Abstract
Context: Silymarin is the main flavonoid extracted from milk thistle, which has been used to treat liver diseases.
Objective: The in vivo effect of silymarin on HFD-induced insulin resistance and fatty liver in mice was studied.
Materials and methods: Male C57BL/6 mice were fed with high-fat diet (HFD) to induce obesity and insulin resistance and treated with 30, 60 mg/kg silymarin for 18 days. Food intake, body weight and the content/histology of epididymal fat and liver tissue were examined; the content of lipids, AST, ALT and inflammatory cytokines in serum were estimated.
Results: Administration of silymarin caused bodyweight loss in diet induced obesity (DIO) mice (HFD group: 47.7 g, 60 mg/kg group: 43.0 g) while the food intake remain unchanged. Silymarin (60 mg/kg) significantly reduced the epididymal fat mass (from 1.75 g to 1.12 g). Elevated plasma lipids (TC 6.1 mM, TG 1.3 mM, LDL 1.2 mM) in DIO mice were all suppressed by silymarin (TC 4.5 mM, TG 0.89 mM, LDL 0.9 mM), as well as insulin (5.1 ng/ml in HFD group to 2.0 ng/ml (60 mg/kg silymarin). Examination of cytokine levels (TNF-α, IL-1β and IL-6) in each group proved that silymarin treatment significantly decreased inflammation in DIO mice. Finally, silymarin effectively protected liver from HFD-induced injury as evidenced by decreasing histological damage and reducing ALT and AST levels, as follows: ALT; 47.4 U/L in HFD group to 28.4 U/L (60 mg/kg silymarin); AST; 150.1 U/L in HFD group to 88.1 U/L (60 mg/kg silymarin) in serum.
Discussion and conclusion: Our results suggested that silymarin-induced alleviation of inflammatory response could be a mechanism responsible for its benefits against liver damage and insulin resistance.
Introduction
Diabetes is a serious and increasing global health burden worldwide as it is projected to rise 592 million by 2035 (Forouhi & Wareham Citation2014; Guariguata et al. Citation2014). Type 1 and type 2 diabetes are the two main types, with type 2 diabetes accounting for the majority (>85%) of total diabetes prevalence. Insulin resistance, a hallmark of type 2 diabetes, is associated with obesity. Obesity gives rise to a state of chronic, low-grade inflammation that contributes to insulin resistance (Kahn & Flier Citation2000; Tateya et al. Citation2013; Minihane et al. Citation2015). Insulin resistance occurs when inadequate response of peripheral tissues to normal circulating level of insulin to regulate whole body glucose homeostasis. Chronic inflammation is found to be closely associated with obesity-induced insulin resistance (Shoelson et al. Citation2006; Shoelson SE et al. Citation2007). Pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) can cause insulin resistance in adipose tissue, skeletal muscle and liver by inhibiting insulin signal transduction (Hotamisligil et al. Citation1993; Rui et al. Citation2001). Moreover, inflammasome-mediated interleukin 1β (IL-1β) production also contributed to insulin resistance (Vandanmagsar et al. Citation2011; Wen et al. Citation2012; Jourdan et al. Citation2013).
Silymarin is a flavonoid complex-containing silybin, silydianin and silychrisin, which are derived from the milk thistle seed, Silybum marianum (Compositae, Silybum marianum genus, Asteraceae). Silymarin has very strong antioxidant activity which can protect liver from both acute and chronic toxicity and injury (Gillessen et al. Citation2014; Abdel-Moneim et al. Citation2015; Luangchosiri et al. Citation2015; Wu et al. Citation2015). Also, silymarin can suppress various inflammatory responses by suppressing NF-κB pathway. For example, silymarin effectively suppressed tumorigenesis via improving oxidative stress and deregulating activation of inflammatory mediators (Wu et al. Citation2008; Toyoda-Hokaiwado et al. Citation2011). Silybin inhibited lipopolysaccharide (LPS)-induced morphological changes and the production of pro-inflammatory cytokines through inhibiting NF-κB signaling pathway (Kim et al. Citation2015; Lovelace et al. Citation2015). In addition, silymarin can improve fructose-induced metabolic abnormalities in rat (Prakash et al. Citation2014). In this study, we found that silymarin alleviated high-fat diet (HFD)-induced obesity and insulin resistance by decreasing inflammation level in high calorie diet-induced obesity model.
Materials and methods
Agents
Silymarin was purchased from Selleck (Houston, TX). Kits for cholesterol (TC), triacylglycerol (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL), glutamic-oxalacetic transaminase (AST), glutamic-pyruvic transaminase (ALT) were purchased from Nanjing Jiancheng Company (Nanjing, China). Human insulin (Novolin) for in vivo treatment was bought from Novo Nordis (Copenhagen, Denmark). Elisa kit for mouse insulin was bought from ALPCO (Illinois, USA). Elisa kits for mouse TNF-α, IL-1β, IL-6 were bought from R&D (Minneapolis, MN). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Animal experiments and drug treatment
Male C57BL/6 mice, 6–8 weeks of age, were purchased from Shanghai Laboratory Animal Center at the China Academy of Sciences. Mice were maintained in an animal facility under standard laboratory conditions for 1 week prior to experiments, with water and standard chow supply. Animal welfare and experimental procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Ministry of Science and Technology of China, 2006, NO. 398). Mice were fed either normal chow diet (NCD) or a high-fat diet (HFD) (40% fat calories) for 14 weeks and had free access to water and food. Concurrently with a HFD, silymarin (30, 60 mg/kg) were given orally for another 3 weeks. Body weight were monitored daily before being sacrificed. The mice were subjected to insulin tolerance test (ITT) and glucose tolerance test (GTT). Fed blood glucose was measured as indicated. After the experimental period, mice were sacrificed and epididymal fat mass was weighted (represent for fat mass). The livers were collected for histological analysis and mRNA extraction.
Insulin tolerance test (ITT) and glucose tolerance test (GTT)
For ITT, the mice were fasted for 4 h and then injected intraperitoneally with insulin solution at a dosage of 0.8 IU/kg. For GTT, the mice were fasted overnight (∼12 h). The next morning, mice were injected intraperitoneally with glucose solution at a dosage of 1.1 g/kg body weight. Blood glucose was measured using a Free Style Lite glucometer.
Histological analysis
To characterize the histological alterations, liver and adipose tissue from four animals in each experimental group were fixed by 10% buffered formalin, embedded in paraffin, and then sectioned. Histopathologic study was executed using H&E-stained sections.
Serum liver functions and blood lipids assay
Serum taken from mice in each group were used for analysis ALT, AST, TC, TG, LDL, HDL, insulin and cytokines according to the manufacturer’s instructions.
Quantitative RT-PCR
Total RNA from each animal was extracted from the liver or adipose tissue using TRIzol (Takara, Dalian, China). RNA samples were reversed to cDNA and analyzed by quantitative PCR, and the relative expression of specific genes was determined using the real-time PCR master mix (Roche, Basel, Switzerland) by using an ABI 7900 Real-Time PCR System. The actin gene was used as an endogenous control to normalize for differences in the amount of total RNA present in the samples. PCR primers were used as follows:
TNF-α, 5′-CTGTGAAGGGAATGGGTGTT-3′ (forward), 5′-CAGGGAAGAATCTGGAAAGGTC-3′ (reverse);
IL-1β, 5′-CTTCAGGCAGGCAGTATCACTC-3′ (forward)and 5′-TGCAGTTGTCTAATGGGAACGT-3′ (reverse);
IL-6, 5′-TTCTTGGGACTGATGCTG-3′ (forward) and 5′-CTGGCTTTGTCTTTCTTGT T -3′(reverse); β-actin, 5′-TGCTGTCCCTGTATGCCTCT-3′ (forward) and 5′-TTTGATGTCACGCACGATTT-3′ (reverse).
Statistical analysis
Data are expressed as mean ± SEM. ANOVA with post hoc two comparisons is used to evaluate the differences between various experimental and control groups. p Values less than 0.05 were considered significant.
Results
Silymarin ameliorated fat accumulation in DIO mice
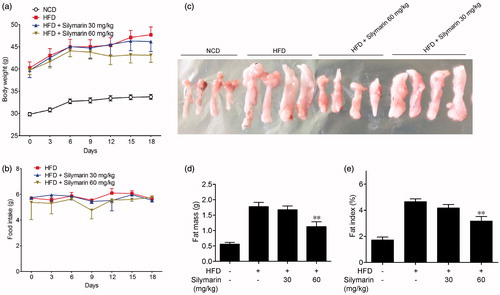
In this study, we used a mice model of HFD-induced obesity to evaluate the effect of silymarin. Mice were fed with HFD or normal chow diet (NCD) for 14 weeks. The bodyweight of HFD-fed mice was significantly higher in comparison with the NCD group. Concurrently with HFD, silymarin (30, 60 mg/kg) were given orally for the last 3 weeks. As shown in , administration of silymarin caused loss of body weight in diet induced obesity (DIO) mice to some extent (from 47.7 to 43.0 g) while the food intake in each group has no difference (). Epididymal fat was taken, weighted and normalized for body mass (). 60 mg/kg silymarin significantly reduced the epididymal fat mass (from 1.75 to 1.12 g) and the fat index (from 4.6 to 3.2%) in DIO mice.
Figure 1. Silymarin ameliorated fat accumulation in HFD-induced obesity mice. Mice were fed with NCD or HFD for 14 weeks, then treated with silymarin for 3 weeks, followed by 12-h fasting. Bodyweight (a) and food intake (b) were recorded. (c) Epididymal fat mass was harvested and weighted (d) and normalized for total body mass (e). Values are means ± SD (n = 8). *p < 0.05, **p < 0.01, compared to HFD group.
Silymarin suppressed blood lipids levels in HFD-induced obesity mice
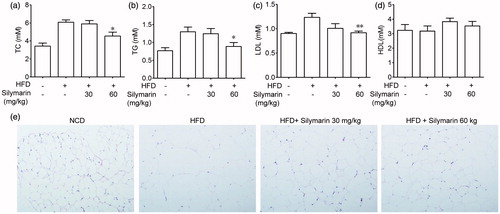
In order to evaluate the blood lipids levels, serum was collected and analyzed. The results showed that the HFD fed induced a great increase of TC, TG and LDL in serum as compared to NCD group. Treatment with silymarin (60 mg/kg) showed a significant decrease of serum level of TC, TG and LDL (), whereas no significant difference in the level of HDL was observed among NCD group, HFD group and silymarin-treated groups (). Adipose sections stained with hematoxylin & eosin showed in proved that silymarin significantly improved lipid metabolism.
Figure 2. Silymarin suppressed blood lipids levels in HFD-induced obesity mice. (a) Mice were fed with NCD or HFD for 14 weeks, then treated with silymarin for 3 weeks, followed by 12-h fasting. (a–d) Serum were collected for TC, TG, LDL and HDL analysis. (e) Epididymal fat was harvested and stained with H&E. Values are means ± SD (n = 8). *p < 0.05, **p < 0.01, compared to HFD group.
Silymarin improved HFD-induced liver injury
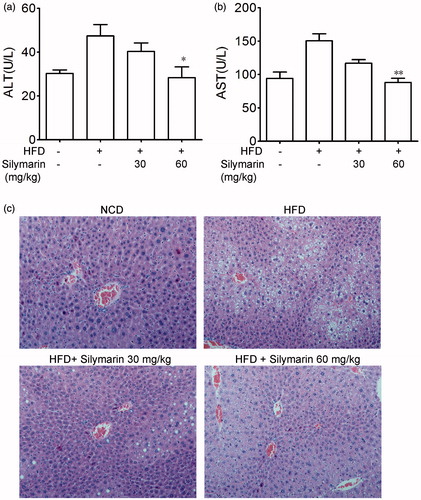
It is well known that HFD can trigger sever liver damage. As shown in DIO mice have a higher level of AST and ALT in serum compared to NCD group. Treatment with silymarin caused a dose-dependent decrease of ALT and AST level in serum. Liver tissue samples of mice in NCD group showed normal lobular architecture with central veins, and no obvious steatosis, inflammation or necrosis was observed (). In HFD group, the structure of hepatic cord was deranged, and a great accumulation of fat droplets, various degrees of diffuse hepatic steatosis could be found obviously. Liver section form silymarin-treated mice showed less degree and extensive of fatty liver than HFD group (). These results suggested that silymarin treatment alleviated fatty liver in DIO mice.
Figure 3. Silymarin improved HFD-induced hepatic injury. Mice were fed with NCD or HFD for 14 weeks, then treated with silymarin for 3 weeks, followed by 12 h fasting. (a) ALT and AST levels in serum were determined. (b) Hematoxylin and eosin staining of liver (400×) was showed. Values are means ± SD (n = 8). *p < 0.05 and **p < 0.01 compared to HFD group.
Silymarin decreased insulin resistance in HFD-induced obesity mice
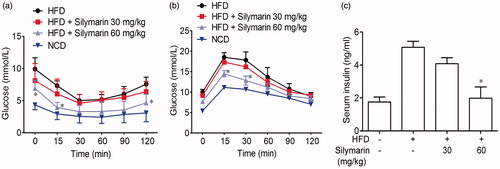
In ITT assay, mice exhibited a state of insulin resistance after 14 weeks of HFD treatment. The silymarin administration significantly improved insulin sensitivity (). Similarly, GTT assay also showed that silymarin increased glucose tolerance as compared with NCD mice (). Moreover, silymarin reversed the elevation of fasting serum insulin concentrations caused by HFD ().
Figure 4. Silymarin decreased insulin resistance in HFD-induced obesity mice. Mice were fed with NCD or HFD for 14 weeks, then treated with silymarin for 3 weeks. Insulin tolerance test (ITT) was performed after a 4-h fasting, and mice were received 0.8 U/kg body weight of insulin (i.p.). Glucose concentration was recorded. (b) For GTT, 1.1 g/kg body weight of glucose was administered by intraperitoneal injection. Blood glucose was measured between 0 and 120 min later. Values are presented as means ± SD (n = 8). *p < 0.05 and **p < 0.01 compared to HFD group.
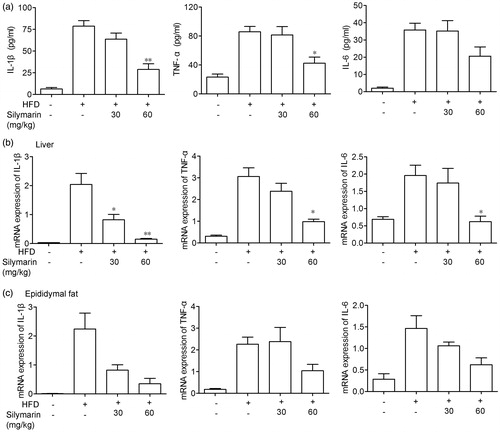
Silymarin counteracted inflammation in HFD-induced obesity mice
Next, the anti-inflammatory property of silymarin was evaluated by determining protein levels of inflammatory cytokines in serum and mRNA levels in liver and adipose tissue, including TNF-α, IL-6 and IL-1β. As shown in , fed with HFD significantly increased the level of TNF-α, IL-6 and IL-1β in serum as well as in liver and adipose tissue while silymarin significantly decreased these cytokine levels.
Figure 5. Silymarin counteracted inflammation in HFD-induced obesity mice. Mice were fed with NCD or HFD for 14 weeks, then treated with silymarin for 3 weeks. (a) TNF-α, IL-6 and IL-1β in serum were tested by ELISA. (b,c) mRNA levels of TNF-α, IL-6 and IL-1β in liver (b) and epididymal fat (c) were examined by RT-PCR. Values are presented as means ± SD (n = 8). *p < 0.05 and **p < 0.01 compared to HFD group.
Discussion
Insulin resistance is found in the metabolic syndrome associated with obesity and type-2 diabetes mellitus, which is the major culprit for the development of diabetes, hypertension and cardiovascular diseases (Matthaei et al. Citation2000; Saltiel Citation2000). Our results showed that administration with silymarin reduced the total body weight while it has no effect on lean body weight, decreased serum TC, TG, and LDL levels and improved insulin resistance in obesity mice. Meanwhile, the reductions in fasting blood glucose, insulin level were obviously observed in silymarin-treated DIO mice. GTT and ITT experiments demonstrated that silymarin significantly improve glucose tolerance and insulin sensitivity. These results suggest that silymarin may be candidate for treatment of insulin resistance.
Insulin resistance is a common pathological state in which target tissues failing to respond properly to physiologic insulin level. Among these tissues, liver is very sensitive to insulin and it is an important regulator of glucose–glycogen homeostasis, thus it precedes other organs to develop insulin resistance triggered by obesity (Bugianesi et al. Citation2005). Therefore, as an important tissue for whole body glucose homeostasis, investigating the pathogenesis of insulin resistance in the liver may be beneficial for the management of diabetes and its associated complications. In our study, liver injury was assessed with histological and biochemical parameters. Our results here showed that obvious liver injury and hepatic steatosis were observed in HFD group. Silymarin could improve the degree of hepatic steatosis and inflammation in liver tissue, and decrease the levels of serum ALT, AST. The results suggested that silymarin could effectively inhibit the HFD-induced fatty liver and improve the liver function. Our results are in agreement with data obtained with a rat model (Zhang et al. Citation2013).
The pathogenic role of inflammation in insulin resistance is increasingly recognized. Several factors such as oxidative stress, inflammation, glucotoxicity, lipotoxicity and adipokine dysregulation have been implicated in the genesis of insulin resistance (Shoelson et al. Citation2007; Piya et al. Citation2013). Inflammatory cytokines have been shown to play an important role in the regulation of glucose homeostasis and insulin resistance. Abnormal changes in these cytokine profiles could reduce insulin sensitivity of remote insulin sensitive organs including liver. Adipose tissue inflammation contributes to insulin resistance through proinflammatory cytokines such as TNF-α, IL-6 and IL-1β (Shoelson et al. Citation2006), while infiltration of proinflammatory cells into pancreatic islets may contribute to beta cell failure (Ehses et al. Citation2007). TNF-α, IL-6 and IL-1β were higher in the serum of obesity mice than in that of control mice. Conversely, silymarin remarkably diminished TNF-α, IL-6 and IL-1β level at both the mRNA and protein level.
In conclusion, when obesity was induced by HFD in mice, co-treatment with silymarin improved hepatic steatosis, serum ALT/AST levels, insulin resistance, and glucose metabolism through the inhibition of inflammation levels in mice. At the same time, silymarin treatment reduced fat accumulation in DIO mice. We deduced that the improvement of silymarin on obesity-induced insulin resistance may dependent on both its effect on inflammation alleviation and fat mitigation. Therefore, silymarin maybe a potent useful herbal in the daily prevention of metabolic syndromes. Further, the detail mechanism and target for silymarin needs to be verified.
Disclosure statement
The authors declare no conflict of interest.
References
- Abdel-Moneim AM, Al-Kahtani MA, El-Kersh MA, Al-Omair MA. 2015. Free radical-scavenging, anti-inflammatory/anti-fibrotic and hepatoprotective actions of taurine and silymarin against CCl4 induced rat liver damage. PLoS One. 10:e0144509.
- Bugianesi E, McCullough AJ, Marchesini G. 2005. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 42:987–1000.
- Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, Ellingsgaard H, Schneider MK, Biollaz G, et al. 2007. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 56:2356–2370.
- Forouhi NG, Wareham NJ. 2014. Epidemiology of diabetes. Medicine (Abingdon). 42:698–702.
- Gillessen A, Herrmann WA, Kemper M, Morath H, Mann K. 2014. Effect of silymarin on liver health and quality of life. Results of a non-interventional study. MMW Fortschr Med. 156:120–126.
- Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. 2014. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 103:137–149.
- Hotamisligil GS, Shargill NS, Spiegelman BM. 1993. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 259:87–91.
- Jourdan T, Godlewski G, Cinar R, Bertola A, Szanda G, Liu J, Tam J, Han T, Mukhopadhyay B, Skarulis MC, et al. 2013. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat Med. 19:1132–1140.
- Kahn BB, Flier JS. 2000. Obesity and insulin resistance. J Clin Invest. 106:473–481.
- Kim EJ, Lee MY, Jeon YJ. 2015. Silymarin inhibits morphological changes in LPS-stimulated macrophages by blocking NF-kappaB pathway. Korean J Physiol Pharmacol. 19:211–218.
- Lovelace ES, Wagoner J, MacDonald J, Bammler T, Bruckner J, Brownell J, Beyer RP, Zink EM, Kim YM, Kyle JE, et al. 2015. Silymarin suppresses cellular inflammation by inducing reparative stress signaling. J Nat Prod. 78:1990–2000.
- Luangchosiri C, Thakkinstian A, Chitphuk S, Stitchantrakul W, Petraksa S, Sobhonslidsuk A. 2015. A double-blinded randomized controlled trial of silymarin for the prevention of antituberculosis drug-induced liver injury. BMC Complement Altern Med. 15:334.
- Matthaei S, Stumvoll M, Kellerer M, Haring HU. 2000. Pathophysiology and pharmacological treatment of insulin resistance. Endocr Rev. 21:585–618.
- Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, Teeling JL, Blaak EE, Fenech M, Vauzour D, et al. 2015. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. 114:999–1012.
- Piya MK, McTernan PG, Kumar S. 2013. Adipokine inflammation and insulin resistance: the role of glucose, lipids and endotoxin. J Endocrinol. 216:T1–T15.
- Prakash P, Singh V, Jain M, Rana M, Khanna V, Barthwal MK, Dikshit M. 2014. Silymarin ameliorates fructose induced insulin resistance syndrome by reducing de novo hepatic lipogenesis in the rat. Eur J Pharmacol. 727:15–28.
- Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF. 2001. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest. 107:181–189.
- Saltiel AR. 2000. Series introduction: the molecular and physiological basis of insulin resistance: emerging implications for metabolic and cardiovascular diseases. J Clin Invest. 106:163–164.
- Shoelson SE, Lee J, Goldfine AB. 2006. Inflammation and insulin resistance. J Clin Invest. 116:1793–1801.
- Shoelson SE, Herrero L, Naaz A. 2007. Obesity, inflammation, and insulin resistance. Gastroenterology. 132:2169–2180.
- Tateya S, Kim F, Tamori Y. 2013. Recent advances in obesity-induced inflammation and insulin resistance. Front Endocrinol (Lausanne). 4:93.
- Toyoda-Hokaiwado N, Yasui Y, Muramatsu M, Masumura K, Takamune M, Yamada M, Ohta T, Tanaka T, Nohmi T. 2011. Chemopreventive effects of silymarin against 1,2-dimethylhydrazine plus dextran sodium sulfate-induced inflammation-associated carcinogenicity and genotoxicity in the colon of gpt delta rats. Carcinogenesis. 32:1512–1517.
- Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. 2011. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 17:179–188.
- Wen H, Ting JP, O'Neill LA. 2012. A role for the NLRP3 inflammasome in metabolic diseases-did Warburg miss inflammation? Nat Immunol. 13:352–357.
- Wu JP, Tsai CC, Yeh YL, Lin YM, Lin CC, Day CH, Shen CY, Padma VV, Pan LF, Huang CY. 2015. Silymarin accelerates liver regeneration after partial hepatectomy. Evid Based Complement Alternat Med. 2015:603529.
- Wu YF, Fu SL, Kao CH, Yang CW, Lin CH, Hsu MT, Tsai TF. 2008. Chemopreventive effect of silymarin on liver pathology in HBV X protein transgenic mice. Cancer Res. 68:2033–2042.
- Zhang Y, Hai J, Cao M, Pei S, Wang J, Zhang Q. 2013. Silibinin ameliorates steatosis and insulin resistance during non-alcoholic fatty liver disease development partly through targeting IRS-1/PI3K/Akt pathway. Int Immunopharmacol. 17:714–720.
