Abstract
Context: Multidrug resistance (MDR) is a major obstacle to efficient therapy of cancers. It is a prime concern for researchers to find compounds with anti-proliferative activity on MDR cell lines. In recent years, annonaceous acetogenins (ACGs) were reported to have anti-proliferative activity. However, the underlying mechanisms are still unknown.
Objective: This study determines the mechanisms of anti-proliferative activity induced by Annosquacin B (AB) against MCF-7/ADR cells.
Material and methods: The cytotoxicity of AB at varying concentrations (0.64, 1.6, 4, 10, 25, 62.5, 156.25 μM) on MCF-7/ADR cells was assessed using the MTT assay. Annexin V-FITC/propidium iodide staining and Acrinidine orange and ethidium bromide (AO/EB) staining were employed to investigate whether AB (14, 7, 3.5 μM) could induce apoptosis in MCF-7/ADR cells. Levels of caspase-3 and caspase-9, Bax, Bcl-2 and MAPKs kinases were evaluated by western blot assay following treatment with various concentrations of AB (3.5, 7, 14 μM) at different time points (0, 0.5, 1, 2, 4, 8, 12 h).
Results and conclusion: MTT assay showed that AB significantly decreased cell viability on MCF-7/ADR (IC50 of 14.69 μM). AB-induced apoptosis in MCF-7/ADR cells through mitochondrial apoptosis pathways. It induced typical apoptosis by morphologic changes; elevate levels of caspase-3, caspase-9 as well as the ratio of Bax/Bcl-2. In addition, AB increased the expression of p-p38 MAPK and decreased the expression of p-JNK, while whether ERK1/2 had an effect on the MCF-7/ADR apoptosis remains to be determined.
Introduction
Multidrug resistance (MDR) is a major impediment to the effective chemotherapy of human malignancies (Choi et al. Citation2002; Ludwig et al. Citation2002; Kowalski et al. Citation2002), a phenomenon that is, not only resistant to the drug first exposed to, but also resistant to the drugs with different structures and mechanisms. Looking for novel compounds with anti-proliferative activity on MDR cell lines is of great significance.
Until now, more than 500 annonaceous acetogenins (ACGs) had been isolated from natural plant sources (Bermejo et al. Citation2005), and reported to be cytotoxic (Yazbak et al. Citation1998), antitumor (Alali et al. Citation1999), pesticidal (Makabe et al. Citation2005), antiparasitic (Takahashi et al. Citation2002) and immunosuppressive (Hwang et al. Citation2005). Their inhibitory activity on MDR cell lines (Hui et al. Citation1989; Oberlies et al. Citation1997; Fu et al. Citation1999; Raynaud et al. Citation1999) has recently attracted the attention of many researchers. However, there are few reports concerning the underlying mechanisms. Here, our group found that Annosquacin B (AB) exerted anti-proliferative activity against MCF-7/ADR cells with an IC50 of 14.69 μM, so we further investigated its mechanisms.
Materials and methods
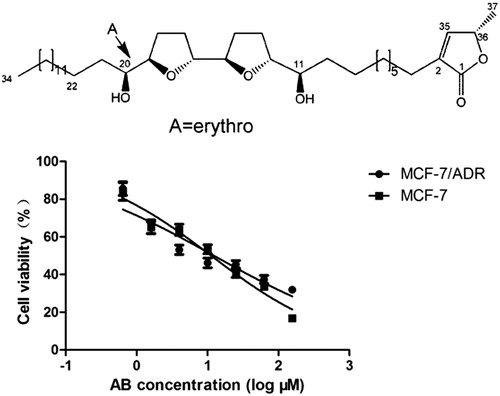
AB was isolated in our laboratory as reported previously (Yang et al. Citation2009; Chen et al. Citation2012a, 2012Citationb, Citation2012c), from the seeds of Annona squamosa L. (Annonaceae), which were collected from Guangdong Province in June, 2014 and identified by Professor Jianwei Chen (College of Pharmacy, Nanjing University of Chinese Medicine, China). A voucher specimen of the plant (No. 083) was deposited in the Herbarium Center, Nanjing University of Chinese Medicine, China. AB is a white powder and its purity was above 98% determined by HPLC and LC-MS analysis. The stereochemistry and configuration were ascertained using combination of 1D and 2D NMR techniques. A stock solution of AB at a concentration of was performed in 0.1% DMSO. The chemical structure is shown in . Cisplatin was purchased from Qilu Pharmaceutical. Adriamycin was purchased from Zhejiang Hisun pharmaceutical.
Figure 1. Chemical structure of AB. AB inhibits cell viability in a concentration-dependent manner on MCF-7/ADR cell line. Values are presented as the mean ± SD of at least three independent experiments. AB: Annosquacin B.
Rabbit antibodies against p-ERK1/2 (1:1000), p-p38 MAPK (1:1000) and p-JNK (1:1000) were purchased from Cell Signaling Technology. Antibodies against ERK1/2 (1:200), p38 MAPK (1:200), JNK (1:500) and c-caspase-3 (1:200), c-caspase-9 (1:1000), Bax (1:500), Bcl-2 (1:500), β-actin (1:1000) were supplied by Bioworld Technology (Nanjing, China), respectively. Annexin V-FITC/propidium iodide Detection Kit and Z-VAD-FMK were purchased from KeyGEN Biotechnology (Nanjing, China). SB202190, SP600125 and U0126 were purchased from Cell Signaling Technology. BCA Protein Assay Kit was purchased from Beyotime Biotechnology (Nanjing, China). ECL Chemiluminescence Kit was purchased from Thermo Fisher (Shanghai, China). All other reagents and chemicals were of analytical grade.
Cell culture
The human breast cancer cell line MCF-7 and its multidrug resistant cell line MCF-7/ADR were kindly provided by Prof. Qinglong Guo, China Pharmaceutical University, and were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U mL−1 penicillin, 100 μg mL−1 streptomycin at 37 °C in a humidified atmosphere of 5% CO2. Adriamycin (ADR) (5 μg mL−1) was added to the medium in order to maintain drug resistance in MCF-7/ADR cells, and was removed two weeks before the experiments. (Zhu et al. Citation2013). The medium was replaced every other day and cells were subcultured after reaching confluence.
MTT assay
The cytotoxicity of AB against MCF-7/ADR cells was assessed by MTT assay. In short, cells were seeded into 96-well plates at a density of 1 × 105 cells mL−1. After an overnight incubation, various concentrations of AB (0.64, 1.6, 4, 10, 25, 62.5, 156.25 μM) were added and incubated for an additional 48 h. 20 μL – MTT solution (5 mg mL−1) was added to each well to form the insoluble formazan crystals, which were dissolved in 150 μL dimethyl sulfoxide (DMSO) 4 h later. The plates were shaken for 10 min and measured immediately at 490 nm using a microplate reader (Spectra MAX190, Molecular Devices, Sunnyvale, CA). The inhibitory ratio was calculated using the following formula: inhibitory ratio = (1 − A/B) × 100%. A and B are mean absorbance of the treated and control wells, respectively. Six replicate wells were used in the experiment. All experiments were performed three times under same the conditions. Cisplatin was used as a positive control.
The multidrug resistance of MCF-7/ADR cell line was also assessed by MTT assay. MCF-7 and MCF-7/ADR cell lines were seeded into 96-well plates at a density of 1 × 105 cells mL−1. After an overnight incubation, various concentrations of adriamycin were added and incubated for an additional 48 h. Resistance index (RI) was calculated using the following formula: RI = IC50 drug-resistant cells/IC50 drug-sensitive cells.
To investigate the roles of MAPKs in AB-induced cell apoptosis on MCF-7/ADR, cells were incubated with SB202190 (p38 MAPK inhibitor, at the concentration of 25 μM), SP600125 (JNK inhibitor, at the concentration of 25 μM) and U0126 (ERK1/2 inhibitor, at the concentration of 10 μM) for 30 min prior to AB (14 μM) treatment, and then subjected to MTT assay.
AO/EB staining assay
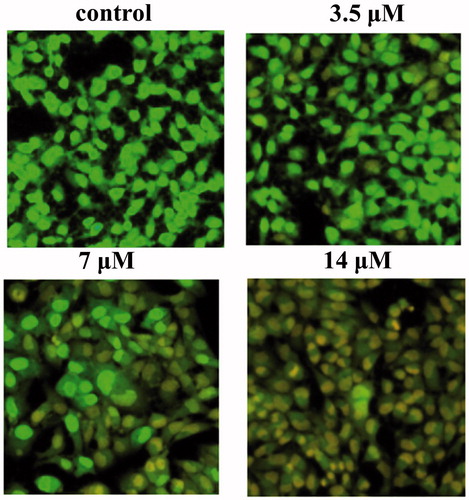
Cell apoptosis was evaluated by Acrinidine orange and ethidium bromide (AO/EB) staining assay. Briefly, after treatment with various concentrations of AB (14, 7, 3.5 μM) for 48 h, MCF-7/ADR cells were washed with PBS twice and stained with AO/EB (100 μg mL−1 in PBS) solutions in dark at room temperature. Then the cells were observed under an inverted fluorescence microscope and photographed.
Annexin V-FITC/propidium iodide assay
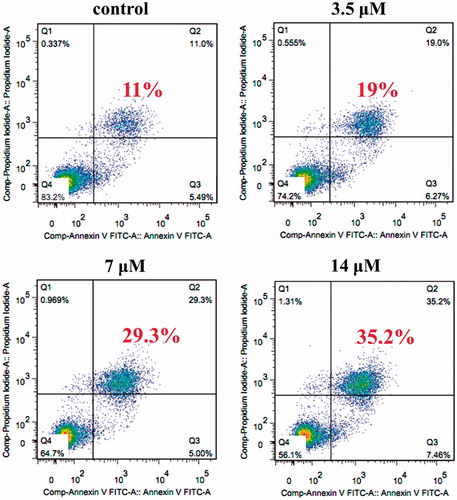
The effects of AB on apoptosis against MCF-7/ADR cells were quantified using Annexin V-FITC/propidium iodide Detection Kit. After 48 h, treatment of AB (14, 7, 3.5 μM), both floating and attached cells were collected and resuspended in the cold binding buffer. 5 μL of Annexin V-FITC and 5 μL of propidium iodide were added, and then incubated for 15 min at room temperature in dark according to the manufacture’s instruction. Then the viable, apoptotic, necrotic cells were classified and counted by FACS flow cytometry (Beckman Coulter, Brea, CA).
Western blot analysis
After treatment with AB, the cells were harvested, washed twice with ice-cold PBS, and then lysed with lysis buffer (Beyotime) containing 1% phenylmethylsulfonyl fluoride (PMSF) for 30 min on ice. In order to obtain pure protein, the whole cell lysate was scraped from the plates and then centrifuged at 12000 rpm for 10 min at 4 °C. The concentrations of protein were determined by BCA Protein Assay Kit. 40 μg of protein was then subjected to SDS-PAGE, and transferred to polyvinylidene fluoride membrane (0.45 μm, Millipore). Afterwards, the membranes were blocked with 5% BSA in TBST buffer for 1.5 h at room temperature, followed by incubation with primary specific antibodies in 5% BSA TBST buffer at 4 °C overnight. After being washed with TBST buffer for three times, the membranes were incubated with HRP-conjugated secondary antibodies for 1 h at room temperature, and then subjected to be washed three times with TBST buffer again prior to detection with an ECL Chemiluminescence Kit on a Chemi Doc XRS Imaging system (BioRad, Hercules, CA).
The effects of AB on the expression of caspase-3 and caspase-9 were affirmed with its inhibitor Z-VAD-FMK by western blot assay.
Statistical analysis
All data were represented as means ± SD. Statistical differences were evaluated using the Student’s t-test. p Values lower than 0.05 were considered to be statistically significant.
Results
AB effectively reduces MCF-7/ADR viability
As shown in , MTT assay showed that MCF-7/ADR cell line was resistant to adriamycin. The IC50 to MCF-7 cells was 0.55 μM, while the IC50 to MCF-7/ADR cells was 46.08 μM, the Resistance index was 83.78.
Table 1. The multidrug resistance of MCF-7/ADR cells.
As shown in and , exposure to AB for 48 h caused MCF-7/ADR and MCF-7 concentration-dependent decrease in cell viability, with an IC50 value of 14.69 μM and 11.58 μM, respectively. It was much more efficient than the positive drug cisplatin, with an IC50 of 232.36 μM and 16.46 μM, respectively. Obviously, AB displayed potent cytotoxicity in vitro on MCF-7/ADR cells, and we chose 3.5, 7, 14 μM AB to study in the present work. The chemical structure of AB is shown in .
AB exerts anti-proliferative activity through cell apoptosis
To investigate whether AB exerted its inhibitory activity against MCF-7/ADR cells through cell apoptosis, AB-treated cells were stained using the fluorescent DNA-binding agents AO/EB. Results showed that cells displayed typical characteristics of apoptosis such as cell shrinkage, chromatin condensation and the increased permeability of cell membranes after the treatment with AB (). These results suggested that apoptosis did occur in AB-treated cells.
Figure 2. Representative images (200×) of fluorescent microscopic analysis of MCF-7/ADR cells without treatment (control) and treated with 3.5, 7, 14 μM of AB. Cells are stained with AO/EB.
On the other hand, various concentrations of AB were used to quantify the ability of AB to induce apoptosis in MCF-7/ADR cells by flow cytometry. As demonstrated in , AB concentrations of 3.5, 7, 14 μM increased the apoptosis rate to 19.0%, 29.3% and 35.2%, respectively. Thus, AB-induced apoptosis on MCF-7/ADR cells, which was in accordance with MTT results.
Figure 3. Apoptosis of MCF-7/ADR cells detected by Annexin V-FITC/propidium iodide assay. Cells were treated with or without AB for 48 h, then the cells were stained with Annexin V-FITC and propidium iodide before subjecting to flow cytometry. Lower right part (Annexin V+/PI−) was considered as early apoptotic cells. Higher right part (Annexin V+/PI+) was considered as late apoptosis cells.
AB triggers mitochondria-dependent apoptosis
As illustrated in , the activation of c-caspase-3 and c-caspase-9 were in concentration-dependent manners, and were reversed by the inhibitor—Z-VAD-FMK (), which indicated the intrinsic apoptosis. These results suggest that AB-induced apoptosis is mediated through caspase-3 and caspase-9 dependent pathway.
Figure 4. Effects of AB on the expression of caspase-3, caspase-9, the ratio of Bax/Bcl-2 on MCF-7/ADR cells. Cells were treated with or without AB for 48 h at indicated concentrations (A). Cells were treated with 14 μM AB in the presence or absence of 10 μM Z-VAD-FMK (B). c-caspase-3: cleaved-caspase-3; c-caspase-9: cleaved-caspase-9; AB: Annosquacin B; Z: Z-VAD-FMK. Values are presented as the means ± SD, n = 3. *p < 0.05, **p < 0.01 versus vehicle control. β-actin was employed as an internal control.
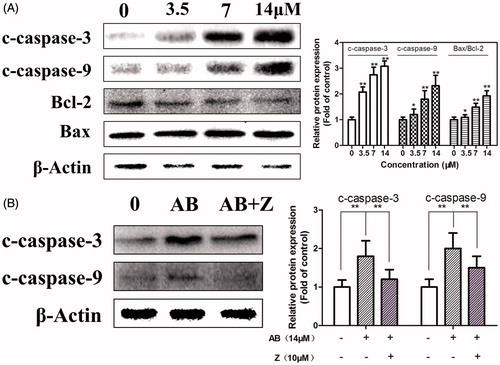
Bax and Bcl-2 play important roles in apoptosis progression, and the increase of Bax/Bcl-2 ratio eventually leads to programed cell death (He et al. Citation2013). To examine the association between Bcl-2 family proteins and apoptosis induced by AB in MCF-7/ADR cells, the expression of anti-apoptosis protein Bcl-2 and pro-apoptosis protein Bax were determined. As shown in AB significantly reduced the expression of Bcl-2 and enhanced the expression of Bax. These results suggested that the apoptosis caused by AB was mediated through the mitochondrial pathway.
The role of MAPKs in AB-induced celluar apoptosis
To investigate the roles of MAPKs in AB-induced cell apoptosis on MCF-7/ADR, cells were incubated with SB202190, SP600125 and U0126 for 30 min prior to the AB (14 μM) treatment and then subjected to MTT assay. As expected, the decrease in cell viability was significantly prevented by SB202190 and enhanced by SP600125 (), and there was no obvious difference in cell number with cotreatment of U0126 and AB compared with cells after treatment with AB alone (). These results demonstrate that the anti-proliferative activity of AB on MCF-7/ADR cells is modulated through p38 and JNK activities.
Figure 5. Cell viability was measured by MTT assay, after treatment with 14 μM AB in the presence or absence of 25 μM SB202190 or SP600125 (A). Cells were treated with 14 μM AB in the presence or absence of 10 μM U0126 (B). Effects of p38 MAPKs pathways in AB-induced apoptosis. Cells were treated with 0–14 μM AB for 24 h (C) or 14 μM AB for 0–12 h (D). AB: Annosquacin B; SB: SB202190; SP: SP600125. Values are presented as the means ± SD, n = 3. *(#)p < 0.05, **(##)p < 0.01 versus vehicle control. β-actin was employed as an internal control.
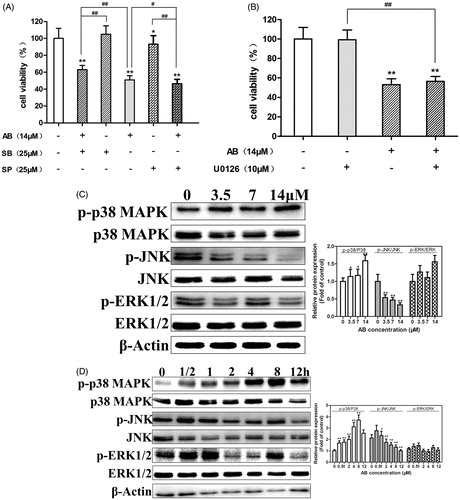
To validate the above conclusion, we carried out western blot assays to detect the expression of p-p38, p-JNK and p-ERK1/2, which modulate the cell apoptosis and survival (Xia et al. Citation1995). As shown in , AB could increase the expression of p-p38 MAPK and decrease the expression of p-JNK. Nevertheless, the expression of p-ERK1/2 did not change significantly. In summary, p38 and JNK signaling are believed to be involved in AB-induced MCF-7/ADR cells apoptosis, the role of ERK1/2 remains still uncertain.
Discussion
Annona squamosa is widely distributed in China, especially in Guangdong, Yunnan, Guangxi and Hainan province. ACGs are always characterized by a long aliphatic chain with an α-, β-unsaturated γ-lactone ring and 0-3 tetrahydrofuran (THF) ring(s). Based on the number and location of the THF, ACGs could be classified into three main classes: mono-THF, bis-adjacent-THF and bis-nonadjacent-THF ACGs. AB belongs to bis-adjacent-THF ACGs, which was reported to be most potent against cancer in general. In this work, AB was found to be able to trigger apoptosis on MCF-7/ADR cells, and characteristic events in apoptosis including morphological changes, high levels of caspase-3 and caspase-9 were also observed. These results suggest that the AB-induced apoptosis may be dependent on an intrinsic apoptosis pathways.
Abnormal apoptosis is believed to contribute to multidrug resistance. Increasing levels of anti-apoptotic proteins such as Bcl-2, or decreasing level of pro-apoptotic proteins such as Bax are believed to be correlated with increased level of multidrug resistance (Green & Kroemer Citation2005). Studies showed that 5-bromotetrandrine, a bromized derivative of tetrandrine, enhanced the sensitivity of Bel7402 cells to Dox, and with a mechanism which was associated with the elevation of Bax/Bcl-2 ratio (Liu et al. Citation2010). In our work, the ratio of Bax/Bcl-2 was also increased after AB treatment, therefore the alteration of the ratio of Bax/Bcl-2 may be another important mechanism for the effect of AB on apoptosis of MCF-7/ADR cells.
Cellular survival signaling pathways including mitogen-activated protein kinase (MAPK) pathway, are often abnormally activated in cancers. Abnormal activation of the MAPKs pathway confers resistance to many cancer types, and is a poor prognostic factor for many types of cancer. There were many reports about the development of MDR, which is often associated with significant changes of MAPK activity (Lee et al. Citation1998; Emanuel et al. Citation1999; Barancik et al. Citation2001; Ding et al. Citation2001). In this work, p38 MAPK and JNK were found increased and decreased, respectively. Furthermore, the effects of AB on cell death and MAPKs were suppressed by an inhibitor of p38 MAPK (SB202190) and enhanced by an inhibitor of JNK (SP600125). These findings support the suggestion that the modulation of AB on MAPK signaling contributes to apoptosis in MCF-7/ADR cells.
As is well known, overexpression of drug efflux pumps, P-glycopretein for instance (Anuchapreeda et al. Citation2002; Tang et al. Citation2005), which is the most common mechanism of drug resistance. In the future work, we would like to analyze the effect of AB on the expression and function of P-glycoprotein, to see whether AB could inhibit activation of P-glycoprotein or affecting its function, and increase drug sensitivity to MDR cells.
Conclusions
In conclusion, our findings suggest that AB exerts high in vitro anti-proliferative activity through mechanisms which include the selective modulation of MAPK signaling pathways, the activation of caspases and the increase in the ratio of Bax/Bcl-2, events which ultimately lead to celluar apoptosis. This study provides useful clues for a better understanding of mechanisms of AB-induced apoptosis against MCF-7/ADR cells.
Acknowledgements
The authors are grateful to Dr. Qinglong Guo, China Pharmaceutical University, for providing MCF-7/ADR and MCF-7 cell lines.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Funding
This study was supported by grants from the National Nature Science Fund Project (81274057, 81573577), National Nature Science Fund Project (81403082), Doctoral Fund of Ministry of Education of China (20113237110009), 2012 Program sponsored for Scientific Innovation research of College Graduate in Jiangsu Province (588) and Nature Science Fund of Jiangsu Province (SBK2012853).
References
- Alali FQ, Rogers L, Zhang Y, Mclaughlin JL. 1999. Goniotriocin and (2,4-cis- and -trans)-xylomaticinones, bioactive annonaceous acetogenins from Goniothalamus giganteus. J Nat Prod. 62:31–34.
- Anuchapreeda S, Leechanachai P, Smith MM, Ambudkar SV, Limtrakul PN. 2002. Modulation of P-glycoprotein expression and function by curcumin in multidrug-resistant human KB cells. Biochem Pharmacol. 64:573–582.
- Barancik M, Bohacova V, Kvackajova J, Hudecova S, Krizanova O, Breier A. 2001. SB203580, a specific inhibitor of p38-MAPK pathway, is a new reversal agent of P-glycoprotein-mediated multidrug resistance. Eur J Pharm Sci. 14:29–36.
- Bermejo A, Figadere B, Zafra-Polo MC, Barrachina I, Estornell E, Cortes D. 2005. Acetogenins from Annonaceae: recent progress in isolation, synthesis and mechanisms of action. Nat Prod Rep. 22:269–303.
- Chen Y, Chen JW, Li X. 2012a. Monotetrahydrofuran annonaceous acetogenins from the seeds of Annona squamosa. Phytochem Lett. 5:33–36.
- Chen Y, Chen JW, Wang Y, Xu SS, Li X. 2012b. Six cytotoxic annonaceous acetogenins from Annona squamosa seeds. Food Chem. 135:960–966.
- Chen Y, Xu SS, Chen JW, Wang Y, Xu HQ, Fan NB, Li X. 2012c. Anti-tumor activity of Annona squamosa seeds extract containing annonaceous acetogenin compounds. J Ethnopharmacol. 142:462–466.
- Choi JH, Lim HY, Joo HJ, Kim HS, Yi JW, Kim HC, Cho YK, Kim MW, Lee KB. 2002. Expression of multidrug resistance-associated protein1, P-glycoprotein, and thymidylate synthase in gastric cancer patients treated with 5-fluorouracil and doxorubicin-based adjuvant chemotherapy after curative resection. Br. J. Cancer. 86:1578–1585.
- Ding S, Chamberlain M, McLaren A, Goh L-b, Duncan I, Wolf CR. 2001. Cross-talk between signalling pathways and the multidrug resistant protein MDR-1. Br J Cancer. 85:1175–1184.
- Emanuel SL, Chamberlin HA, Cohen D. 1999. Antimitotic drugs cause increased tumorigenicity of multidrug resistant cells. Int J Oncol. 14:487–494.
- Fu LW, Tan BY, Liang YJ, Pan QC, Huang HB, Feng GK. 1999. The circumvention of tumor multidrug resistance by bullatacin and its mechanism. Acta Pharm Sin. 34:268–271.
- Green DR, Kroemer G. 2005. Pharmacological manipulation of cell death: clinical applications in sight. J Clin Invest. 115:2610–2617.
- He H, Zang L, Feng YS, Chen LX, Kang N, Tashiro S, Onodera S, Qiu F, Ikejima T. 2013. Physalin A induces apoptosis via p53-Noxa-mediated ROS generation, and autophagy plays a protective role through p38-NF-KB survival pathway in A375-S2 cells. J. Ethnopharmacol. 148:544–555.
- Hui YH, Rupprecht JK, Liu YM, Anderson JE, Smith DL, Chang CJ, Mclaughlin JL. 1989. Bullatacin and bullatacinone: two highly potent bioactive acetogenins from Annona bullata. J Nat Prod. 52:463–477.
- Hwang CH, Keum G, Sohn K, Lee DH, Lee E. 2005. Stereoselective synthesis of (-)-jimenezin. Tetrahedron Lett. 46:6621–6623.
- Kowalski P, Stein U, Scheffer GL, Lage H. 2002. Modulation of the atypical multidrug-resistant phenotype by a hammerhead ribozyme directed against the ABC transporter BCRP/MXR/ABCG2. Cancer Gene Ther. 9:579–586.
- Lee YJ, Galoforo SS, Berns CM, Chen JC, Davis BH, Sim JE, Corry PM, Spitz DR. 1998. Glucose deprivation-induced cytotoxicity and alterations in mitogen-activated protein kinase activation are mediated by oxidative stress in multidrug-resistant human breast carcinoma cells. J Biol Chem. 273:5294–5299.
- Liu XD, Sun H, Liu GT. 2010. 5-Bromotetrandrine enhances the sensitivity of doxorubicin-induced apoptosis in intrinsic resistant human hepatic cancer Bel7402 cells. Cancer Lett. 292:24–31.
- Ludwig A, Dietel M, Lage H. 2002. Identification of differentially expressed genes in classical and atypical multidrug-resistant gastric carcinoma cells. Anticancer Res. 22:3213–3221.
- Makabe H, Higuchi M, Konno H, Murai M, Miyoshi H. 2005. Synthesis of (4R, 15R, 16R, 21S)- and (4R, 15S, 16S, 21S)-rollicosin. Tetrahedron Lett. 46:4671–4675.
- Oberlies NH, Croy VL, Harrison ML, Mclaughlin JL. 1997. The annonaceous acetogenin bullatacin is cytotoxic against multidrug-resistant human mammary adenocarcinoma cells. Cancer Lett. 115:73–79.
- Raynaud S, Nemati F, Miccoli L, Michel P, Poupon M, Fourneau C, Laurens A, Hocquemiller R. 1999. Antitumoral effects of squamocin on parental and multidrug resistant MCF7 (human breast adenocarcinoma) cell lines. Life Sci. 65:525–533.
- Takahashi S, Kubota A, Nakata T. 2002. Total synthesis of muconin. Tetrahedron Lett. 43:8661–8664.
- Tang XQ, Bi H, Feng JQ, Hu B, Feng JQ, Cao JG. 2005. Effect of curcumin on multidrug resistance in resistant human gastric carcinoma cell line SGC7901/VCR. Acta Pharmacol Sin. 26:1009–1016.
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. 1995. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 270:1326–1331.
- Yang HJ, Li X, Zhang N, Chen JW, Wang MY. 2009. Two new cytotoxic acetogenins from Annona squamosa. J Asian Nat Prod Res. 11:250–256.
- Yazbak A, Sinha SC, Keinan E. 1998. Total synthesis of Uvaricin. J Org Chem. 63:5863–5868.
- Zhu LT, Zhao L, Wang H, Wang Y, Pan D, Yao J, Li ZY, Wu GZ, Guo QL. 2013. Oroxylin A reverses P-glycoprotein-mediated multidrug resistance of MCF7/ADR cells by G2/M arrest. Toxicol Lett 219:107–115.
