Abstract
Context: The genus Hymenocrater Fisch. et Mey. (Lamiaceae) contains over 21 species in the world. Some species have been used in folk medicine around the world. The present review comprises the ethnopharmacological, phytochemical and therapeutic potential of various species of Hymenocrater.
Objective: This review brings together most of the available scientific research regarding the genus Hymenocrater. Through this review, the authors hope to attract the attention of natural product researchers throughout the world to focus on the unexplored potential of Hymenocrater species.
Methods: This review has been compiled using references from major databases such as Chemical Abstracts, Medicinal and Aromatic Plants Abstracts, ScienceDirect, SciFinder, Google Scholar, Scopus, PubMed, Springer Link and books, without limiting the dates of publication. General web searches were also carried out using Google and Yahoo search engines by applying some related search terms (e.g., Hymenocrater spp., phytochemical, pharmacological, extract, essential oil and traditional uses). The articles related to agriculture, ecology, and synthetic works and those using languages other than English or Persian have been excluded.
Results: The genus Hymenocrater contains essential oil. Flavonoids, phenolic acids and terpenoids are important constituents of this genus. The pharmacological studies confirmed that the species of the genus Hymenocrater showed antimicrobial, antiparasitic, antioxidant, anticancer and antidiabetic activities.
Conclusion: This review discusses the current knowledge of Hymenocrater species that review therapeutic potential, especially their effects on the cancer cells and gaps offering opportunities for future research.
Introduction
Since ancient times, plants have been one of the first and most available resources used for treating illnesses, and throughout history, there has always been a close relationship between man and plants, and the medicinal effects of plants and their uses are well known. Currently, according to the World Health Organization (WHO), as much as 80% of the world's people depend on traditional medicine for their primary health care needs. There are considerable economic benefits in the development of indigenous medicines and in the use of medicinal plants for the treatment of various diseases. Traditional medicine involves the use of plant parts in crude form, either fresh or dried for preventing or healing various forms of ailments (Joharchi & Amiri Citation2012). The potential of plants as a source of new drugs is still largely unexplored (Morteza-Semnani Citation2015). Some species of the Hymenocrater Fisch. et Mey. (Lamiaceae) genus have been investigated from different points of view: identification of essential oils composition, antimicrobial, antifungal, and antioxidant activities, anatomical and pollen morphology, ornamentation and palynological studies (El-Gazzar & Watson Citation1968; Fazly Bazzaz & Haririzadeh Citation2003; Firouznia et al. Citation2005; Zaidi & Crow Citation2005; Barazandeh Citation2006; Ryding Citation2007; Akramian et al. Citation2008; Moon et al. Citation2008a,Citationb; Jafari & Jafarzadeh Citation2008; Ahmadi et al. Citation2010; Miguel Citation2010; Ryding Citation2010a,Citationb; Prakash et al. Citation2015). In our continuing research on the plants of the genus Hymenocrater (Morteza-Semnani et al. Citation2010, Citation2012), some of the main reports on the botanical, ethnopharmacological, phytochemical and biological activities of the species of Hymenocrater were reviewed. This is the first comprehensive review of the genus Hymenocrater. The authors hope to attract the attention of natural product researchers throughout the world to focus on the unexplored potential of Hymenocrater species. This genus needs to be investigated systematically so that potential species can be exploited as therapeutic agents.
Distribution of the genus Hymenocrater
Hymenocrater, Stachyioideae subfamily, has 21 species in the world (Taherpour et al. Citation2011; Shahriari et al. Citation2013); it is found in Iran, Iraq, Pakistan, Afghanistan, Turkey and Turkmenistan (Mozaffarian Citation1996; Satil et al. Citation2007; Jafari & Jafarzadeh Citation2008; Zaidi & Crow Citation2012; Serpooshan et al. Citation2014). There are 12 species distributed in the southern part of central Asia, the Caucasus, Asia Minor and Iran (Hassanzadeh et al. Citation2011; Drew & Sytsma Citation2012). It is represented by 11 species in Flora Iranica, nine of them, H. bituminosus Fisch. & C.A. Mey., H. calycinus (Boiss.) Benth., H. elegans Bunge, H. incanus Bunge, H. longiflorus Benth., H. oxyodontus Rech. f., H. platystegius Rech. f., H. sessilifolius Benth. and H. yazdianus Rech. f., have been widely distributed in Iran, four species including H. yazdianus, and H. incanus, H. platystegius and H. oxyodontus are endemic to Iran, two species in Flora of the USSR and one species in east Turkey (Rechinger Citation1982; Mozaffarian Citation1996; Kashipazha et al. Citation2004; Firouznia et al. Citation2005; Satil et al. Citation2007; Zarezadeh et al. Citation2007; Jamzad Citation2009; Baghestani Maybodi et al. Citation2010; Azimova & Glushenkova Citation2012; Esmaili et al. Citation2012; Serpooshan et al. Citation2014; Soodmand et al. Citation2015). The genus Hymenocrater is named Gol-e-Arvaneh in Persian (Mozaffarian Citation1996; Razzaghi-Abyaneh & Rai Citation2013). Hymenocrater spp. has been found in Razavi, North and South Khorasan, Azerbaijan, Fars, Golestan, Isfahan, Kermanshah, Kurdistan, Mazandaran, Qazvin, Tehran and Yazd Provinces (Rechinger Citation1982; Mirza et al. Citation2001; Ghelichnia Citation2002; Ghahreman et al. Citation2006; Ghollassi Citation2008; Jankju et al. Citation2011; Nadaf & Mortazavi Citation2011; Esmaili et al. Citation2012; Ekrami Citation2013; Sadeghian et al. Citation2015).
General morphology
There are low shrubs and perennial herbs (Mirza et al. Citation2001), subshrubs, glabrous or pubescent with gray or yellowish-gray bark, numerous erect branches and ovate or broad ovate, dentate, acute leaves. Flowers are usually numerous, sessile or short-pediceled, cylindrical or infundibular, corolla long and indistinctly 2-lipped, in 2–7 flowered cymes or semiverticles forming a pyramidal or subspicate inflorescens. Nutless ovoid, smooth of finely tuberculate (Hassanzadeh et al. Citation2011).
Ethnopharmacology
Prior to the development of modern medicine, traditional medicine systems that have evolved over the centuries among various communities were still maintained as a great traditional knowledge basis in herbal medicines (Amiri et al. Citation2012). Plants belonging to this genus are pharmacologically active and have been used in folk medicine all around the world (Al-Anee et al. Citation2014). Hymenocrater genus surprisingly possess antioxidant properties as well as antidiabetic, anticlotting, anti-inflammatory and anticancer activities, because of the presence of important metabolites including rosmarinic acid and rutin in the aerial branches (Safamansouri et al. Citation2014). Hymenocrater bituminosus is an ornamental plant (Azimova & Glushenkova Citation2012). It is reported in the Flora of the USSR that H. bituminosus has commercial value due to a lemon aroma (Satil et al. Citation2007). Traditional uses of the species of the genus Hymenocrater from different origins are summarized in .
Table 1. Traditional uses of Hymenocrater spp. from different origins.
Phytochemistry
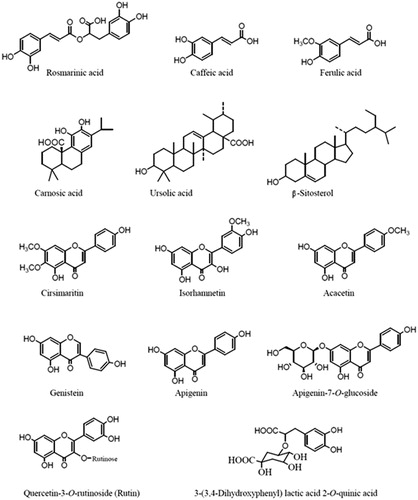
Flavonoids and essential oils are important constituents of the genus Hymenocrater (Zaidi & Crow Citation2012). Fazly Bazzaz et al. (Citation1997) reported the presence of alkaloids (+, +, 2+), flavonoids (+, +, +), saponins (+, 3+, 3+) and tannins (3+, 2+, 2+) in the aerial parts of H. bituminosus, H. calycinus and H. platystegius, respectively, collected from the various parts of Khorasan Province in Iran by the qualitative chemical analysis. The total phenolic and flavonoid contents of methanol extract of the aerial parts of H. calycinus collected from Bojnurd, North Khorasan Province in Iran, were found to be more than those of ethyl acetate and dichloromethane extracts (Soodmand et al. Citation2015). Some compounds have been isolated from the extracts of Hymenocrater spp. so far (). Rosmarinic acid has been reported from the genus Hymenocrater (Pedersen Citation2000). Rosmarinic acid, β-sitosterol, ursolic acid, quercetin-3-O-rutinoside (rutin) and 3-(3,4-dihydroxyphenyl) lactic acid 2-O-quinic acid were identified in the extracts of H. calycinus grown in Iran (Gohari et al. Citation2009, Citation2011). β-Sitosterol was also found in H. bituminosus (Azimova & Glushenkova Citation2012). Ten compounds including cirsimaritin, rosmarinic acid, apigenin-7-O-glucoside, genistein, apigenin, acacetin, carnosic acid, caffeic acid, ferulic acid and isorhamnetin were identified as flavonoids and phenolic acids in the methanol extract of H. longiflorus grown in Iraq (Al-Anee et al. Citation2015).
There are several studies on the chemical composition of the essential oil of Hymenocrater spp. (Mirza et al. Citation2001; Firouznia et al. Citation2005; Barazandeh Citation2006; Akramian et al. Citation2008; Akhlaghi et al. Citation2009; Masoudi et al. Citation2009; Firouznia et al. Citation2009; Ahmadi et al. Citation2010; Morteza-Semnani et al. Citation2010; Taherpour et al. Citation2011; Azimova & Glushenkova Citation2012; Masoudi et al. 2012; Morteza-Semnani et al. Citation2012; Sabet Teimouri et al. Citation2012; Shahriari et al. Citation2013). Chemically, essential oils are very complex natural mixtures which may contain components at quite different concentrations. They are characterized by major components at fairly high concentrations, compared with other components present in trace amounts. Generally, these major components determine the biological properties of the essential oil. The components include two groups with different biosynthetical origins: the main group is composed of terpenes, and the other of aromatic and aliphatic constituents, all characterized by their low molecular weight (Abad et al. Citation2012). Phytol, spathulenol, β-caryophyllene, hexacosane and heneicosane were found as main components of the essential oil from the aerial parts of H. platystegius collected from six different locations of Khorasan-e-razavi in Iran (Sabet Teimouri et al. Citation2012). Citral was identified in H. bituminosus oil (Azimova & Glushenkova Citation2012). summarizes reported major components (>5%) of Hymenocrater species and illustrates the chemical structures of the most commonly occurring major volatile compounds.
Figure 2. Chemical structures of the most commonly occurring major volatile components of Hymenocrater species.
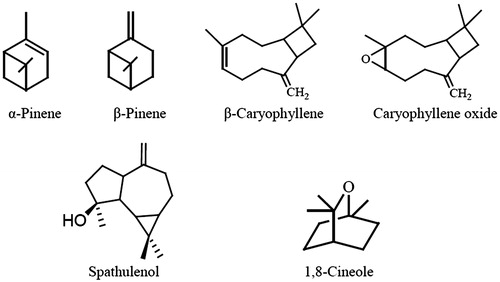
Table 2. Major essential oil components (>5%) of Hymenocrater species.
Biological activities
Antimicrobial activity
The methanol extract of the aerial parts of H. calycinus and H. platystegius collected form Khorasan Province in Northeast of Iran were tested for antimicrobial activity using the cylinder plate assay method; H. calycinus extract showed significant activity against Escherichia coli, Klebsiella pneumoniae, Morganella morganii, Pseudomonas aeruginosa and Staphylococcus aureus but H. platystegius extract had no effect on micro-organisms tested (Fazly Bazzaz & Haririzadeh Citation2003). The extract of H. sessilifolius collected from Balochistan Province of Pakistan showed good activity against Candida albicans and also significant activity against Bacillus subtilis, P. aeruginosa and E. coli (Zaidi & Crow Citation2005). Rosmarinic acid, as the main component of H. calycinus extract, had an antifungal property against C. albicans (Gohari et al. Citation2009). Isolated 3-(3,4-dihydroxyphenyl) lactic acid 2-O-quinic acid from H. calycinus extract was not active against S. aureus and E. coli at any concentration below 1 mg/disk but it (500 μg/disc) enhanced antibacterial effect of Ampicillin, Ciprofloxacin, Vancomycin and Cefepime against S. aureus and activated the effects of Ampicillin and Vancomycin against E. coli (Gohari et al. Citation2010). Ether extract of H. bituminosus showed antifungal activity (Azimova & Glushenkova Citation2012). The methanol extract of the stem and leaves of H. longiflorus collected from Iran showed antibacterial activity against some tested bacteria (Abedini et al. Citation2014). Ethanol extract of the flowers of H. longiflorus collected from Kurdistan region in North of Iraq showed antifungal activity against C. albicans and Cryptococcus neoformans at some concentrations (Abu-Mejdad Citation2014). The polar and essential oil fractions of H. longiflorus collected from Kermanshah Province in Iran showed moderate activity against the S. aureus. The results from the measurements of minimal inhibition concentration (MIC) indicate that the polar sub-fraction was more sensitive with a lower MIC value (40 μg/mL) than the essential oil (an MIC value of 120 μg/mL). Furthermore, the results showed a resistance of all micro-organisms against the non-polar sub-fraction of plant. This weak spectrum of antibacterial activity of essential oil may be due to low or very rare presence of antibacterial constituents, especially 1,8-cineole (eucalyptol) in the oil. The results of antifungal activity assays showed that only the essential oil have significant inhibitory effects on the growth of Aspergillus niger and C. albicans with MIC values of 480 and 240 μg/mL, respectively. This inhibition effect of the essential oil against the two fungal species may be due to the presence of a relatively high proportion of oxygenated monoterpenes in the oil (Ahmadi et al. Citation2010). The H. elegans oil exhibited concentration-dependent antibacterial activity on B subtilis, S. aureus, E. coli and Salmonella typhi with MIC values of 0.8, 0.8, 1.6 and 1.6 mg/mL, respectively; the essential oil did not show antifungal activity against A. niger and C. albicans (Morteza-Semnani et al. Citation2010). The H. calycinus oil exhibited concentration-dependent antibacterial activity on B. subtilis, S. aureus, E. coli and S. typhi with MIC values of 1.6, 0.8, 1.6 and 1.6 mg/mL, respectively; the essential oil did not show antifungal activity against A. niger and C. albicans (Morteza-Semnani et al. Citation2012). Essential oil of H. bituminosus had antibacterial property (Azimova & Glushenkova Citation2012). The leaf oil of H. yazdianus collected from Yazd Province in Iran showed inhibitory activity against S. epidermidis, E. faecalis, E. coli and S. paratyphi B (MIC values of 31.25 μg/mL) (Masoudi et al. Citation2012).
Antiparasitic activity
The essential oil and the methanol extract of H. longiflorus exhibited significant toxic activity against the larvae of Echinococcus granulosus with LC50 values of 79.68 and 135.88 μm/mL, respectively (Taran et al. Citation2013).
Antioxidant activity, anticancer activity and cytotoxicity
The antioxidant activity of essential oil, polar sub-fraction and non-polar sub-fraction H. longiflorus collected from Kermanshah Province in Iran was also determined by 1,1-diphenyl-2-picryl-hydrazyl (DPPH) free radical scavenging, β-carotene linoleic acid assay and reducing power (Ahmadi et al. Citation2010). The free radical scavenging activity of the methanol extract of H. longiflorus grown in Iraq was evaluated using DPPH assay; the antioxidant activities of the extract may be attributed to its polyphenolic composition (Al-Anee et al. Citation2015). The aerial parts of H. calycinus collected from Bojnurd in Iran are a potential source of natural antioxidants, phenolic and flavonoids; the total phenolic content and antioxidant ability were depending on the type of solvent (Soodmand et al. Citation2015).
Hoshyar et al. (Citation2015) reported the correlation of anticancer effects of H. platystegius extract on human breast adenocarcinoma cells with antioxidant properties. The aqueous extract of H. platystegius collected from South Khorasan in Iran possessing high total antioxidant activity, total phenolic content and DPPH radical scavenging activity dramatically decreased the cancer cell viability. The extract inhibited significantly growth of breast cancer cells in a dose- and time-dependent manner.
In the test of induction of lambda-phage formation in lysogenic E. coli K-12(λ), the methanol extract of the aerial parts of H. platystegius collected from Turkman Sahra in Iran showed positive effect. The active extract causes the prophage to be released from the host genome and the phage reverts to the lytic mode and lyses cells. Production of plaque shows this effect. The induction test may provide a useful screen for the detection of potential DNA-reactive agents. Evidence supports a relationship between an agent’s inducing capability and its antitumour activity (Taghvaei et al. Citation2009). The cytotoxicity of the six fractions of the extract of H. sessilifolius collected from Balochistan Province of Pakistan was determined by flow cytometry on 2 × 106 CFU/mL of C. albicans; the extracts of H. sessilifolius showed non-significant activity against C. albicans and was not toxic for brine shrimps (Zaidi & Crow Citation2012). The results of in vitro studies of the cytotoxicity and cell cycle arrest of the methanol extract of H. longiflorus collected from north of Iraq on colon cancer (RKO) cell line revealed that this plant had a significant cytotoxicity and showed cell-cycle arrest in the G1 phase at 40 μg/mL 3 h, 40 μg/mL 6 h, 80 μg/mL 3 h and 80 μg/mL 6 h (29.07, 34.54, 38.00 and 56.10%, respectively) as compared with non-treated cells (26.69%). Hymenocrater longiflorus extract decreased the viability of RKO cells and the decrement was in a significant concentration, as well as, time-dependent manner. The cytotoxicity of H. longiflorus extract may belong to its active compounds like phenols, alkaloids, flavonoids, tannins and resins. Some plants secondary metabolites are known as potential anticancer drugs through direct cytotoxicity toward cancer cells or inhibition of tumour development (Al-Anee et al. Citation2014). Al-Anee et al. (Citation2015) demonstrated that the methanol extract of H. longiflorus grown in Iraq exerts potent antioxidant, cytotoxic and apoptotic effects on the osteosarcoma (U2OS) cells; extract decreased cell viability, and the effect was concentration and time dependent. Exposure to a high concentration (100 μg/mL) of H. longiflorus extract for 24 h induced detachment of a number of cells from the walls of the wells and appeared to cause a form of cell death called anoikis. Anoikis is a cell death program that largely shares its molecular program with apoptosis, but occurs only when the cells are no longer attached to the substrate. Ten compounds detected by HPLC-ESI/MS were also identified as flavonoids and phenolic acids in this extract. High cytotoxic is most likely due to its polyphenolic composition. Flavonoids are considered the most abundant and most effective antioxidant compounds in plants. Apigenin and related flavonoids are potentially useful as antioxidant and may have therapeutic applications in various diseases. Many natural phenols have been reported to scavenge reactive oxygen species, which are implicated in DNA damage, cancer and accelerated cell aging.
It seems likely that the species of Hymenocrater may act at various therapeutic levels, but further studies are required to evaluate the practical values of therapeutic application.
Antidiabetic activity
Natural α-amylase inhibitors of herbal origin are an attractive therapeutic approach to control post-prandial hyperglycaemia via reducing the glucose release from starch and delaying carbohydrate absorption. These compounds are able to inhibit the activity of the carbohydrate hydrolyzing enzymes in the small intestine and potentially useful in control of diabetes. Many species of Lamiaceae (Labiatae) family possess medicinal properties and have been used traditionally for treatment of chronic illnesses including diabetes. The ethyl acetate extract of the aerial parts of H. bituminosus grown in Iran showed significant α-amylase inhibitory activity with IC50 value of 6.6 mg/mL. This study has been carried out on a “model α-amylase” and the results should be checked in future studies on mammalian amylase or in vivo models (Safamansouri et al. Citation2014).
Discussion and conclusion
Flavonoids, phenolic acids and terpenoids are important constituents of the genus Hymenocrater. The different parts of Hymenocrater spp. of different origins yielded 0.03–2.3% (w/w) essential oil with a pale yellow color and a distinct sharp odour. The quality and yield of essential oils from Hymenocrater spp. is influenced by the harvesting season, fertilizer and pH of soils, the choice and stage of drying conditions, the geographic location, chemotype or subspecies, choice of plant part or genotype, or extraction method (Morteza-Semnani et al. Citation2004, Citation2009; Abad et al. Citation2012). The studies confirm that there is a positive correlation between the chemical content of Hymenocrater spp. and their biological activities.
Rosmarinic acid, β-sitosterol, ursolic acid, quercetin-3-O-rutinoside (rutin), 3-(3,4-dihydroxyphenyl) lactic acid 2-O-quinic acid, cirsimaritin, apigenin, apigenin-7-O-glucoside, genistein, acacetin, carnosic acid, caffeic acid, ferulic acid and isorhamnetin were identified in the extracts of Hymenocrater spp. (Gohari et al. Citation2009, Citation2011; Al-Anee et al. Citation2015). α-Pinene, β-pinene, β-caryophyllene, caryophyllene oxide, spathulenol and 1,8-cineole were found as the most commonly occurring major volatile components in the essential oil of many species of Hymenocrater.
A broad range of biological and physiological activities have been reported for rosmarinic acid, including as an antioxidant, anti-inflammatory, antimutagenic, antibacterial, anticarcinogenic and antiviral, and is used in therapy of herpes simplex and human immunodeficiency virus type 1 (HIV-1) infections (Gohari et al. Citation2011). α-Pinene, β-pinene, 1,8-cineole, β-caryophyllene, β-sitosterol, apigenin, rutin, caffeic acid, ferulic acid and isorhamnetin have been reported to possess antibacterial effects; α- and β-pinene, 1,8-cineole, β-caryophyllene, caryophyllene oxide, caffeic acid, ferulic acid and genistein possess fungicidal properties; apigenin, rutin, ursolic acid, caffeic acid, ferulic acid, isorhamnetin and genistein have been reported to display antioxidant activity; α-pinene, caffeic acid, ferulic acid, β-sitosterol, apigenin, rutin, isorhamnetin and genistein possess anticancer effects; rutin also shows antidiabetic property (Duke et al. Citation2003; Rivas da Silva et al. Citation2012). The appreciable presence of these compounds in the genus Hymenocrater could explain its various activities. In addition, some components that occur in lesser amount may also contribute to the biological activities of Hymenocrater spp., involving probably some type of synergism with the other active compounds.
Pharmacological reports support medicinal potential of genus Hymenocrater for developing new drugs. These plants may have some novel antibiotics, antifungal, antiparasitic, anticancer or antidiabetic compounds that may be effective for treating diseases. Recently, this genus has drawn more attention due to the anticancer effects (Al-Anee et al. Citation2014, Citation2015; Hoshyar et al. Citation2015). Identification of active compounds of Hymenocrater spp. and their mode of action require further investigation for drug development.
Disclosure statement
The authors report that there is no conflict of interest.
References
- Abad MJ, Bedoya LM, Apaza L, Bermejo P. 2012. The Artemisia L. genus: a review of bioactive essential oils. Molecules. 17:2542–2566.
- Abedini A, Roumy V, Mahieux S, et al. 2014. Antimicrobial activity of selected Iranian medicinal plants against a broad spectrum of pathogenic and drug multiresistant micro-organisms. Lett Appl Microbiol. 59:412–421.
- Abu-Mejdad NMJA. 2014. Antifungal activity of some plant extracts against two yeasts isolates in vitro. Res J Pharm Biol Chem Sci. 5:1992–1998.
- Ahmadi F, Sadeghi S, Modarresi M, Abiri R, Mikaeli A. 2010. Chemical composition, in vitro anti-microbial, antifungal and antioxidant activities of the essential oil and methanolic extract of Hymenocrater longiflorus Benth., of Iran. Food Chem Toxicol. 48:1137–1144.
- Akhlaghi H, Saiidi Asl MR, Mohamad-Hosseini M. 2009. Composition of the essential oil of Hymnocrater platystegius in Iran. Chem Nat Compd. 45:448–449.
- Akramian M, Nejad Ebrahimi S, Joharchi MR. 2008. Essential oil composition of Hymenocrater platystegius Rech. f. from Iran. J Essent Oil Bear Pl. 11:199–202.
- Al-Anee RSA, Sulaiman GM, Al-Sammarrae KW, et al. 2014. In vitro studies of the cytotoxicity and cell cycle arrest of Hymenocrater longiflorus plant extract on colon cancer (RKO) cell line. Curr Res Microbiol Biotechnol. 2:367–372.
- Al-Anee RS, Sulaiman GM, Al-Sammarrae KW, Napolitano G, Bagnati R, Lania L, Passoni A, Majello B. 2015. Chemical characterization, antioxidant and cytotoxic activities of the methanolic extract of Hymenocrater longiflorus grown in Iraq. Z Naturforsch C J Biosci. 70:227–235.
- Amiri MS, Jabbarzadeh P, Akhondi M. 2012. An ethnobotanical survey of medicinal plants used by indigenous people in Zangelanlo district, Northeast Iran. J Med Plants Res. 6:749–753.
- Azimova SS, Glushenkova AI. 2012. Lipids, lipophilic components and essential oils from plant sources. New York: Springer.
- Baghestani Maybodi N, Mirvakili SM, Zarezadeh A. 2010. Introduction to the flora, life form and plant geographical distribution in the steppic rangelands (Case study: Khod-niok catchment in Yazd Province, Iran). Renew Nat Resources Res. 1:43–58.
- Barazandeh MM. 2006. Volatile constituents of the essential oil of Hymenocrater elegans Bunge. J Essent Oil Res. 18:284–285.
- Drew BT, Sytsma KJ. 2012. Phylogenetics, biogeography, and staminal evolution in the tribe Menthae (Lamiaceae). Am J Bot. 99:933–953.
- Duke JA, Bogenschutz-Godwin MJ, duCellier J, Duke PK. 2003. Handbook of medicinal spices. Boca Raton (FL): CRC Press.
- Ekrami A. 2013. Plant species diversity in Gonabad. Int J Adv Biol Biom Res. 1:1590–1600.
- El-Gazzar A, Watson L. 1968. Labiatae: taxonomy and susceptibility to Puccinia menthae Pers. New Phytol. 67:739–743.
- Esmaili A, Vaezi J, Ejtehadi H, et al. 2012. A taxonomic study on the genus Hymenocrater Fisch. & C. A. Mey. (Lamiaceae) in Khorasan region. Taxon Biosyst. 4:61–72.
- Fazly Bazzaz BS, Haririzadeh G. 2003. Screening of Iranian plants for antimicrobial activity. Pharm Biol. 41:573–583.
- Fazly Bazzaz BS, Haririzadeh G, Imami SA, Rashed MH. 1997. Survey of Iranian plants for alkaloids, flavonoids, saponins, and tannins [Khorasan Province]. Int J Pharmacogn. 35:17–30.
- Firouznia A, Rustaiyan A, Masoudi S, et al. 2009. Volatile constituents of Salvia limbata, Stachys turcomanica, Scutellaria litwinowii and Hymenocrater elegans four Lamiaceae herbs from Iran. J Essent Oil Bear Pl. 12:482–489.
- Firouznia A, Rustaiyan A, Nadimi M, et al. 2005. Composition of the essential oil of Hymenocrater calycinus (Boiss.) Benth. from Iran. J Essent Oil Res. 17: 527–529.
- Ghahreman A, Heydari J, Attar F, Hamzehee B. 2006. A floristic study of the southwestern slopes of Binaloud elevations (Iran: Khorasan Province). J Sci (Univ Tehran). 32:1–12.
- Ghelichnia H. 2002. The study of dispersion and ecology of aromatic plants in Mazandaran Province. Iran J Med Aromatic Plants. 13:81–95.
- Ghollassi MS. 2008. A contribution to some ethnobotanical aspects of Birjand flora (Iran). Pak J Bot. 40:1783–1791.
- Gohari AR, Saeidnia S, Hajimehdipoor H, et al. 2011. Isolation and quantification of rosmarinic acid from Hymenocrater calycinus. J Herbs Spices Med Plants. 17:132–138.
- Gohari AR, Saeidnia S, Mollazadeh Moghaddam K, et al. 2010. Isolation of a new quinic acid derivative and its antibacterial modulating activity. DARU. 18:69–73.
- Gohari AR, Saeidnia S, Shahverdi AR, et al. 2009. Phytochemistry and antimicrobial compounds of Hymenocrater calycinus. EurAsia J BioSci. 3:64–68.
- Hassanzadeh MK, Emami SA, Asili J, Tayarani Najaran Z. 2011. Review of the essential oil composition of Iranian Lamiaceae. J Essent Oil Res. 23:1–40.
- Hoshyar R, Mostafavinia SE, Zarban A, et al. 2015. Correlation of anticancer effects of 12 Iranian herbs on human breast adenocarcinoma cells with antioxidant properties. Free Rad Antiox. 5:65–73.
- Jafari A, Jafarzadeh F. 2008. Anatomical and pollen ornamentation study on Hymenocrater species in north east of Iran. Pak J Biol Sci. 11:2149–2153.
- Jamzad Z. 2009. New species and new plant records of Lamiaceae from Iran. Iranian J Bot. 15:51–56.
- Jankju M, Mellati F, Atashgahi Z. 2011. Flora, life form and chorology of winter and rural range plants in the Northern Khorasan Province, Iran. J Rangeland Sci. 1:269–284.
- Joharchi MR, Amiri MS. 2012. Taxonomic evaluation of misidentification of crude herbal drugs marketed in Iran. Avicenna J Phytomed. 2:105–112.
- Kashipazha AM, Asri Y, Moradi HM. 2004. Introduction to the flora, lifeformes and chorology of Bagheshad Region, Iran. Pajouhesh Sazandegi. 63:95–103.
- Masoudi S, Azad L, Arabshahi B, et al. 2009. Volatile constituents of Micromeria persica Boiss., Hymenocrater platystegius Rech. f. and Scutellaria pinnatifida A. Hamilt. subsp. pinnatifida, three Labiatae herbs growing wild in Iran. J Essent Oil Res. 21:515–518.
- Masoudi S, Rustaiyan A, Mohebat R, Mosslemin MH. 2012. Composition of the essential oils and antibacterial activities of Hymenocrater yazdianus, Stachys obtusicrena and Nepeta asterotricha three Labiatae herbs growing wild in Iran. Nat Prod Commun. 7:117–120.
- Miguel MG. 2010. Antioxidant and anti-inflammatory activities of essential oils: a short review. Molecules. 15:9252–9287.
- Mirza M, Ahmadi L, Tayebi M. 2001. Volatile constituents of Hymenocrater incanus Bunge. an Iranian endemic species. Flavour Fragr J. 16:239–240.
- Moon HK, Vinckier S, Smets E, Huysmans S. 2008a. Comparative pollen morphology and ultrastructure of Mentheae subtribe Nepetinae (Lamiaceae). Rev Palaeobot Palynol. 149:174–186.
- Moon HK, Vinckier S, Smets E, Huysmans S. 2008b. Palynological evolutionary trends within the tribe Mentheae with special emphasis on subtribe Menthinae (Nepetoideae: Lamiaceae). Plant Syst E. 275:93–108.
- Morteza-Semnani K. 2004. The essential oil composition of Perovskia abrotanoides from Iran. Pharm Biol. 42:214–216.
- Morteza-Semnani K. 2015. A review on Chenopodium botrys L.: traditional uses, chemical composition and biological activities. Pharm Biomed Res. 1:1–9.
- Morteza-Semnani K, Saeedi M, Akbarzadeh M. 2009. The essential oil composition of Clinopodium vulgare L. from Iran. J Essent Oil Res. 21:31–32.
- Morteza-Semnani K, Saeedi M, Akbarzadeh M. 2010. Chemical composition and antimicrobial activity of the essential oil of Hymenocrater elegans Bunge. J Essent Oil Bear Pl. 13:260–266.
- Morteza-Semnani K, Saeedi M, Akbarzadeh M. 2012. Chemical composition and antimicrobial activity of the essential oil of Hymenocrater calycinus (Boiss.) Benth. J Essent Oil Bear Pl. 15:708–714.
- Mozaffarian V. 1996. A dictionary of Iranian plant names. Tehran, Iran: Farhang Mo’aser Publishers.
- Nadaf M, Mortazavi SM. 2011. Investigation flora and life from of plants in protected region Sarigol (North Khorasan Province, Iran). Pak J Biol Sci. 14:78–81.
- Pedersen JA. 2000. Distribution and taxonomic implications of some phenolics in the family Lamiaceae determined by ESR spectroscopy. Biochem Syst Ecol. 28:229–253.
- Prakash B, Kedia A, Mishra PK, Dubey NK. 2015. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities – potentials and challenges. Food Control. 47:381–391.
- Razzaghi-Abyaneh M, Rai M. 2013. Antifungal metabolites from plants. Berlin, Heidelberg: Springer.
- Rechinger KH. 1982. Flora Iranica. Graz, Austria: Akademische Druck-U.Verlagsanstalt.
- Rivas da Silva AC, Lopes PM, Barros de Azevedo MM, Costa DC, Alviano CS, Alviano DS. 2012. Biological activities of α-pinene and β-pinene enantiomers. Molecules. 17:6305–6316.
- Ryding O. 2007. Amount of calyx fibres in Lamiaceae, relation to calyx structure, phylogeny and ecology. Plant Syst E. 268:45–58.
- Ryding O. 2010a. Pericarp structure and phylogeny of tribe Mentheae (Lamiaceae). Plant Syst E. 285:165–175.
- Ryding O. 2010b. Crystals in calyces of Lamiaceae and their phylogenetic and adaptive significance. Plant Syst E. 290:201–215.
- Sabet Teimouri M, koocheki A, Nassiri Mahallati M. 2012. Comparison of essential oil percent of Gol-e Arvaneh Bezghi (Hymenocrater platistegius Rech. F.) in six habitats of Khorasan province, Iran. Int J Agric Crop Sci. 4:643–646.
- Sadeghian M, Hakimi MH, Sodaeizadeh H. 2015. Effect of drought stress on some physiological and morphological characteristics of Hymenocrater yazdianus. J Biol Environ Sci. 7:110–119.
- Safamansouri H, Nikan M, Amin G, Sarkhail P, Gohari AR, Kurepaz-Mahmoodabadi M, Saeidnia S. 2014. α-Amylase inhibitory activity of some traditionally used medicinal species of Labiatae. J Diabetes Metab Disord. 13:114.
- Sarangzai AM, Ahmed A, Laghari SK. 2013. Traditional uses of some useful medicinal plants of Ziarat District Balochistan, Pakistan. Fuuast J Biol. 3:101–107.
- Satil F, Ünal M, Hopa E. 2007. Comparative morphological and anatomical studies of Hymenocrater bituminosus Fisch. & C.A.Mey. (Lamiaceae) in Turkey. Turk J Bot. 31:269–275.
- Serpooshan F, Jamzad Z, Nejadsattari T, Mehregan I. 2014. Taxonomic significance of nutlet and leaf characters in Hymenocrater, Nepeta sect. Psilonepeta and Lophanthus (Nepetinae, Nepetoideae: Lamaceae). Iran J Bot. 20:80–95.
- Shahriari S, Khanahmadi M, Tahvilian R. 2013. The study of essential oil of Hymenocrater longiflorus Benth growing in Paveh. J Rep Pharm Sci. 2:111–115.
- Soodmand M, Mohamadi Sani A, Jalilvand MR. 2015. Phytochemical analysis, total phenolic and flavonoid content, and antioxidant activity from aerial parts of Hymenocrater calycinus (Boiss). J Appl Environ Biol Sci. 4:141–145.
- Taghvaei M, Naghibi F, Mosaddegh M, et al. 2009. Prophage induction in Escherichia coli K-12(λ) by some plants from Iran. Ethno–Med. 3:57–59.
- Taherpour A, Maroofi H, Changizi M, et al. 2011. Chemical compositions of the essential oil and calculation the biophysicochemical coefficients of the components of Hymenocrater longiflorus Benth. of Iran. Nat Sci. 3:104–108.
- Taran M, Karimi N, Abdi J, et al. 2013. Larvicidal effects of essential oil and methanolic extract of Hymenocarter longiflorus (Lamiaceae) against Echinococcus granulosus. J Essent Oil Bear Pl. 16:85–91.
- Zaidi MA, Crow SA Jr. 2005. Biologically active traditional medicinal herbs from Balochistan, Pakistan. J Ethnopharmacol. 96:331–334.
- Zaidi MA, Crow SA Jr. 2012. Cytotoxicity of four medicinal plants of Pakistan. Pak J Bot. 44:395–397.
- Zarezadeh A, Rezaee MB, Mirhosseini A, Shamszadeh M. 2007. Ecological investigation of some aromatic plants from Lamiaceae family in Yazd Province. Iran J Med Aromatic Plants. 23:432–442.