubation period, the spectrophotometer was stabilized against a DPPH control (i.e., 1 mL of methanol), and the absorbance of the sample mixture was read spectrophotometrically at 517 nm. The scavenging activity was calculated using the equation:
where quercetin was used as the standard.
Reducing power assay
A modified version of the method of Hsu et al. (Citation2003) was used to determine the reducing power of the extract. Briefly, 2 g of extract were dissolved in 80 mL of methanol. The mixture was left overnight and then filtered through Whatman No. 1 filter paper with a vacuum pump. The filtrate was brought up to 100 mL with methanol to give a concentration of 20 mg/mL and then diluted to the following concentrations: 50, 100, 150, 200 and 250 μg/mL, respectively. The resulting solutions were used for reducing power assay. Briefly, 1 mL of extract, 0.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of potassium ferricyanide (instead of potassium hexacyanoferrate) solution (1% v/v in water) were placed in a test tube and incubated for 20 min at 50 °C. The tubes were cooled in crushed ice and 0.5 mL of trichloroacetic acid (10% in water) was added to each tube. After centrifugation at 3000g for 10 min, 1 mL of the supernatant was mixed with an equal volume of distilled water and 0.1 mL of ferric chloride solution (0.1% in water). The mixture was incubated for 10 min and the absorbance was measured at 700 nm using a UV spectrophotometer. A higher reaction mixture absorbance indicates a greater reducing power. Quercetin was also used as the standard.
Statistical analysis
The statistical package for social sciences (SPSS), version 17.0 (SPSS Inc., Chicago, IL) was used to analyse all data. Results of the polyphenols and antioxidant assays are presented as means ± standard deviations and values were considered to be significant when p < 0.05.
Results
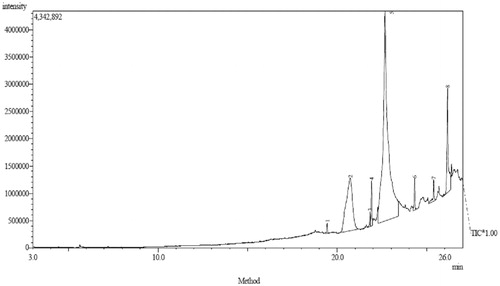
The GC–MS chromatogram of the methanol/chloroform extract of the flour of C. esculenta revealed the presence of various compounds with corresponding peaks at different retention times (), while the structures of the compounds identified in the extract are shown in The compounds identified in the extract as shown in were as follows:
Table 1. GC–MS analysis of methanol/chloroform extract of C. esculenta.
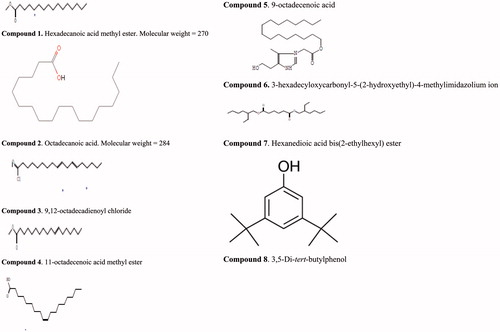
Compound 1 was found to be hexadecanoic acid methyl ester, a fatty acid methyl ester and it made up 0.43% of the extract.
Compound 2, identified as octadecanoic acid, a fatty acid made up 20.91% of the extract.
Compound 3 was found to be 9, 12-octadecadienoyl chloride, a linoleoyl chloride and it made up 0.77% of the extract.
Compound 4 was found to be 11-octadecenoic acid methyl ester and it made up 2.12% of the extract.
Compound 5 was found to be 9-octadecenoic acid, a fatty acid and it made up 64.37% of the extract.
Compound 6 was found to be 3-hexadecyloxycarbonyl-5-(2-hydroxylethyl)-4-methylimidazolium ion and it made up 1.36% of the extract.
Compound 7 was found to be hexanedioic acid, bis(2-ethylhexyl) ester and it made up 1.36% of the extract.
Compound 8 was found to be 3, 5-di-t-butyl phenol and it made up 3.27% of the extract.
The major constituents of the extract with identified biological activities were: 9-octadecenoic (64.37%), 9,12-octadecadienoyl chloride (0.77%), hexadecenoic acid (0.43%), octadecanoic acid (20.91%) and 3,5-di-tert-butyl phenol (3.27%), respectively, making up 89.75% of the extract. The remaining identified compounds that made up 4.84% of the extract had unknown biological activities while the remaining compounds that made up 5.41% of the extract could not be identified.
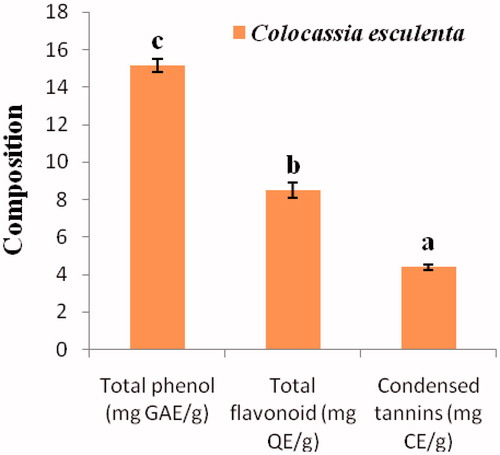
The result of the total phenolic, flavonoid and condensed tannin contents of C. esculenta is shown in . The total phenolic content was 15.15 ± 0.35 mg Gallic Acid Equivalence/g dry weight, the total flavonoid content was 8.50 ± 0.42 mg Quercetin Equivalence/g dry weight, while the total content condensed tannin was 4.40 ± 0.14 mg Catechin Equivalence/g dry weight. The total phenolic content of C. esculenta was significantly higher (p < 0.05) than its total flavonoid and condensed tannin contents.
Figure 3. Polyphenolic composition of methanol extract of C. esculenta. Values are the means ± SD of three determinations. a–cp < 0.05 (Significantly different from each other). QE: quercetin equivalence; GAE: gallic acid equivalence; CE: catechin equivalence.
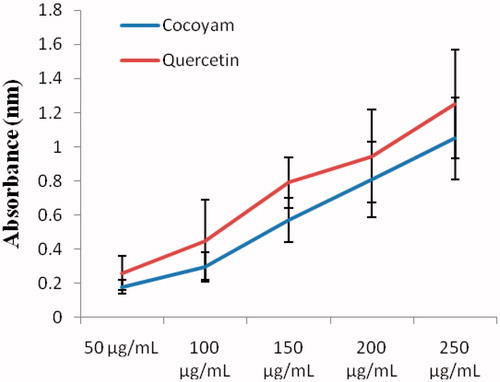
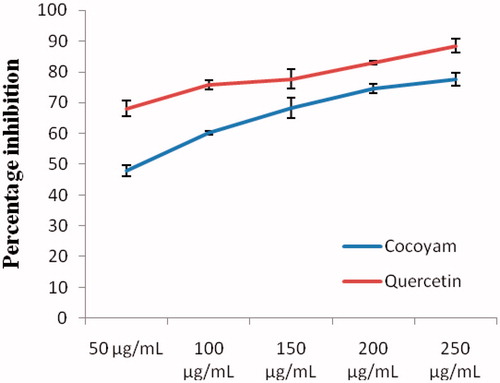
As observed in this study, the inhibitory activity of the methanol extract of the flour of C. esculenta on DPPH radical on the average was 65.73 ± 11.94% compared with standard quercetin that recorded 78.57 ± 7.73% inhibition of DPPH radical ().
Figure 4. Inhibition of methanol extract of C. esculenta and quercetin on DPPH radical. Values are the means ± SD of three determinations.
The reducing power of the methanol extract of the flour of C. esculenta on the average was 0.58 ± 0.36 nm compared with standard quercetin that had an average reducing power of 0.740 ± 0.394 nm ().
Discussion
Medicinal plants constitute the cornerstone of traditional practice worldwide and they have been used for decades as remedies for human diseases because they contain natural products of therapeutic values (Rajagopal et al. Citation2014). According to the World Health Organization (WHO), more than 80% of the world’s population relies on traditional medicine for their primary healthcare needs (Sermakkani & Thangapandian Citation2012). Furthermore, GC–MS assay has proven to be a reliable tool for the identification of these natural products in medicinal plants (Cong et al. Citation2007).
Previous studies indicated that 9-octadecenoic acid, that constituted 64.37% of the extract, possesses anti-inflammatory, antialopecic, 5-α-reductase inhibitory, anemiagenic, α-reductase inhibitory, antitumour, immunostimulatory, anti-leucotriene-D4, antiandrogenic, lipoxygenase inhibitory, and hypocholesterolemic properties (Omotoso et al. Citation2014).
Kundu et al. (Citation2012) reported in their studies that water soluble extract of C. esculenta demonstrated antimestatic activity by inhibiting the proliferation of some breast and prostate cancer cell lines. Their study further showed that tumour cell migration was completely blocked by the C. esculanta extract. Studies have also shown that inhibitors of 5α-reductase, which catalyses the reductive conversion of testosterone to 5α-dihydrotestosterone, may be useful in the treatment of androgen-dependent diseases, one of which is benign prostatic hyperplasia (Chul-Ho et al. Citation2010).
Therefore, the presence of 9-octadecenoic acid as the major natural product isolated from this species of C. esculenta tuber suggests that it may possess anti-inflammatory, anemiagenic, antialopecic, anti-leucotriene-D4, immunostimulatory properties and also provides a biochemical rationale for the inhibition of breast and prostate cancer cell lines demonstrated by C. esculenta as reported by Kundu et al. (Citation2012) and for the hypocholesterolemic actions also demonstrated by C. esculenta as reported by Eleazu et al. (Citation2016).
9,12-Octadecadienoyl chloride, a linoleoyl chloride that constituted 0.77% of the extract was reported to possess antisecretory, choleretic, contraceptive, antispermatogenic and antitubercular properties (Kalaivani et al. Citation2012). The presence of this compound in the extract suggests that this species of C. esculenta could possess antisecretory, choleretic, contraceptive, antispermatogenic and antitubercular properties.
Hexadecanoic acid methyl ester that constituted 0.43% of the extract was reported to possess antioxidant, antibacterial properties, hypocholesterolemic, pesticide and haemolytic 5-α-reductase-inhibitory properties (Kalaivani et al. Citation2012; Neha & Vibha Citation2013; Mohamed et al. Citation2014; Omotoso et al. Citation2014). The presence of this compound in C. esculenta suggests it could possess these properties, and further re-affirms its hypocholesterolemic properties as previously reported (Eleazu et al. Citation2016); it also suggests the antioxidant potentials of C. esculenta.
According to Karikalan and Rajangam (Citation2014), octadecanoic acid which constituted 20.91% of the extract possesses antibacterial and antifungal actions. The considerable amount of this compound in the tuber of C. esculenta suggests the antibacterial and antifungal properties of this plant.
tert-Butylphenols are widely used as inhibitors of free radicals. The mechanism of their physiological action is associated with the stable phenoxyl radical formation in the process of hydrogen atom abstraction by highly reactive peroxyl radicals of lipids (Niki et al. Citation2005).
3,5-Di-tert-butyl phenol, a phenolic compound was reported to possess antioxidant, and antimicrobial properties (Mamza et al. Citation2012). The presence of this phenolic compound in the extract of C. esculenta further reaffirms the antioxidant potentials of this plant.
Crude extracts of plants rich in phenolics are increasingly of interest in the food industry because they retard oxidative degradation of lipids and thus improve the quality and nutritional values of food (Javanmardia et al. Citation2003). Flavonoids and phenolic acids are known to possess antioxidant activities due to the presence of hydroxyl groups in their structures and their redox properties (Zahid et al. Citation2015). Furthermore, flavonoids as the largest group of phenolics identified in fruits, vegetables and other plant parts have been linked to reducing the risk of major degenerative diseases (Liu Citation2003). Tannins are water-soluble high molecular weight phenolic compounds found in many plants that are important in herbal medicine due to their wound healing properties (Nguyi Citation1988). Studies have also indicated their antioxidant, antiradical, antimicrobial and antimutagenic properties (Ryszard Citation2007). Values obtained in this study with respect to the phenolic, flavonoid and condensed tannin contents of C. esculanta suggest that C. esculenta contains considerable amounts of these phenolic compounds.
The DPPH assay is a widely accepted method for the determination of the antioxidant activities of various food substances. Results obtained with respect to DPPH scavenging activity by the methanol extract of the C. esculenta suggests the antioxidant potentials of C. esculenta although it was lower than that of quercetin.
The DPPH assay is limited by colour interference and sample solubility (Dorman & Hiltunen Citation2004) and this informed the assay of the reducing power of C. esculenta.
Reducing power is an efficient, fast, stable, reliable and convenient choice for assay of antioxidants in plants (Fang et al. Citation2004). The principle of this assay involves the reduction of Fe3+ to Fe2+ through electron transfer. Results obtained with respect to reducing power of methanol extract of C. esculenta further reaffirm the antioxidant activity of C. esculenta which could be attributed to the presence of hexadecanoic acid methyl ester and tert-butylphenols in the plant.
Conclusion
The presence of 9-octadecenoic acid as the major natural product isolated from C. esculenta tuber places a research spot light on C. esculenta as a novel plant with prospective antiprostatic and hypocholesterolemic properties.
Disclosure statement
The author reports no declarations of interest.
References
- Adegunwa MO, Alamu EO, Omitogun LA. 2011. Effect of processing on the nutritional contents of yam and cocoyam tubers. J Applied Biosci. 6:3086–3092.
- Blois MS. 1985. Antioxidant determination by use of stable free radicals. Nature. 181:1199–1200.
- Chatterjee A, Pakrashi SC. 2001. The treatise on Indian medicinal plant. New Delhi: National Institute of Science Communication. p. 32.
- Chul-Ho C, Jong-Sup B, Yong-Ung K. 2010. 5α-Reductase inhibitory components as antiandrogens from herbal medicine. J Acupunct Meridian Stud. 3:116–118.
- Cong Z, Meiling Q, Qinglong S, Shan Z, Ruonong F. 2007. Analysis of the volatile compounds in Ligusticum chuanxiong Hort using HS-SPME-GCMS. Pharm Biomed Anal. 44:464–470.
- Dorman HJD, Hiltunen R. 2004. Fe(II) reductive and free radical scavenging properties of summer savory (Satureja hortensis L.) extract and sub-fractions. Food Chem. 88:193–199.
- Eleazu CO, Eleazu KC, Iroaganachi MA. 2016. Effect of cocoyam (Colocasia esculenta), unripe plantain (Musa paradisiaca) or their combination on glycated hemoglobin, lipogenic enzymes, and lipid metabolism of streptozotocin-induced diabetic rats. Pharm Biol. 54:91–97.
- Fang X, Cao W, Gao G. 2004. Effects of Premna microphylla Turcz root extraction on the proliferation of T lymphocyte in test mice. J Biol. 21:33–34.
- Halliwell B, Gutteridge JMC. 1998. Free radicals in biology and medicine. 3rd ed. Oxford: Oxford University Press.
- Hsu CL, Chen W, Weng YM, Tseng CY. 2003. Chemical composition, physical properties, and antioxidant activities of yam flours as affected by different drying methods. Food Chem. 83:85–92.
- Igwe OU, Okwu DE. 2013. GC–MS evaluation of bioactive compounds and antibacterial activity of the oil fraction from the seeds of Brachystegia eurycoma (HARMS). Asian J Plant Sci Res. 3:47–54.
- Javanmardia J, Stushnoff C, Locke E, Vivanco JM. 2003. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 83:547–550.
- Kaensombath L, Lindberg JE. 2012. Effect of replacing soybean protein by taro leaf (Colocasia esculenta (L.) Schott) protein on growth performance of exotic (Landrace × Yorkshire) and native (Moo Lath) Lao pigs. Trop Anim Health Proc. 45:45–51.
- Kalaivani CS, Sathish SS, Janakiraman N, Johnson M. 2012. GC–MS studies on Andrographis paniculata (Burm.f.) Wall. Ex Nees – a medicinally important plant. Int J Med Aromatic Plants. 2:69–74.
- Karikalan G, Rajangam U. 2014. GC–MS analysis of phytocompounds of leaf and stem of Marsilea quadrifolia (L.). Int J Biochem Res Rev. 4:517–526.
- Kumawat NS, Chaudhari SP, Wani NS, Deshmukh TA, Patil VR. 2010. Antidiabetic activity of ethanol extract of Colocasia esculenta leaves in alloxan induced diabetic rats. Int J Pharm Tech Res. 2:1246–1249.
- Kundu N, Campbell P, Hampton B, Lin C-Y, Ma X, Ambulos N, Zhao XF, Goloubeva O, Holt D, Fulton AM. 2012. Antimetastatic activity isolated from Colocasia esculenta (taro). Anti-Cancer Drugs. 23:200–211.
- Liu RH. 2003. Health benefits of fruits and vegetables are from additive and synergistic combination of phytochemicals. Am J Clin Nutr. 78:517s–520s.
- Mamza UT, Sodipo OA, Khan IZ. 2012. Gas chromatography–mass spectrometry (GC–MS) analysis of bioactive components of Phyllanthus amarus leaves. Int Res J Plant Sci. 3:208–215.
- Meda A, Lamien CE, Romito M, Millogo JF, Nacoulma OG. 2005. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 91:571–577.
- Mohamed ZZ, Fasihuddin BA, Wei-Seng H, Shek-Ling P. 2014. GC–MS analysis of phytochemical constituents in leaf extracts of Neolamarckia Cadamba (Rubiaceae) from Malaysia. Int J Pharm Pharm Sci. 6:123–127.
- Mooza A, Nora A, Shah AK. 2014. GC–MS analysis, determination of total phenolics, flavonoid content and free radical scavenging activities of various crude extracts of Moringa peregrina (Forssk.) Fiori leaves. Asian Pac J Trop Biomed. 4:964–970.
- Neha A, Vibha R. 2013. GC–MS analysis of bioactive components in the ethanolic and methanol extract of Syzygium Cumini. Int J Pharm Bio Sci. 4:296–304.
- Nguyi A. 1988. Tannins of some Nigerian flora. J Biotechnol. 6:221–226.
- Niba LL. 2003. Processing effects on susceptibility of starch to digestion in some dietary starch sources. Int J Food Sci Nutr. 54:97–109.
- Niki E, Yoshida Y, Saito Y, Noguchi N. 2005. Lipid peroxidation: mechanisms, inhibition and biological effects. Biochem Biophy Res Commun. 338:668–676.
- Olajide R, Akinsoyinu AO, Babayemi OJ, Omojola AB, Abu AO, Afolabi KD. 2011. Effect of processing on energy values, nutrient and anti-nutrient components of wild cocoyam (Colocasia esculenta (L.) Schott] corm. Pakistan J Nutr. 10:29–34.
- Omotoso AE, Ezealisiji K, Mkparu KI. 2014. Chemometric profiling of methanol leaf extract of Cnidoscolus aconitifolius (Euphorbiaceae) using UV–VIS, FTIR and GC–MS techniques. Peak J Medicinal Plant Res. 2:6–12.
- Procházková D, Boušová I, Wilhelmová N. 2011. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 82:513–523.
- Rajagopal G, Periyasamy M, Rameshbabu B. 2014. Antimicrobial potent and bioactive constituents from aerial parts of Vitis setosa Wall. J Med Plant Res. 8:454–460.
- Rui HL. 2003. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 78:517S–520S.
- Ryszard A. 2007. Tannins: the new natural antioxidants? Eur J Lipid Sci Technol. 109:549–555.
- Sefa-Dedeh S, Emmanuel KA. 2004. Chemical composition and the effect of processing on oxalate content of cocoyam Xanthosoma sagittifolium and Colocasia esculenta cormels. Food Chem. 85:479–487.
- Sermakkani M, Thangapandian V. 2012. GC–MS analysis of Cassia italica leaf methanol extract. Asian J Pharm Clin Res. 5:90–94.
- Singleton VL, Orthofer R, Lamuela-Raventos RM. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 299:152–178.
- Sun B, Richardo-Da-Silvia JM, Spranger I. 1998. Critical factors of vanillin assay for catechins and proanthocyanidins. J Agric Food Chem. 46:4267–4274.
- Willett WC. 1994. Diet and health: what should we eat? Science. 254:532–537.
- Willett WC. 1995. Diet, nutrition, and avoidable cancer. Environ Health Perspect. 103:165–170.
- Zahid KA, Shalini S, Mohamed IS, Nahla Z, Hasibur R, Abid AA. 2015. Phytochemical, antioxidant and mineral composition of hydroalcoholic extract of chicory (Cichorium intybus L.) leaves. Saudi J Bio Sci. 22:322–332.