Abstract
Context: Chrysobalanus icaco L. (Chrysobalanaceae) has been used for the treatment of abdominal pain and cramps.
Objective: Assess the chemical and pharmacological profile of the lyophilized aqueous extract from C. icaco leaves (AEC).
Materials and methods: Chromatographic methods were used to assess compounds from AEC. Mice were treated with vehicle (control group) or AEC (100, 200 or 400 mg/kg, p.o.) (group with 7–8 mice) and the analgesic profile was assessed employing the acetic acid-induced writhing, formalin, hot plate tests and hyperalgesia induced by carrageenan (CG) or tumour necrosis factor-alpha. The animal motor performance was assessed using rota-rod and grip strength tests.
Results: The chromatographic profile of AEC demonstrated the presence of terpenoid compounds. The acute pretreatment with AEC, at all doses, produced a significant (p < 0.01) inhibition of painful bahaviour (11.4 ± 3.6; 10.3 ± 2.8; 11.3 ± 2.2) when compared to the control group (24.7 ± 4.7) in acetic acid-induced writhing test. In the formalin test, AEC were effective in the second phase (p < 0.01) (57.2 ± 10.3; 56.3 ± 9.2; 54.7 ± 8.9) when compared to control group (121.9 ± 18.5). No response was observed in the hot plate test. The higher dose of AEC produced a significant (p < 0.01 or p < 0.05) inhibitory effect on the mechanical hyperalgesia test. AEC did not affect the motor performance of the mice.
Discussion: The terpenoids from AEC are known for its analgesic and anti-inflammatory properties. So, these results corroborate the experiments using the AEC in inflammatory pain protocols.
Conclusion: Our results suggest that AEC act against inflammatory pain.
Introduction
Historically, medicinal plants have been employed in the prevention and treatment of various undesirable clinical conditions. Their medicinal use has considerably increased among populations, since it is believed to be both beneficial and have few significant side effects (Rates Citation2001; Hart Citation2005). However, the concept that these products are ‘natural’ is neither adequate nor sufficient to assure safety and efficacy (Rates Citation2001). Additionally, the use of medicinal plants as an optional treatment has been increasing, especially in developing countries. Hence, chemical and pharmacological studies are essential for the usage to become safe and improve effectiveness (Guimaraes et al. Citation2013, Citation2014; Quintans et al. Citation2014).
Chrysobalanus icaco L. (Chrysobalanaceae), popularly kwon as ‘abajeru’ or ‘grageru’, is a medicinal plant common in tropical areas. The Chrysobalanaceae encompasses 17 genera and about 450 species represented by trees and shrubs growing in tropical and subtropical lowlands. In northeastern Brazil, infusion or decoction of C. icaco fruits, leaves, bark or roots cures chronic diarrhoea, dysentery, haemorrhages, inflammatory and pain disorders (abdominal pain and cramps), and leucorrhoea (Castilho & Kaplan Citation2011; Barbosa et al. Citation2013). C. icaco leaf infusion is also used popularly as diuretic and hypoglycemic (Vargas et al. Citation2010; Barbosa et al. Citation2013). In addition, previous reports showed the presence of diterpenes in the roots of C. icaco (Gustafson et al. Citation1991) and anthocyanins in its fruits (Brito et al. Citation2007) that may contribute to a better understanding of the pharmacological effects. Terpenes have been promising substances in the study of new chemical entities targeting analgesic and anti-inflammatory drugs (Guimaraes et al. Citation2013, Citation2014).
In this regard, the chemical composition of Chrysobalanaceae species includes flavonoids, terpenoids (triterpenes and diterpenes), steroids and tannins (Castilho & Kaplan Citation2008). However, there are few phytochemical studies on the C. icaco species. From ‘grageru’ leaves, sterols, triterpenes and 7-O-methylkaempferol (Fernandes et al. Citation2003; Castilho & Kaplan Citation2011) have been found, as well as the flavonoids rutin, myricetin, quercetin and other myricetin and quercetin derivatives (Barbosa et al. Citation2006). Some biological activities have been attributed to the terpenoids found in the C. icaco species, such as the anti-HIV activity (Gustafson et al. Citation1991). Recently, our group has demonstrated in vivo and in vitro protocols that the lyophilized aqueous extract (AEC) obtained from C. icaco leaves possesses a remarkable anti-diabetic effect and anti-oxidant profile; those properties may be related with the presence of phenolic compounds (Barbosa et al. Citation2006, Citation2013).
Besides the extensive folk medicine use of C. icaco leaves (mainly as tea) to treat inflammatory and painful conditions, we did not find studies that supported the traditional use of ‘grageru’ in Brazilian traditional medicine as an analgesic and anti-inflammatory. Thus, we evaluated the chemical composition and pharmacological effects of AEC in experimental protocols.
Materials and methods
Plant material and preparation of the aqueous extract
Chrysobalanus icaco leaves were collected from the village Jatoba (Latitude: 10°47′50″ S and Longitude: 36°50′44″ W), state of Sergipe, Brazil, in February 2008 and were identified by Dr. Ana Paula Prata. A voucher specimen (ASE 11855) has been deposited in the Herbarium of the Federal University of Sergipe. The aqueous extract was obtained from C. icaco (AEC) dried leaves and prepared according to Barbosa et al. (Citation2013). The extract was dried in vacuo (lyophilized with 16% yield) and administered to mice immediately after suspension in distilled water.
High performance liquid chromatography-photo diode array analysis (HPLC-PDA analysis)
The chromatographic analysis was performed using a Shimadzu HPLC system (Shimadzu, Columbia, MD) composed by two pumps with an online degasser, an autoinjector and a C18 column (4.6 mm ×250 mm, 5 μm) coupled to a DAD-UV/Vis detector. The sample volume of 20 μL (1 mg/mL) dissolved in HPLC grade methanol was subjected to gradient elution with mobile phases consisting of water and methanol from 5% to 100%, during 60 min in a wavelength range from 220 to 400 nm. The Shimadzu LC Solution software (Kyoto, Japan) was used for the system control and also to acquire data.
Animals
Experimental protocols were performed using male Swiss mice (25–30 g) obtained from the Animal Facilities of the Federal University of Sergipe (UFS). Mice were housed in controlled-temperature rooms (21 ± 2 °C), under a 12/12-h light–dark cycle, with access to water and food ad libitum until use. All behaviour experiments were performed under blind conditions and all efforts were made to minimize the number of animals used and their discomfort. Experimental procedures were approved by the Ethics Committee on Animal Care in the Federal University of Sergipe (CEPA/UFS # 67/09).
Drugs and reagents
The drugs and reagents used in this study were λ-carrageenan (CG), tumour necrosis factor-alpha (TNF-α), acetic acid (Sigma-Aldrich, St. Louis, MO), diazepam and morphine (MOR) (União Química, São Paulo, Brazil) and formaldehyde (Merck, Boston, MA).
Pharmacological protocols
Acetic acid-induced abdominal writhes
The writhing test was carried out according to the method previously described by Koster et al. (Citation1959). Abdominal writhing in mice (n = 8, per group) was induced by intraperitoneal injection (i.p.) of 0.8% solution of acetic acid (0.1 mL/10 g body weight). The animals were previously treated with different doses of AEC (100, 200 or 400 mg/kg; gavage, p.o.), distilled water as control group (0.1 mL/10 g; gavage, p.o.) or indomethacin (INDO; 10 mg/kg, gavage, i.p.) 1 h before stimulation with acetic acid. The number of muscular contractions was counted for 15 min after injection and the data represent the average of the total number of writhes observed.
After that, the animals were treated with the highest dose of AEC (400 mg/kg, p.o.) and vehicle to evaluate the time of action. After 0.5, 1, 2, 4, 8 or 24 h the mice received the acetic acid injection (i.p.), which was evaluated as described by Quintans et al. (Citation2013).
Formalin-induced nociceptive behaviour in mice
Mice were injected with formalin (2%, 20 μL) in the subplantar area of the right hind paw. The time of paw licking (in seconds) was determined over 0–5 min (early phase – neurogenic pain) and 15–30 min (late phase – inflammatory pain) after formalin injection (Hunskaar & Hole Citation1987). Mice (n = 7, per group) were previously treated with AEC (100, 200 or 400 mg/kg, gavage, p.o.) or distilled water as control group (0.1 mL/10 g; gavage, p.o.) 120 min before intraplantar injection of the stimulus or the reference compound, aspirin (200 mg/kg, gavage, p.o.), 1 h before the administration of formalin.
Hot plate test
The hot plate test was performed according to that described by Eddy and Leimbach (Citation1953) and was assessed using a hot plate device (EFF-361 Insight, São Paulo, Brazil). Mice were placed on a metal plate maintained at 55 ± 1 °C and the time until licking, stomping, shaking the hind paw or jumping off from the surface were measured as a latency time. Mice were pretreated with AEC (100, 200 or 400 mg/kg, gavage, p.o.), distilled water as control group (0.1 mL/10 g; gavage, p.o.) or MOR (5 mg/kg, i.p.) and evaluated at times 60, 90, 120 and 240 min after treatment. In order to avoid tissue damage, the maximal time standing on the plate was limited to 30 s.
Hyperalgesia induced by CG or TNF-α
The mechanical hyperalgesia procedure was performed as described by Guimaraes et al. (Citation2012) and Santana et al. (Citation2013). The mice were divided into five groups (n = 7, per group), which were treated with distilled water as control group (0.1 mL/10 g; gavage, p.o.), AEC (100, 200 or 400 mg/kg, gavage, p.o.) or INDO (10 mg/kg, i.p.). Sixty minutes after treatment, 20 μL of CG (300 μg/paw) or TNF-α (100 pg/paw) was injected subcutaneously into the subplantar region of the hindpaw. The paw withdrawal threshold was evaluated at times 30, 60, 120 and 240 min after the injection of hyperalgesic agents. Mechanical hyperalgesia was assessed in mice as reported by Cunha et al. (Citation2004).
Rota-rod and grip strength tests
To assess the possible interference of AEC on animal motor performance, the rota-rod and grip strength tests were performed (Nascimento et al. Citation2014, Citation2015). For the rota-rod test, mice were trained on the apparatus for 2 days before the experiment. For both tests, the animals were treated with distilled water as control group (0.1 mL/10 g; gavage, p.o.), AEC (100, 200 or 400 mg/kg, gavage, p.o.) or diazepam (1.5 mg/kg, i.p.) and evaluated 60 min after treatment.
Statistical analysis
The data obtained were evaluated through one-way or two-way analysis of variance (ANOVA) followed by Tukey’s test. In all cases, differences were considered significant if p < 0.05. All statistical analyses were carried using Graph Pad Prism 5.01 (Graph Pad Prism Software Inc., San Diego, CA). The percent inhibition by an anti-nociceptive agent (to acetic acid-induced abdominal writhes and formalin-induced pain tests) was determined using the equation as described earlier (Reanmongkol et al. Citation1994).
Results and discussion
Chrysobalanus icaco is a medicinal plant broadly used in northeastern Brazil for the treatment of diabetes, chronic diarrhoea, dysentery, inflammation or painful conditions. Despite its wide therapeutic usage in folk medicine, its pharmacological effects are poorly understood. Hence, this is the first report of oral pretreatment of lyophilized aqueous extract from leaves of C. icaco (AEC) as an analgesic agent in experimental protocol with rodents.
Assessing the chemical profile of AEC, a gradient elution of the mobile phase (5–100%, 60 min) is a fundamental condition for detecting components of a sample due to different polarities. Therefore, the results obtained were initial parameters for further analysis (Lima Citation2007). The chromatographic profile () showed the sample as surely having terpenoid compounds eluted between the retention time 21 and 57 min, mainly. This evidence is viewed in the UV spectra (). Absorption spectra in the UV lower than 200 nm are characteristic the series of sesquiterpenes and diterpenes, especially when seen coupled to photodiode detector (Kong et al. Citation2001; Urano et al. Citation2012).
Figure 1. Chromatographic profile of the lyophilized aqueous extract obtained from C. icaco leaves (AEC). Conditions for elution: mobile phase methanol–water (5–100%), 1 h, C18 analytical column (25 cm × 0.46 mm, 5 μm), detection at 330 nm DAD-UV-Vis, 1 mL/min.
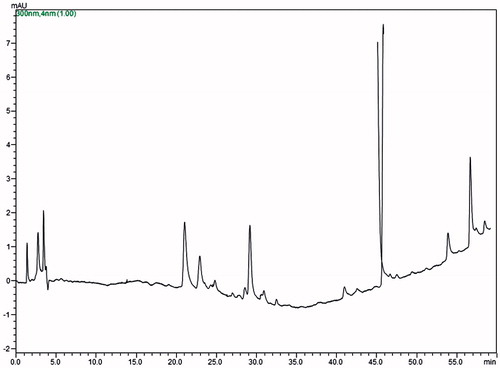
Figure 2. UV absorption spectrum of the compound eluting at retention time 21.22 (A), 29.18 (B), 46.55 (C) and 57.47 (D) from of the lyophilized aqueous extract obtained from C. icaco leaves (AEC).
After identifying the phytochemical profile of AEC, pharmacological evaluations were performed. Thus, the acetic acid-induced writhing reaction in mice is described as a typical model of inflammatory pain, and it has long been used as a screening tool for the assessment of analgesic or anti-inflammatory properties of new potential drugs. At the cellular level, protons depolarize sensory neurones by directly activating a non-selective cationic channel located on cutaneous, visceral and other types of nocisponsive peripheral afferent C fibres (Le Bars et al. Citation2001). Local irritation also induces the lease of endogenous mediators such as bradykinin, prostaglandins and cytokines (TNF-α, IL-1β and IL-8), which stimulate the nociceptive neurons. This method shows good sensitivity but poor specificity because the abdominal writhing response may be suppressed by muscle relaxants and other drugs, leaving scope for the misinterpretation of results (Le Bars et al. Citation2001). Thus, acute treatment with AEC produced a significant (p < 0.01) analgesic effect against acetic acid-induced writhing at all doses when compared with control group (). Additionally, the anti-nociceptive effect lasted up to 8 h after administration with AEC, as demonstrated in .
Figure 3. Time–response curve for the anti-nociceptive effect of the lyophilized aqueous extract obtained from C. icaco leaves (AEC; 400 mg/kg, p.o.) on acetic acid-induced writhing response in mice. Writhings were counted over 15 min following i.p. injection of acetic acid (0.8%). AEC (400 mg/kg, p.o.) was administered p.o. 0.5, 1, 2, 4, 8 or 24 h before acid acetic injection (0.8%). Control animals received an injection of vehicle by p.o. route. Each column represents mean ± SEM (n = 8, per group). *p < 0.05 versus control (ANOVA followed by Tukey’s test).
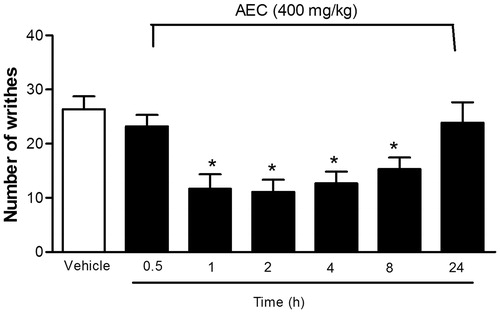
Table 1. Effect of the lyophilized aqueous extract obtained from C. icaco leaves (AEC) or indomethacin (INDO), reference drug, on writhing induced by acetic acid- and formalin-induced nociceptive behaviour in mice.
The formalin test is widely used for pharmacological evaluation of analgesic drugs because involves a biphasic response. The intraplantar injection of formalin promotes two distinct phases of pain sensitivity. The first phase (neurogenic pain) occurs through direct chemical stimulation promoted by formalin on nociceptors, in type C and part of the Aδ afferent fibres, and it is associated with the release of excitatory aminoacids, nitric oxide and substance P, among others. The second phase (inflammatory pain) is characterized as inflammatory pain, associated with the release of chemical mediators such as histamine, serotonin, bradykinin, prostaglandins and excitatory aminoacids (Hunskaar & Hole Citation1987). Our results demonstrated that AEC were effective in the second phase of the formalin test (), which suggests that its effectiveness occurs apparently during the inflammatory phase (inflammatory pain) of this model.
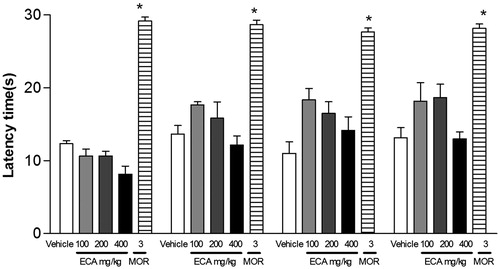
In addition, the hot plate test was used to assess the possible central analgesic action of AEC. As demonstrated in , the pretreatment with AEC, at all doses, did not produce a significant analgesic effect in the hot plate test. This effect corroborates the findings in the formalin test, where the AEC also showed no significant effect in the first phase, ruling out the possible central analgesic effect of ‘grageru’ extract.
Figure 4. Effect of acute administration of vehicle, lyophilized aqueous extract obtained from C. icaco leaves (AEC; 100, 200 or 400 mg/kg, p.o.) or morphine (MOR, 5 mg/kg, i.p.) on hot plate test in mice. The bars shown the latency time to animals express pain behaviour, so each column represents the mean ± SEM (n = 8, per group). *p < 0.001 versus control (ANOVA followed by Tukey’s test).
Recently, Oliveira et al. (Citation2014) demonstrated that the aqueous extract of the bark of C. icaco, at the same doses used in this study, also showed a potent anti-inflammatory action evidenced by the abdominal writhing test and the formalin test. Furthermore, the aqueous extract was able to reduce the concentration of nitric oxide in the inflammatory exudate.
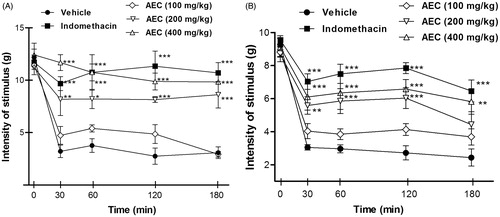
The effect of pretreatment with AEC was further evaluated in a model of inflammatory hyperalgesia induced by intraplantar injection of CG and TNF-α. CG injection induced mechanical hyperalgesia through the synthesis and release of a cascade of inflammatory mediators such as prostaglandins, histamine, neuropeptides, proinflammatory cytokines (IL-1β and TNF-α), nitric oxide and transcription factors (NF-κB) (Ferreira et al. Citation1993). Thus, the formation of ‘inflammatory soup’ leads to activation of primary afferent fibres Aδ and type C, increasing the local flow and vascular permeability by the release of substance P and neurokinin A (Cunha et al. Citation2005). Our results showed that the pretreatment with AEC, in higher doses, has an inhibitory activity on CG-induced inflammatory pain reducing significantly (p < 0.05 or p < 0.01) nociceptive behaviour ().
Figure 5. Effect of acute administration of vehicle, lyophilized aqueous extract obtained from C. icaco leaves (AEC; 100, 200 or 400 mg/kg, p.o.) or indomethacin (IND, 10 mg/kg) on mechanical hyperalgesia induced by CG (300 μg/paw) (A) or TNF-α (100 pg/paw) (B). Each point represents the mean ± SEM of the variation of paw withdrawal threshold (in grams) to tactile stimulation of the ipsilateral hind paw. **p < 0.05 and ***p < 0.01, compared with vehicle-treated group (two-way ANOVA followed by Tukey’s test).
TNF-α plays a key role in the hyperalgesia caused by inflammatory mediators. This proinflammatory cytokine is produced in large quantities and rapidly by macrophages in response to inflammatory stimuli. Moreover, TNF-α is able to induce the release of other cytokines such as IL-1β that also contributes to the onset of hyperalgesia (Brown et al. Citation2006; Verri et al. Citation2006). Our findings showed an inhibitory effect of AEC (200 and 400 mg/kg, p.o.) on the mechanical hyperalgesia induced by TNF-α (shown in ).
The anti-hyperalgesic effect produced by the pretreatment with AEC in the CG and TNF-α models confirms that the compound studied may be acting similarly to NSAIDs inhibiting the synthesis or release of inflammatory mediators. These results do not rule out the possible anti-inflammatory effect of the lowest dose shown in screening tests such as abdominal writhing and formalin, however, no significant effect on mechanical hyperalgesia test can be explained by the high specificity of the test, since the paw withdrawal threshold is measured. These results corroborate previous findings of Oliveira et al. (Citation2014), which demonstrated analgesic and anti-inflammatory profiles of aqueous extract of the bark of C. icaco, however, this study suggested that these effects do not seem to be related to the levels of cytokines, such as TNF-α or IL-6.
Recent phytochemical investigations have reported the presence of myricetin as one of the major components of the AEC (White, Araújo, et al. Citation2016; White, Cercato, et al. Citation2016). Moreover, the presence of diterpene and triterpene has also been confirmed (Oliveira et al. Citation2014). Due to the consistent presence of flavonols and terpenes in AEC, it is possible to suggest that these compounds contributed to the analgesic effect, since several studies have described the analgesic and anti-inflammatory profiles of these compounds (Guimaraes et al. Citation2013; Sá et al. Citation2013, Citation2014; Souza et al. Citation2014).
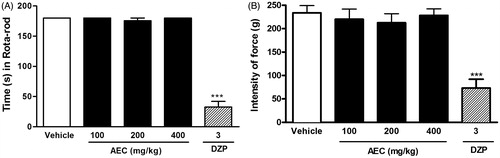
In the rota-rod and grip strength tests, AEC-treated mice produced no significant alteration in motor performance or muscle tension (). The rota-rod test is widely used to evaluate the motor coordination of rodents, and is especially sensitive in detecting cerebellar dysfunction (Shiotsuki et al. Citation2010). The grip strength test is able to detect if the study drug has a muscle relaxant effect. These results discard a possible deficit on motor coordination or relaxing muscle-schelectric activity, which could impair the comprehension of the analgesic profile found by ‘grageru’ extract.
Figure 6. Effect of acute administration of vehicle, lyophilized aqueous extract obtained from C. icaco leaves (AEC; 100, 200 or 400 mg/kg, p.o.) or diazepam (DZP, 3 mg/kg, i.p.) on rota-rod test (A) or grip strength meter (B) in mice. ***p < 0.01, compared with vehicle-treated group (one-way ANOVA followed by Tukey’s test).
Together, our results support that the lyophilized aqueous extract from C. icaco leaves bears analgesic profile mainly to inflammatory pain. This effect has no relation with motor coordination or muscle relaxant action. The probable analgesic profile of AEC may play a role in the action of synthesis or release inflammatory mediators, and also might involve redox-mediated mechanisms, as previously shown by Barbosa et al. (Citation2013) and White, Araújo, et al. (Citation2016) and White, Cercato, et al. (Citation2016). In addition, the high presence of flavonols and terpenes compounds in AEC may help explain this analgesic profile. Considering the widespread use of C. icaco leaves in folk medicinal preparations such as tea, its analgesic profiles may be of nutraceutical interest, and should be confirmed in future clinical studies.
Acknowledgements
Authors thank Douglas Prado for the technical support. We thank teacher Abilio Borghi for the grammar review on the manuscript.
Disclosure statement
The authors declare no conflict of interest.
Funding
We would like to thank CNPq, CAPES and FAPITEC/SE, all from Brazil, for the financial support.
References
- Barbosa AP, Silveira Gde O, de Menezes IA, Rezende Neto JM, Bitencurt JL, Estavam Cdos S, de Lima Ado C, Thomazzi SM, Guimarães AG, Quintans LJ Jr, dos Santos MR. 2013. Antidiabetic effect of the Chrysobalanus icaco L. aqueous extract in rats. J Med Food. 16:538–543.
- Barbosa WLR, Peres A, Gallori S, Vincieri FF. 2006. Determination of myricetin derivatives in Chrysobalanus icaco L. (Chrysobalanaceae). Rev Bras Farmacogn. 16:333–337.
- Brito ES, Araujo MC, Alves RE, Carkeet C, Clevidence BA, Novotny JA. 2007. Anthocyanins present in selected tropical fruits: acerola, jambolão, jussara, and guajiru. J Agric Food Chem. 55:9389–9394.
- Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF. 2006. Neutrophils in development of multiple organ failure in sepsis. Lancet. 368:157–169.
- Castilho RO, Kaplan MAC. 2008. Constituintes químicos de Licania tomentosa Benth. (Chrysobalanaceae) [Chemical constituents of Licania tomentosa Benth. (Chrysobalanaceae)]. Quím Nova. 31:66–69.
- Castilho RO, Kaplan MAC. 2011. Phytochemical study and antimicrobial activity of Chrysobalanus icaco. Chem Nat Compd. 47:436–437.
- Cunha TM, Verri WA Jr, Silva JS, Poole S, Cunha FQ, Ferreira SH. 2005. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci USA. 102:1755–1760.
- Cunha TM, Verri WA Jr, Vivancos GG, Moreira IF, Reis S, Parada CA, Cunha FQ, Ferreira SH. 2004. An electronic pressure-meter nociception paw test for mice. Braz J Med Biol Res. 37:401–407.
- Eddy NB, Leimbach D. 1953. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther. 107:385–393.
- Fernandes J, Castilho RO, da Costa MR, Wagner-Souza K, Coelho Kaplan MA, Gattass CR. 2003. Pentacyclic triterpenes from Chrysobalanaceae species: cytotoxicity on multidrug resistant and sensitive leukemia cell lines. Cancer Lett. 190:165–169.
- Ferreira SH, Lorenzetti BB, Poole S. 1993. Bradykinin initiates cytokine-mediated inflammatory hyperalgesia. Br J Pharmacol. 110:1227–1231.
- Guimaraes AG, Quintans JSS, Quintans Junior LJ. 2013. Monoterpenes with analgesic activity – a systematic review. Phytother Res. 27:1–15.
- Guimaraes AG, Serafini MR, Quintans-Junior LJ. 2014. Terpenes and derivatives as a new perspective for pain treatment: a patent review. Expert Opin Ther Pat. 24:243–265.
- Guimaraes AG, Xavier MA, de Santana MT, Camargo EA, Santos CA, Brito FA, Barreto EO, Cavalcanti SC, Antoniolli AR, Oliveira RC, Quintans-Júnior LJ. 2012. Carvacrol attenuates mechanical hypernociception and inflammatory response. Naunyn Schmiedebergs Arch Pharmacol. 385:253–263.
- Gustafson KR, Munro MHG, Blunt JW, Cardellina JH, McMahon JB, Gulakowski RJ, Cragg GM, Cox PA, Brinen LS, Clardy J, Boyd MR. 1991. HIV inhibitory natural products. 3. Diterpenes from Homalantus acuminatus and Chrysobalanus icaco. Tetrahedron. 47:4547–4554.
- Hart BL. 2005. The evolution of herbal medicine: behavioural perspectives. Anim Behav. 70:975–989.
- Hunskaar S, Hole K. 1987. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 30:103–114.
- Kong L, Li X, Zou H, Wang H, Mao X, Zhang Q, Ni J. 2001. Analysis of terpene compounds in Cimicifuga foetida L. by reversed-phase high-performance liquid chromatography with evaporative light scattering detection. J Chromatogr A. 936:111–118.
- Koster R, Anderson M, De Debeer EJ. 1959. Acetic acid for analgesic screening. Fed Proc. 18:412–416.
- Le Bars D, Gozariu M, Cadden SW. 2001. Animal models of nociception. Pharmacol Rev. 53:597–652.
- Lima MD. 2007. Perfil cromatográfico dos extratos brutos das sementes de Annona muricata L. e Annona squamosa L. através da cromatografia líquida de alta eficiência [Chromatography profile of the crude extracts of Annona muricata L. and Annona squamosa L. seeds by High-performance liquid chromatography] [Dissertação (Mestre em Química e Biotecnologia)]. Maceió: Universidade Federal de Alagoas; 2007. p. 102 f.
- Nascimento SS, Araujo AA, Brito RG, Serafini MR, Menezes PP, DeSantana JM, Lucca W Jr, Alves PB, Blank AF, Oliveira RC, et al. 2015. Cyclodextrin-complexed Ocimum basilicum leaves essential oil increases Fos protein expression in the central nervous system and produce an antihyperalgesic effect in animal models for fibromyalgia. Int J Mol Sci. 16:547–563.
- Nascimento SS, Camargo EA, DeSantana JM, Araújo AA, Menezes PP, Lucca-Júnior W, Albuquerque-Júnior RL, Bonjardim LR, Quintans-Júnior LJ. 2014. Linalool and linalool complexed in β-cyclodextrin produce anti-hyperalgesic activity and increase Fos protein expression in animal model for fibromyalgia. Naunyn Schmiedebergs Arch Pharmacol. 387:935–942.
- Oliveira TB, de Carvalho Júnior CRH, Mota FVB, Gonçalves-Silva T. 2014. Anti-inflammatory and antinociceptive effects of the aqueous extract of the bark of Chrysobalanus icaco Linnaeus. Brit J Pharm Res. 4:1253–1268.
- Quintans JS, Antoniolli AR, Almeida JR, Santana-Filho VJ, Quintans-Júnior LJ. 2014. Natural products evaluated in neuropathic pain models – a systematic review. Basic Clin Pharmacol Toxicol. 114:442–450.
- Quintans JSS, Menezes PP, Santos MR, Bonjardim LR, Almeida JR, Gelain DP, Araújo AA, Quintans-Júnior LJ. 2013. Improvement of p-cymene antinociceptive and anti-inflammatory effects by inclusion in beta-cyclodextrin. Phytomedicine. 20:436–440.
- Rates SM. 2001. Plants as source of drugs. Toxicon. 39:603–613.
- Reanmongkol W, Matsumoto K, Watanabe H, Subhadhirasakul S, Sakai S. 1994. Antinociceptive and antipyretic effects of alkaloids extracted from the stem bark of Hunteria zeylanica. Biol Pharm Bull. 17:1345–1350.
- Sá RCS, Andrade LN, de Sousa DP. 2013. A review on anti-inflammatory activity of monoterpenes. Molecules. 18:1227–1254.
- Sá RCS, Andrade LN, Oliveira RRB, de Sousa DP. 2014. A review on anti-inflammatory activity of phenylpropanoids found in essential oils. Molecules. 19:1459–1480.
- Santana MT, Oliveira MG, Santana MF, De Sousa DP, Santana DG, Camargo EA, de Oliveira AP, Almeida JR, Quintans-Júnior LJ Jr. 2013. Citronellal, a monoterpene present in Java citronella oil, attenuates mechanical nociception response in mice. Pharm Biol. 51:1144–1149.
- Shiotsuki H, Yoshimi K, Shimo Y, Funayama M, Takamatsu Y, Ikeda K, Takahashi R, Kitazawa S, Hattori N. 2010. A rotarod test for evaluation of motor skill learning. J Neurosci Methods. 189:180–185.
- Souza MT, Almeida JR, Araujo AA, Duarte MC, Gelain DP, Moreira JC, dos Santos MR, Quintans-Júnior LJ. 2014. Structure–activity relationship of terpenes with anti-inflammatory profile – a systematic review. Basic Clin Pharmacol Toxicol. 115:244–256.
- Urano RPM, Rodrigues FT, Berlinck RGS. 2012. Utilização de detecção por espalhamento de luz evaporativo para análise de produtos naturais [Use of evaporative light-scattering detector for the analysis of natural products]. Quím Nova. 35:1198–1208.
- Vargas CE, Mendes MF, Azevedo DA, Pessoa FLP, Uller AC. 2010. Extraction of the essential oil of abajeru (Chrysobalanus icaco) using supercritical CO2. J Supercrit Fluids. 54:171–177.
- Verri WA Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. 2006. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther. 112:116–138.
- White PA, Araújo JM, Cercato LM, Souza LA, Barbosa AP, Quintans-Junior LJ, Machado UF, Camargo EA, Brito LC, Santos MR. 2016. Chrysobalanus icaco L. leaves normalizes insulin sensitivity and blood glucose and inhibits weight gain in high-fat diet-induced obese mice. J Med Food. 19:155–160.
- White PA, Cercato LM, Batista VS, Camargo EA, De Lucca W Jr, Oliveira AS, Silva FT, Goes TC, Oliveira ER, Moraes VR, et al. 2016. Aqueous extract of Chrysobalanus icaco leaves, in lower doses, prevent fat gain in obese high-fat fed mice. J Ethnopharmacol. 179:92–100.