Abstract
Context: Hepatocellular carcinoma (HCC) is a common cancer around the world, with high mortality rate. Currently, there is no effective drug for the therapy of HCC. Ursolic acid (UA) is a natural product which exists in various medicinal herbs and fruits, exhibiting multiple biological effects such as its outstanding anticancer and hepatoprotective activity, which has drawn many pharmacists’ attention.
Objective: This paper summarizes the current status of the hepatoprotective activity of UA analogues and explains the related mechanism, providing a clear direction for the development of novel anti-HCC drugs.
Methods: All of the data resources were derived from PubMed. By comparing the IC50 values and analyzing the structure–activity relationships, we listed compounds with good pharmacological activity from the relevant literature, and summarized their anti-HCC mechanism.
Results: From the database, 58 new UA derivatives possessing wonderful anticancer and hepatoprotective effects were listed, and the relevant anti-HCC mechanism were discussed.
Conclusion: UA’s anti-HCC effect is the result of combined action of many mechanisms. These 58 new UA derivatives, particularly compounds 45 and 53, can be used as potential drugs for the treatment of liver cancer.
Introduction
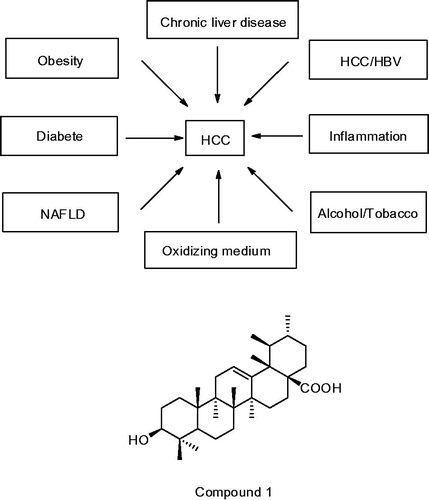
Along with the improvement of people’s living standards, the risk of liver cancer increases. HCC is the fifth most common cancer in men and the seventh in women (El-Serag Citation2012), and the third leading cause of cancer-related death, only behind the lung and the stomach cancer (Ferlay et al. Citation2010). Liver cancer is caused by many factors (), including infection with HBV and HCV (Honda et al. Citation2001; Goossens & Hoshida Citation2015; Wang et al. Citation2015), nonalcoholic liver disease (Ekstedt et al. Citation2006), exposure to aflatoxin B1 and cadmium (Liu & Wu Citation2010; Satarug Citation2012), hemochromatosis, alpha-antitrypsin deficiency, autoimmune hepatitis, some porphyrias, and Wilson′s disease (El-Serag Citation2011). Moreover, alcohol, tobacco and obesity are also independent risk factors for HCC in patients, and they interact synergistically to increase the risk of HCC (Marrero et al. Citation2005). In addition to the above factors, El-Serag et al. (Citation2004) found that diabetes doubled the risk of chronic liver disease and hepatocellular carcinoma (HCC). Sasaki (Citation2010) reported that insulin resistance was a consequence of HCC because it easily caused obesity and hepatic inflammation, both of which could promote hepatocarcinogenesis by themselves, through the production of cytokine and/or oxidative stress. Garcia-Compean et al. (Citation2009) held that insulin resistance could decrease cure rates in patients with chronic hepatitis C (CHC) and enhance liver fibrosis. In genetics, lacking of the enzyme glucose-6-phosphatase, which is essential for glucose release from liver glycogen, is also a cause of HCC and hepatic adenomas (Bannasch Citation2010).
Throughout the ages, there are four main methods of treating liver cancer, including chemotherapy, drug treatment, liver resection and liver transplantation. Natural products, a rich source of compounds, was applied in various fields of medical treatment including cancer therapy (Cragg et al. Citation2009), and played a dominant role in the development of sophisticated traditional medicine systems. In our diet, pentacyclic triterpenoids are considered relatively nontoxic to humans (Kuttan et al. Citation2011). As we all known, ursolic acid (UA, compound 1) is the most predominant representative of pentacyclic triterpenoids and possesses considerable pharmacological activities, including: anti-HIV (Slamenová et al. Citation2006), anticancer (Rashid et al. Citation2013), anti-inflammatory (Jung et al. Citation2013), antidiabetic (Alqahtani et al. Citation2013; Lee et al. Citation2014; Wu et al. Citation2014), and antibacterial activities (Zhao et al. Citation2014). Importantly, it is also reported to have hepatoprotective activity (Liu Citation1995). Moreover, traditional Chinese medicine containing UA has been used for the treatment of liver disease for several decades. Here, we will discuss the anti-HCC activity and the mechanism of UA and its derivatives.
The anti-hepatocellular carcinoma mechanism of UA
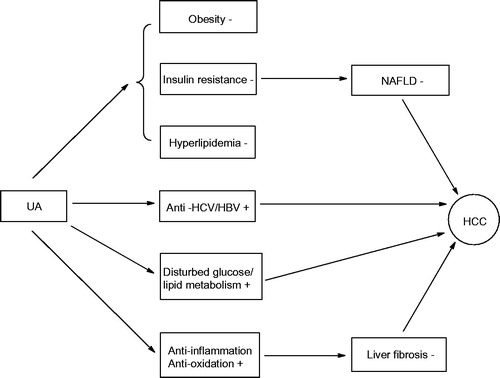
UA widely exists in fruits and herbs with low toxicity, which makes it suitable for cancer metastatic chemoprevention (Novotny et al. Citation2001). Accumulating mechanistic studies indicate that the anti-HCC effect of UA is attributed to its direct inhibition of HCC cells growth processes and indirect suppression of carcinogenic factor (). In terms of inhibition of HCC cell growth, UA can inhibit HCC cell proliferation, invasion and metastasis. Ramos et al. (Citation2008) found that UA prevented DNA damage via increase of DNA repair and anti-proliferative properties in HepG2 cells. Lin et al. (Citation2011) found that UA reversed the invasion and migration of Hep3B and Huh7 cell lines at 4 μmol/L. Son et al. (Citation2013) reported that UA blocked HepG2 cellular metabolism via AMPK activation and GSK3b phosphorylation, and thus induced apoptosis of HepG2 cells. On the suppression of carcinogenic side, UA could prevent HCC from nonalcoholic fatty liver disease (NAFLD), insulin resistance, inflammation and oxidation stress. UA was demonstrated to decrease diet-induced obesity, glucose intolerance and NAFLD by increasing skeletal muscle mass, fibre size, brown fat weight and energy expenditure (Kunkel et al. Citation2012). NAFLD has high risk of liver fibrosis and cirrhosis, even hepatocarcinoma (Brunt Citation2005). But, nowadays many results showed UA may ameliorate disease progression in patients with NAFLD. Li et al. (Citation2015) suggested that UA treatment (10–30 μg/mL) inhibited the development of NAFLD via increasing lipid β oxidation and inhibiting the hepatic endoplasmic reticulum stress. Jia et al. (Citation2011) showed that UA was a PPAR-α agonist which regulated hepatic lipid metabolism and, therefore, significantly reduced intracellular triglyceride concentrations in hepatocytes. In addition, Wang et al. (Citation2015) and Sundaresan et al. (Citation2014) found that the combination of UA with artesunate or rosiglitazone exhibited the same pharmacological effect and free of toxic side effects. Li et al. (Citation2014) indicated that UA reversed high-fat diet-induced hepatic steatosis. For HCC caused by insulin resistance, Wang et al. (Citation2012) proved that UA ameliorated insulin resistance in liver of KKAy mice via activation of peroxisome proliferator-activated receptors α and γ. Castro et al. (Citation2015) showed that UA as insulin secretagogue and insulin-mimetic also regulated the glucose balance in the liver of KKAy mice. Both liver inflammation and oxidation are regarded as hallmark of early-stage fibrosis, which aggravate fibrosis and cirrhosis and eventually deteriorate into liver cancer. However, Ma et al. (Citation2014, Citation2015) demonstrated that UA protected mouse liver against CCl4-induced oxidative stress and inflammation by the MAPK/NF-κB pathway, and meliorated the symptom of liver fibrosis via the Nrf2/ARE pathway. Liu et al. (Citation2014) also revealed that UA, Viili exopolysaccharides (VEPS), Astragalus polysaccharides (APS) and any combination of these reduced the activities of COX-2, SOD, PGE2 as well as markedly decreased MDA. Hence, the reduction of internal anti-oxidative elements created an over-oxidative environment for HepG2 cells, triggering HepG2 cells apoptosis and the inhibition of HepG2 cell proliferation.
As a promising antifibrosis agent, UA treats liver fibrosis via inhibition of hepatic stellate cells (HSCs) (Shyu et al. Citation2008). He et al. (Citation2015) proved that UA reduced the accumulation of type I collagen in rat HSCs through attenuating the activity of NOX4 and downregulating ERK, PI3K/Akt, and p38 MAPK signalling pathways in HSCs. Meanwhile, successful antimicrobial therapy is good for liver cancer through declining the morbidity and mortality of HCC and reducing the risk of liver failure in patients with advanced fibrosis (Rutter et al. Citation2015). Kong et al. (Citation2013) discovered that UA significantly suppressed the replication of HCV genotype 1b replicon and HCV genotype 2a JFH1 virus by suppressing HCV NS5B RdRp activity as noncompetitive inhibitors. Li et al. (Citation2012) also confirmed that UA showed inhibitory activity for HBV, another pathogenic virus, with IC50 values of 89.91 μM for HBsAg and 97.61 μM for HBeAg at no cytotoxicity. Wu et al. (Citation2011) supported that UA could break hepatitis B virus X protein (HBx)-mediated tumourigenic effects. Besides that, UA could directly accelerate liver mass recovery and induce the hepatocyte proliferation after partial hepatectomy by the stimulation of C/EBPβ (Jin et al. Citation2012).
The modification of UA
UA exhibits low water solubility, rapid metabolism and low bioavailability, which limit its anti-HCC activity. Because of its structural constraints, considerable structural modification has been performed on UA and potentially important derivatives have emerged. Shao et al. (Citation2011) concluded from a series novel UA derivatives (compounds 2–7, ) that: (1) keeping a polar group at either the 3-OH and/or 17-COOH position was necessary for antitumour effect; (2) increasing the length of the carbon chain decreased the derivatives’ inhibitory effect on the cancer cells due to long chains could obstruct cells from contacting with the derivatives′ mother nucleus; (3) connecting diethanolamine at the 17-COOH position and acetylating the 3-OH was beneficial to the anticancer activities. Moreover, piperazine ring had better pharmacodynamic effects than the piperidine ring at the 17-COOH position; (4) introducing a more bulky group decreased UA derivatives’ binding ability to the target because of steric hindrance. Analyzing the mechanism of compound 3 demonstrated that it suppressed HepG2 cells by cell-cycle arrest at the S phase, and increased the activity of caspase-3 at the dosage of 100 mg/kg.
Table 1. UA derivatives with diethanolamine conjugation suppressed tumour growth.
2-Deoxy-d-glucose (2-DG), as an essential inhibitor of glucose metabolism, successfully inhibits tumour growth in animal experiments and human clinical trials (Maschek et al. Citation2004; Zhong et al. Citation2009; Raez et al. Citation2013). Selective targeting of cancer glucose metabolism provided an alternative strategy of anticancer drug development with minimum side effects on normal cells. Dong et al. (Citation2014) showed that piperazine moiety-modified UA (compound 8, ) further enhanced the therapeutic effects of 2-DG probably through synergistic suppression of cancer cell glucose metabolism. Subsequently, Yang et al. (Citation2015) demonstrated that UA bound to cyclins D1 (Cyc D1) and cyclin-dependent kinases (CDK6), while compound 9 targeted at glucokinase (GK), glucose transporter 1 (GLUT1) and ATPase. Therefore, modification at the C-28 position of UA with the piperazine moiety (compounds 9–11) exhibited better anticancer activity than against HepG2 by interfering with glucose metabolism in a dose-dependent manner. Wang et al. (Citation2014) also synthesized a series of UA derivatives (compounds 12–19), and found that UA diamine derivative combined with 2-DG showed synergistic inhibition for hepatoma cell proliferation by dual-targeting of apoptosis and glycolysis. Furthermore, explicating its structure–activity relationship helped diamine connected at C-28 enhance efficacy and selectivity of UA; additionally, the length of six carbon chains (6C) of diamine might represent the most optimal length for UA to bind the active sites of hexokinase and show the most excellent competitiveness, hence downregulating the glycolysis metabolism of cancer cells. Xiang et al. (Citation2015) further proved that the mechanism of compound 14 inhibiting HepG2 cell attributed to its suppression of cell-surface CAMs and integrin α6β1. The adjustment of ester metabolism and free radical scavenging also associated with the treatment of liver cancer. Recently, Kazmi et al. (Citation2013) noticed that UA stearoyl glucoside (UASG, compound 20), a newly discovered triterpene in Lantana camara, significantly suppressed diethylnitrosamine (DENA)-induced hepatocellular carcinogenesis by scavenging the hydroxyl radicals, modulating the levels of lipid peroxidation (LPO) and increasing the endogenous antioxidant enzymes level. UASG was also able to repair the HCC-caused histological changes at concentrations of 40 and 80 mg/kg.
Table 2. UA derivatives inhibit HCC through glucose and ester metabolism pathway.
Thiourea derivatives have potential anti-cancer activity (Huang et al., 2013a, Citation2013b). Accordingly, Hua et al. (Citation2015) synthesized a series of piperazine-thiourea UA derivatives (compounds 21–40, ) by combining thiourea, piperazine and UA, and reached the following conclusions: (1) the synchronous introduction of piperazine-thiourea at C-28 and an acyl group at C-3 was significant for improving their activity, however, only introduction of an acyl piperazine thiourea at C-28 led to the opposite effect on anti-cancer activity; (2) the activity of introducing electron-donating group to phenyl ring was higher than electron-withdrawing group; (3) para-substituted phenyl ring showed better anti-cancer activity than meta-substituted. Among these piperazine-thiourea UA derivatives, trimethoxyphenyl thiourea UA (compound 38) showed the best efficiency. Moreover, compound 38 displayed higher levels of anti-proliferative activities compared with UA and 5-fluorouracil (5-FU) (IC50 was 5.4 ± 0.79 μM) and exhibited much lower cytotoxicity than 5-FU in HepG2 cells line.
Table 3. Piperazine-thiourea UA derivatives.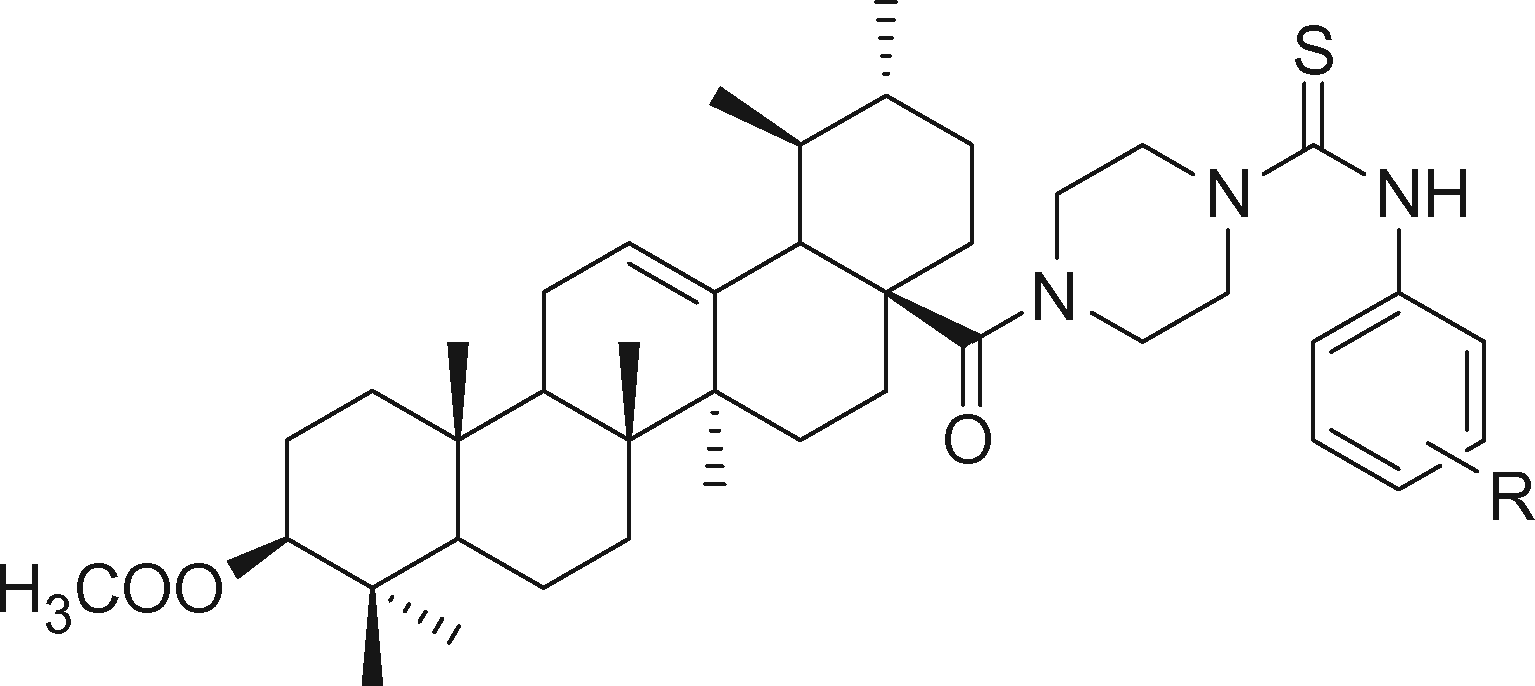
Carbazole, a nitrogen-containing aromatic heterocyclic compound, attracted more attention owing to its excellent anti-cancer activity (Shaikh et al. Citation2015). Prompted by the above facts, Gu et al. (Citation2015) synthesized a series of novel carbazole derivatives of UA (compounds 40–46, ). Among these derivatives, some compounds showed significant cytotoxic activity to HepG2 cells with IC50 values below 10 μM. Especially in compound 45, IC50 was only 1.26 ± 0.17 μM. This outstanding result indicated that: (1) the incorporation of carbazole moiety to the molecule was beneficial to the anticancer activity; 2) the N-(dimethylamino) propyl moiety was crucial for their cytotoxic activity. But, esterification at the C-28 carboxyl group led to reduction of activities; (3) the indole benzene rings with lipophilic substitutes exhibited stronger inhibitory activity than hydrophilic substitutes; (4) the indole benzene rings with p-substitution had higher cytotoxicity than o-substitution. Similarly, Leal et al. (Citation2012) introduced a nitrogen-containing heterocyclic ring(s) to UA, such as imidazole and triazole, and obtained compounds 47–51 () displaying better anti-proliferative effects than UA.
Table 4. Carbazole derivatives of UA.
Table 5. Imidazole and triazole derivatives of UA.
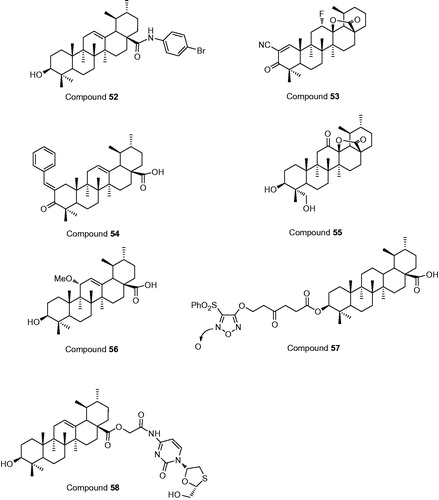
Kalani et al. (Citation2014) used an anti-HepG2 test and showed that 4-bromoanilamide derivative of UA (compound 52, ) was 212 times more efficient than the starting material, and 11 times more efficient than the positive control, paclitaxel. Amide derivatives were better than the ester derivatives against HepG2 cancer cell lines. A UA fluorolactone derivative (compound 53), 2-cyano-3-oxo-12a-fluoro-urs-1-en-13,28b-olide, improved the anti-proliferative activity against HepG2 cell lines, with IC50 value lower than 1 μM (Leal et al. Citation2012). Functional liver cancer stem cells (LCSCs) could rapidly self-renew and infinitely proliferate. CD133+, as a surface marker, isolated LCSCs. UA chalcone (compound 54) targeted PLC/PRF/5 hepatoma cell line and Huh7 LCSCs, inhibiting the expression of CD133+ in a dose- and time-dependent manner. The inhibition was significant at 50 μM and on day 8 (Lin et al. Citation2015). Concurrently, in natural, two new ursane-type triterpenes, microfokienoxane C (compound 55) and 3β, 28-dihydroxy-11α-methoxyurs-12-ene (compound 56), had recently been isolated from the leaves of Microtropis fokienensis and Ilex cornuta, respectively. Compound 55 was found to be the strongest cytotoxic activity against HepG2 and Hep3B cancer cell lines, with IC50 values of 3.8 and 4.5 μg/ml, respectively. Compound 56 also exhibited cytotoxic activity against the HepG2 cell line, with IC50 values of 4.6 μg/ml (Chen et al. Citation2006).
High concentrations of NO can promote HCC cells apoptosis via intervening with liver inflammatory. Thence, the combination of NO and UA probably protect hepatocytes from inflammation and toxicant-mediated liver damage. On this basis, Chen et al. (Citation2011) connected UA with furoxan for a series of novel NO-releasing compounds, which could produce high levels of NO in the liver. Among these NO-releasing compounds, compound 57 showed the best anti-cancer activity against HepG2 cells, with IC50 values of 3.2 μM. A novel codrug (compound 58), connecting lamivudine (LMV) with UA via ester linkage, had the dual action of anti-hepatitis B virus activity and hepatoprotective effects against acute liver injury. Moreover, in vitro, the high concentration of LMV and UA indicated that the ester bond of the codrug could be easily broken by esterases (Zhong et al. Citation2012).
Except chemical structural modification, great efforts also had been put in the modification of dosage form and drug delivery system. PEGylation is an effective method for increasing solubility in water, controlling permeability through biological barriers, longevity in the blood stream, and controlling release (Greenwald et al. Citation2003). Therefore, Zacchigna et al. (Citation2014) synthesized mPEG-UA which increased solubility in water and stability in physiological buffer and delivered free and active UA in a prolonged manner in plasma. Zhao et al. (Citation2015) found that PEG-modified UA liposomes not only possessed higher solubility but also lower cytotoxic effect than conventional UA liposomes. Nanotechnology-based drug delivery systems are effective methods to increase the solubility and dissolution rate of water-insoluble compounds. Then, Song et al. (Citation2014) loaded UA into nanocrystals without stabilizer. Compared with the raw material, the UA nanocrystals exhibited improved aqueous dispensability and dissolution rate. Moreover, the suspension of UA nanocrystals showed better physically stability after storage at 4 °C for 7 weeks. Zhang et al. (Citation2015) loaded UA into amphiphilic poly (N-vinylpyrrolidone)-block-poly (ɛ-caprolactone) nanoparticles (UA-NPs). The UA-NPs significantly delayed tumour growth and localized to the tumour site via altering the in vivo biodistribution and prolong the retention time of UA. The UA non-covalent complex with hydrophilic cyclodextrins not only improved water solubility but also optimized bioavailability and stability (Soica et al. Citation2008). However, in pharmacokinetic study, changing UA delivery carrier improved its bioavailability. UA liposomes and UA nanoliposomes were released and accumulated in the liver; therefore, Zhu et al. (Citation2013) suggested UA nanoliposomes for safely dose levels within the range of 37–98 mg/m2. In this drug concentration range, no drug accumulated was observed even with 14 days of continuous IV infusion. Moreover, the dose of UA liposomes for a phase II clinical trial recommended by Qian et al. (Citation2015) is 98 mg/m2.
Discussion
With many pharmacological activities, UA’s hepatoprotective effect possesses great potential in the future for the development of anti-HCC drugs. The mechanism of UA’s resistance to HCC was proved of great complexity. UA mainly blocks HCC cells growth and migration through inhibiting HCC cells glucose, ester metabolism and replication of genetic material. UA also has the function of promoting liver cells regeneration to repair damaged liver. Additionally, UA possesses anti-inflammatory, antioxidation, anti-HCV/HBV and antidiabetic activities which can assist in treating HCC. It could also relieve obesity, insulin resistance and hyperlipidemia-induced NAFLD, and therefore, decreases the incidence of HCC. However, in spite of the virtue of low toxicity, the clinical application of UA is still limited because of its low water solubility, which results in the compromised bioavailability and therapeutic efficacy. Therefore, there have been many research works on structural modification of UA due to its structural limitations. So far the modification of UA focused on the C-3 hydroxyl group, the C-28 carboxyl group and the double bond. Upon the modification of the C-3 hydroxyl group, some research works found that acylation was better than hydrolysis, and substituted nitrogen heterocyclic at C-3 was better than at C-28. In the C-28 carboxyl group, the anti-HCC activity of UA derivatives was improved by introducing nitrogen-containing group. But, attention still needed to be paid to the size and length of the added carbon chain, otherwise, the steric blocking would decrease the activity. C-1/C-21 and C-7/C-21 dihydroxylation is useless for anti-HCV activity, as well as C-1 hydroxylation and simultaneous introduction of carbonyl group at C-21 (Fu et al. Citation2013). In addition to chemical structural modification, covalent modification is also an important method to enhance the activity of UA, which includes conjugating highly water-soluble macromolecular groups, like PEG, NP and cyclodextrin to improve UA′s aqueous solubility as well as using nanoparticles, nanocrystals and nanosuspension to prolong the retention time and enhance dissolution velocity. However, the surface of polyamidoamine (PAMAM) conjugated with UA and folic acid (FA) showed no effect on the cellular uptake for HepG2 cells (Gao et al. Citation2015). Despite the low toxicity of UA, we should closely monitor their doses in clinic.
Conclusion
UA is a natural product with many pharmacological activities, including anticancer and hepatoprotective activity. To date, UA has been applied in liver disease treatment. However, its in vitro pharmacological activities reduced greatly because of its limited water solubility. Therefore, in order to find new effective anti-HCC compounds, many studies have focused on the structural modification of UA. The acetylation of the C-3 hydroxyl group and amidation of the C-28 carboxyl group are common and effective ways to improve its bioavailability and activities. Among these derivatives, compounds 45 and 53 showed outstanding anti-HCC activity with IC50 values of 1.26 ± 0.17 μM and lower than 1 μM, respectively, which is worth developing further.
Disclosure statement
The authors report that they have no conflict of interest.
Funding
This research was supported by the National Natural Science Foundation of China (No. 81273537), Scientific Research Fund of Hunan Provincial Education Department (No. 12K095), the key disciplines of Hunan Province and the Project is sponsored by Zhengxing scholar program of the University of South China.
References
- Alqahtani A, Hamid K, Kam A, Wong KH, Abdelhak Z, Razmovski-Naumovski V, Chan K, Li KM, Groundwater PW, Li GQ. 2013. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr Med Chem. 20:908–931.
- Bannasch P. 2010. Hepatocellular glycogenosis and hepatic neoplasms. Toxicol Pathol. 38:1000–1002.
- Brunt EM. 2005. Pathology of nonalcoholic steatohepatitis. Hepatol Res. 33:68–71.
- Castro AJ, Frederico MJ, Cazarolli LH, Mendes CP, Bretanha LC, Schmidt ÉC, Bouzon ZL, de Medeiros Pinto VA, da Fonte Ramos C, Pizzolatti MG, et al. 2015. The mechanism of action of ursolic acid as insulin secretagogue and insulinomimetic is mediated by cross-talk between calcium and kinases to regulate glucose balance. Biochim Biophys Acta. 1850:51–61.
- Chen IH, Chang FR, Wu CC, Chen SL, Hsieh PW, Yen HF, Du YC, Wu YC. 2006. Cytotoxic triterpenoids from the leaves of Microtropis fokienensis. J Nat Prod. 69:1543–1546.
- Chen L, Qiu W, Tang J. 2011. Synthesis and bioactivity of novel nitric oxide-releasing ursolic acid derivatives. Chin Chem Lett. 22:413–416.
- Cragg GM, Grothaus PG, Newman DJ. 2009. Impact of natural products on developing new anticancer agents. Chem Rev. 109:3012–3043.
- Dong H, Yang X, Xie J, Xiang L, Li Y, Ou M, Chi T, Liu Z, Yu S, Gao Y, et al. 2014. UP12, a novel ursolic acid derivative with potential for targeting multiple signaling pathways in hepatocellular carcinoma. Biochem Pharmacol. 93:151–162.
- Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. 2006. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 44:865–873.
- El-Serag HB. 2011. Hepatocellular carcinoma. N Engl J Med. 365:1118–1127.
- El-Serag HB. 2012. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 142:1264–1273.
- El-Serag HB, Tran T, Everhart JE. 2004. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 126:460–468.
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. 2010. Estimates of worldwide burden of cancer in 2008: Globocan 2008. Int J Cancer. 127:2893–2917.
- Fu SB, Yang JS, Cui JL, Sun DA. 2013. Biotransformation of ursolic acid by syncephalastrum racemosum CGMCC 3.2500 and anti-HCV activity. Fitoterapia. 86:123–128.
- Gao Y, Li Z, Xie X, Wang C, You J, Mo F, Jin B, Chen J, Shao J, Chen H, et al. 2015. Dendrimeric anticancer prodrugs for targeted delivery of ursolic acid to folate receptor-expressing cancer cells: Synthesis and biological evaluation. Eur J Pharm Sci. 70:55–63.
- Garcia-Compean D, Jaquez-Quintana JO, Gonzalez-Gonzalez JA, Maldonado-Garza H. 2009. Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. 15:280–288.
- Goossens N, Hoshida Y. 2015. Hepatitis C virus-induced hepatocellular carcinoma. Clin Mol Hepatol. 21:105–114.
- Gu W, Hao Y, Zhang G, Wang SF, Miao TT, Zhang KP. 2015. Synthesis, in vitro antimicrobial and cytotoxic activities of new carbazole derivatives of ursolic acid. Bioorg Med Chem Lett. 25:554–557.
- Greenwald RB, Choe YH, McGuire J, Conover CD. 2003. Effective drug delivery by PEGylated drug conjugates. Adv Drug Deliv Rev. 55:217–250.
- He W, Shi F, Zhou ZW, Li B, Zhang K, Zhang X, Ouyang C, Zhou SF, Zhu X. 2015. A bioinformatic and mechanistic study elicits the antifibrotic effect of ursolic acid through the attenuation of oxidative stress with the involvement of ERK, PI3K/Akt, and p38 MAPK signaling pathways in human hepatic stellate cells and rat liver. Drug Des Devel Ther. 9:3989–4104.
- Honda M, Kaneko S, Kawai H, Shirota Y, Kobayashi K. 2001. Differential gene expression between chronic hepatitis B and C hepatic lesion. Gastroenterology. 120:955–966.
- Hua SX, Huang RZ, Ye MY, Pan YM, Yao GY, Zhang Y, Wang HS. 2015. Design, synthesis and in vitro evaluation of novel ursolic acid derivatives as potential anticancer agents. Eur J Med Chem. 95:435–452.
- Huang XC, Wang M, Pan YM, Tian XY, Wang HS, Zhang Y. 2013. Synthesis and antitumor activities of novel α-aminophosphonates dehydroabietic acid derivatives. Bioorg Med Chem Lett. 23:5283–5289.
- Huang XC, Wang M, Pan YM, Yao GY, Wang HS, Tian XY, Qin JK, Zhang Y. 2013. Synthesis and antitumor activities of novel thiourea α-aminophosphonates from dehydroabietic acid. Eur J Med Chem. 69:508–520.
- Jia Y, Bhuiyan MJ, Jun HJ, Lee JH, Hoang MH, Lee HJ, Kim N, Lee D, Hwang KY, Hwang BY, et al. 2011. Ursolic acid is a PPAR-α agonist that regulates hepatic lipid metabolism. Bioorg Med Chem Lett. 21:5876–5880.
- Jin YR, Jin JL, Li CH, Piao XX, Jin NG. 2012. Ursolic acid enhances mouse liver regeneration after partial hepatectomy. Pharm Biol. 50:523–528.
- Jung K, Chin YW, Yoon K, Chae HS, Kim CY, Yoo H, Kim J. 2013. Anti-inflammatory properties of a triterpenoidal glycoside from Momordica cochinchinensis in LPS-stimulated macrophages. Immunopharmacol Immunotoxicol. 35:8–14.
- Kalani K, Yadav DK, Singh A, Khan F, Godbole MM, Srivastava SK. 2014. QSAR guided semi-synthesis and in vitro validation of anticancer activity in ursolic acid derivatives. Curr Top Med Chem. 14:1005–1013.
- Kazmi I, Narooka AR, Afzal M, Singh R, Al-Abbasi FA, Ahmad A, Anwar F. 2013. Anticancer effect of ursolic acid stearoyl glucoside in chemically induced hepatocellular carcinoma. J Physiol Biochem. 69:687–695.
- Kong L, Li S, Liao Q, Zhang Y, Sun R, Zhu X, Zhang Q, Wang J, Wu X, Fang X, et al. 2013. Oleanolic acid and ursolic acid: novel hepatitis C virus antivirals that inhibit NS5B activity. Antiviral Res. 98:44–53.
- Kunkel SD, Elmore CJ, Bongers KS, Ebert SM, Fox DK, Dyle MC, Bullard SA, Adams CM. 2012. Ursolic acid increases skeletal muscle and brown fat and decreases diet induced obesity, glucose intolerance and fatty liver disease. PLoS One. 7:e39332.
- Kuttan G, Pratheeshkumar P, Manu KA, Kuttan R. 2011. Inhibition of tumor progression by naturally occurring terpenoids. Pharm Biol. 49:995–1007.
- Leal AS, Wang R, Salvador JA, Jing Y. 2012. Synthesis of novel ursolic acid heterocyclic derivatives with improved abilities of antiproliferation and induction of p53, p21waf1 and NOXA in pancreatic cancer cells. Bioorg Med Chem. 20:5774–5786.
- Leal AS, Wang R, Salvador JA, Jing Y. 2012. Semisynthetic ursolic acid fluorolactone derivatives inhibit growth with induction of p21waf1 and induce apoptosis with upregulation of NOXA and downregulation of c-FLIP in cancer cells. Chem Med Chem. 7:1635–1646.
- Lee J, Lee HI, Seo KI, Cho HW, Kim MJ, Park EM, Lee MK. 2014. Effects of ursolic acid on glucose metabolism, the polyol pathway and dyslipidemia in non-obese type 2 diabetic mice. Indian J Exp Biol. 52:683–691.
- Li JS, Wang WJ, Sun Y, Zhang YH, Zheng L. 2015. Ursolic acid inhibits the development of nonalcoholic fatty liver disease by attenuating endoplasmic reticulum stress. Food Funct. 6:1643–1651.
- Li LQ, Li J, Huang Y, Wu Q, Deng SP, Su XJ, Yang RY, Huang JG, Chen ZZ, Li S. 2012. Lignans from the heartwood of Streblus asper and their inhibiting activities to Hepatitis B virus. Fitoterapia. 83:303–309.
- Li S, Liao X, Meng F, Wang Y, Sun Z, Guo F, Li X, Meng M, Li Y, Sun C. 2014. Therapeutic role of ursolic acid on ameliorating hepatic steatosis and improving metabolic disorders in high-fat diet-induced non-alcoholic fatty liver disease rats. PLoS One. 9:e86724
- Lin CC, Huang CY, Mong MC, Chan CY, Yin MC. 2011. Antiangiogenic potential of three triterpenic acids in human liver cancer cells. J Agric Food Chem. 59:755–762.
- Lin RX, Gong LL, Fan LM, Zhao ZK, Yang SL. 2015. Role of ursolic acid chalcone, a synthetic analogue of ursolic acid, in inhibiting the properties of CD133+ sphere-forming cells in liver stem cells . Int J Clin Exp Pathol. 8:1427–1434.
- Liu J. 1995. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 49:57–68.
- Liu L, Zhang J, Li M, Zhang X, Zhang J, Li Z, Wang L, Wu J, Luo C. 2014. Inhibition of HepG2 cell proliferation by ursolic acid and polysaccharides via the downregulation of cyclooxygenase-2. Mol Med Rep. 9:2505–2511.
- Liu Y, Wu F. 2010. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ Health Perspect. 118:818–824.
- Ma JQ, Ding J, Zhang L, Liu CM. 2014. Ursolic acid protects mouse liver against CCl4-induced oxidative stress and inflammation by the MAPK/NF-κB pathway. Environ Toxicol Pharmacol. 37:975–983.
- Ma JQ, Ding J, Zhang L, Liu CM. 2015. Protective effects of ursolic acid in an experimental model of liver fibrosis through Nrf2/ARE pathway. Clin Res Hepatol Gastroenterol. 39:188–197.
- Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS. 2005. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 42:218–224.
- Maschek G, Savaraj N, Priebe W, Braunschweiger P, Hamilton K, Tidmarsh GF, De Young LR, Lampidis TJ. 2004. 2-Deoxy-d-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res. 64:31–34.
- Novotny L, Vachalkova A, Biggs D. 2001. Ursolic acid: an anti-tumorigenic and chemopreventive activity. Minireview. Neoplasma. 48:241–246.
- Pang R, Poon RT. 2006. Angiogenesis and antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett. 242:151–167.
- Qian Z, Wang X, Song Z, Zhang H, Zhou S, Zhao J, Wang H. 2015. A phase I trial to evaluate the multiple-dose safety and antitumor activity of ursolic acid liposomes in subjects with advanced solid tumors. Biomed Res Int. 2015:809714.
- Raez LE, Papadopoulos K, Ricart AD, Chiorean EG, Dipaola RS, Stein MN, Rocha Lima CM, Schlesselman JJ, Tolba K, Langmuir VK, et al. 2013. A phase I dose-escalation trial of 2-deoxy-d-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 71:523–530.
- Ramos AA, Lima CF, Pereira ML, Fernandes-Ferreira M, Pereira-Wilson C. 2008. Antigenotoxic effects of quercetin, rutin and ursolic acid on HepG2 cells: evaluation by the comet assay. Toxicol Lett. 177:66–73.
- Rashid S, Dar BA, Majeed R, Hamid A, Bhat BA. 2013. Synthesis and biological evaluation of ursolic acid-triazolyl derivatives as potential anti-cancer agents. Eur J Med Chem. 66:238–245.
- Rutter K, Stättermayer AF, Beinhardt S, Scherzer TM, Steindl-Munda P, Trauner M, Ferenci P, Hofer H. 2015. Successful anti-viral treatment improves survival of patients with advanced liver disease due to chronic hepatitis C. Aliment Pharmacol Ther. 41:521–531.
- Shaikh MS, Karpoormath R, Thapliyal N, Rane RA, Palkar MB, Faya AM, Patel HM, Alwan WS, Jain K, Hampannavar GA. 2015. Current perspective of natural alkaloid carbazole and its derivatives as antitumor agents. Anticancer Agents Med Chem. 15:1049–1065.
- Sasaki Y. 2010. Insulin resistance and hepatocarcinogenesis. Clin J Gastroenterol. 3:271–278.
- Satarug S. 2012. Long-term exposure to cadmium in food and cigarette smoke, liver effects and hepatocellular carcinoma. Curr Drug Metab. 13:257–271.
- Shao JW, Dai YC, Xue JP, Xue JP, Wang JC, Lin FP, Guo YH. 2011. In vitro and in vivo anticancer activity evaluation of ursolic acid derivatives. Eur J Med Chem. 46:2652–2661.
- Shyu MH, Kao TC, Yen GC. 2008. Hsian-tsao (Mesona procumbens Heml.) prevents against rat liver fibrosis induced by CCl4 via inhibition of hepatic stellate cells activation. Food Chem Toxicol. 46:3707–3713.
- Slamenová D, Horváthová E, Bartková M, Krajcovicová Z, Lábaj J, Kosíková B, Masterová I. 2006. Reduction of DNA-damaging effects of anti-HIV drug 3'-azido-3'-dideoxythymidine on human cells by ursolic acid and lignin biopolymer. Neoplasma. 53:485–491.
- Soica C, Dehelean C, Peev C, Coneac G, Gruia A. 2008. Complexation with hydroxipropil γ cyclodextrin of some pentacyclic triterpenes, characterisation of their binary products. Farmacia. 56:182–190.
- Son HS, Kwon HY, Sohn EJ, Lee JH, Woo HJ, Yun M, Kim SH, Kim YC. 2013. Activation of AMP-activated protein kinase and phosphorylation of glycogen synthase kinase 3β mediate ursolic acid induced apoptosis in HepG2 liver cancer cells. Phytother Res. 27:1714–1722.
- Song J, Wang Y, Song Y, Chan H, Bi C, Yang X, Yan R, Wang Y, Zheng Y. 2014. Development and characterisation of ursolic acid nanocrystals without stabiliser having improved dissolution rate and in vitro anticancer activity. AAPS PharmSciTech. 15:11–19.
- Sundaresan A, Radhiga T, Pugalendi KV. 2014. Effect of ursolic acid and Rosiglitazone combination on hepatic lipid accumulation in high fat diet-fed C57BL/6J mice. Eur J Pharmacol. 741:297–303.
- Wang CH, Wey KC, Mo LR, Chang KK, Lin RC, Kuo JJ. 2015. Current trends and recent advances in diagnosis, therapy, and prevention of hepatocellular carcinoma. Asian Pac J Cancer Prev. 16:3595–3604.
- Wang J, Jiang Z, Xiang L, Li Y, Ou M, Yang X, Shao J, Lu Y, Lin L, Chen J, et al. 2014. Synergism of ursolic acid derivative US597 with 2-deoxy-D-glucose to preferentially induce tumor cell death by dual-targeting of apoptosis and glycolysis. Sci Rep. 4:5006.
- Wang L, Wang GL, Liu JH, Liu JH, Li D, Zhu DZ, Wu LN. 2012. Effects of ursolic acid in ameliorating insulin resistance in liver of KKAy mice via peroxisome proliferator-activated receptors α and γ. Zhong Xi Yi Jie He Xue Bao. 10:793–799.
- Wu HY, Chang CI, Lin BW, Yu FL, Lin PY, Hsu JL, Yen CH, Liao MH, Shih WL. 2011. Suppression of hepatitis B virus X protein-mediated tumorigenic effects by ursolic acid. J Agric Food Chem. 59:1713–1722.
- Wu P, He P, Zhao S, Huang T, Lu Y, Zhang K. 2014. Effects of ursolic acid derivatives on Caco-2 cells and their alleviating role in streptozocin-induced type 2 diabetic rats. Molecules. 19:12559–12576.
- Xiang L, Chi T, Tang Q, Yang X, Ou M, Chen X, Yu X, Chen J, Ho RJ, Shao J, et al. 2015. A pentacyclic triterpene natural product, ursolic acid and its prodrug US597 inhibit targets within cell adhesion pathway and prevent cancer metastasis. Oncotarget. 6:9295–9312.
- Yang X, Li YF, Jiang W, Ou M, Chen Y, Xu Y, Wu Q, Zheng Q, Wu F, Wang L, et al. 2015. Synthesis and biological evaluation of novel ursolic acid derivatives as potential anticancer prodrugs. Chem Biol Drug Des. 86:1397–1404.
- Zacchigna M, Cateni F, Drioli S, Procida G, Altieri T. 2014. PEG-ursolic acid conjugate: synthesis and in vitro release studies. Sci Pharm. 82:411–421.
- Zhang H, Zheng D, Ding J, Xu H, Li X, Sun W. 2015. Efficient delivery of ursolic acid by poly(N-vinylpyrrolidone)-block-poly (ɛ-caprolactone) nanoparticles for inhibiting the growth of hepatocellular carcinoma in vitro and in vivo. Int J Nanomed. 10:1909–1920.
- Zhao CH, Xu J, Zhang YQ, Zhao LX, Feng B. 2014. Inhibition of human enterovirus 71 replication by pentacyclic triterpenes and their novel synthetic derivatives. Chem Pharm Bull. 62:764–771.
- Zhao T, Liu Y, Gao Z, Gao D, Li N, Bian Y, Dai K, Liu Z. 2015. Self-assembly and cytotoxicity study of PEG-modified ursolic acid liposomes. Mater Sci Eng C Mater Biol Appl. 53:196–203.
- Zhong DS, Xiong L, Liu TR, Liu X, Liu X, Chen J, Sun SY, Khuri FR, Zong Y, Zhou Q, et al. 2009. The glycolytic inhibitor 2-deoxyglucose activates multiple prosurvival pathways through IGF1R. J Biol Chem. 284:23225–23233.
- Zhong Y, Dai ZH, Xu Y, Teng Y, Wu B. 2012. Synthesis, stability and pharmacological evaluation of a novel codrug consisting of lamivudine and ursolic acid. Eur J Pharm Sci. 45:110–115.
- Zhu Z, Qian Z, Yan Z, Zhao C, Wang H, Ying G. 2013. A phase I pharmacokinetic study of ursolic acid nanoliposomes in healthy volunteers and patients with advanced solid tumors. Int J Nanomed. 8:129–136.