Abstract
Context: Seaweeds are rich in bioactive compounds in the form of vitamins, phycobilins, polyphenols, carotenoids, phycocyanins and polysaccharides; many of these are known to have advantageous applications in human health. 3-Hydroxy-4,7-megastigmadien-9-one (comp) was isolated from Ulva pertusa (U. pertusa) Kjellman (Ulvaceae), which is a familiar edible green seaweed.
Objective: This study evaluates the anti-inflammatory activity of comp in CpG DNA-stimulated bone marrow-derived dendritic cells (BMDCs).
Materials and methods: For evaluating the effect of comp on cytokines production, BMDCs were treated with doses of comp (0, 0.5, 1, 2, 5, 10, 25 and 50 μM) for 1 h before stimulation with CpG DNA (1 μM). Cytokine production was measured by ELISA. Western blotting was conducted for evaluating effect of comp (50 μM) on MAPKs and NF-κB pathways. Luciferase reporter gene assay was conducted for effect of comp (0, 5, 10 and 25 μM) on transcriptional activity of AP-1 and NF-κB.
Results: Comp exhibited strong inhibition of interleukin (IL)-12 p40, IL-6 and TNF-α cytokine production with IC50 values of 6.02 ± 0.35, 27.14 ± 0.73, and 7.56 ± 0.21 μM, respectively. It blocked MAPKs and NF-κB pathways by inhibiting the phosphorylation of ERK1/2, JNK1/2, p38 and IκBα. In addition, it strongly inhibited the transcriptional activity of AP-1 and NF-κB with IC50 values of 8.74 ± 0.31 and 12.08 ± 0.24 μM, respectively.
Discussion and conclusion: Taken together, these data suggest that comp has a significant anti-inflammatory property and warrants further studies concerning the potential of comp for medicinal use.
Introduction
The mammalian immune system consists of two types, innate and adaptive immunity. The innate immunity is the first line of defence in combating invading pathogens and is mediated by phagocytes including macrophages and dendritic cells (DCs). Acquired immunity eliminates pathogens in the late phase of infection and generates immunological memory (Akira et al. Citation2006). Chronic inflammation results from continuous exaggerated immune reaction to injury or exposure to foreign pathogens (Uronis et al. Citation2009; Katoh et al. Citation2013). Microbial pathogen recognition by innate immune system is important for activation of microbicidal effectors and progression of adaptive immunity (Honstettre et al. Citation2004). Toll-like receptors (TLRs) consist of a family of pattern recognition receptor that sense conserved molecular pattern of microbes. They are crucial for recognition of microbial infection and initiating inflammatory and immune responses (Rakoff-Nahoum et al. Citation2004; Yuk & Jo Citation2011). TRL9 recognizes unmethylated DNA having CpG island derived from viruses and bacteria (Takeuchi & Akira Citation2010). Stimulation of immune cell with microbial product causes the production of pro-inflammatory cytokines, such as TNF-α and various interleukins and is maintained for the duration of inflammatory response (Ma et al. Citation2013; Shi et al. Citation2015).
TLR stimulation causes the phosphorylation and activation of extracellular signal regulated kinase (ERK1/2), c-Jun NH2-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPKs) signalling pathways (Intayoung et al. Citation2016). Activator protein (AP-1), a heterodimeric transcription factor composed of c-Fos, c-Jun, ATF and JDP families, is activated by MAPK signalling pathway. AP-1 upregulate the transcription of genes encoding pro-inflammatory mediators (Hossen et al. Citation2015). NF-κB pathway is one of the well-established inflammatory pathways (Greten et al. Citation2004; Zaki et al. Citation2011). Its activation requires phosphorylation and subsequent degradation of IκB, allowing the nuclear translocation of NF-κB, where it causes the transcription and release of pro-inflammatory cytokines (Saba et al. Citation2015).
In search for new drugs from natural products, marine organisms, including seaweeds and marine microorganisms have been the focus of numerous studies (Khan et al. Citation2008). Ulva pertusa (U. pertusa) Kjellman (Ulvaceae), an edible green seaweed, is consumed by local inhabitants of Asia (Shi et al. Citation2013). It has been authorized for human utilization by French authorities because of nutritional interests, i.e., rich in dietary fibres, vitamins, oligoelement and minerals (Pengzhan et al. Citation2003). Previous studies found that ulvan extracted from U. pertusa possessed antihyperlipidemic and antioxidant activities (Qi et al. Citation2012). Sulphated polysaccharides from U. pertusa have immune modulatory and anti-avian influenza virus activities (Song et al. Citation2016). 3-Hydroxy-4,7-megastigmadien-9-one (comp), a norisoprenoid, is the degradation product of carotenoids (Machida & Kikauchit Citation1996). As part of on-going research on evaluating biological effects, in this study, comp was isolated from U. pertusa, and was examined for anti-inflammatory activities for the first time. The detailed mechanisms of anti-inflammatory potential of comp have not been reported yet. Here, we investigated the anti-inflammatory potential of comp, especially on TLR9-mediated inflammatory response in bone marrow derived dendritic cell.
Materials and methods
Preparation of 3-hydroxy-4,7-megastigmadien-9-one
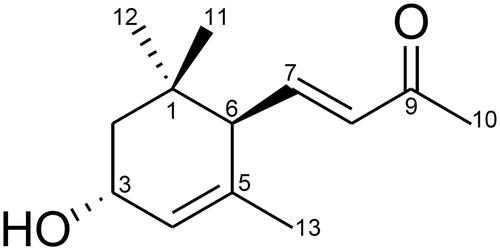
U. pertusa was collected on Jeju Island, South Korea. A voucher specimen (JBRI10253) has been deposited in herbarium of the Jeju Biodiversity Research Institute. Dried U. pertusa was subjected to solvent extraction in 70% aqueous ethanol. The resultant solvent suspension was extracted with n-hexane, ethyl acetate (EtoAc), n-butanol and water successively that gave the respective fraction extract. A portion of EtoAc-soluble fraction was subsequently subjected to medium pressure liquid chromatography (MPLC) by reserved-phase silica gel through water–methanol gradients to give 45 fractions. Fraction 18 (47.9 mg), by silica gel with n-Hex/EtOAc =1/1 to give 3-hydroxy-4,7-megastigmadien-9-one (comp). Structure of comp was identified by spectroscopic studies and by comparing the obtained data with literature values (Machida & Kikauchit Citation1996).
Cell cultures and measurement of cytokine production
To grow BMDCs, wild-type 6-week-old female C57BL/6 mice were used as previously described (Chae et al. Citation2013; Manzoor et al. Citation2014a). All animal procedures were approved by and performed according to the guidelines of the Institutional Animal Care and Use Committee of Jeju National University, Jeju, South Korea (#2010-0028). Briefly, bone marrow cells were differentiated in RPMI 1640 (BD, Grand Island, NY) medium containing granulocyte-macrophage colony-stimulating factor for DCs generation. For BMDCs, on day 6 of incubation the cells were harvested and seeded in 48-well plates at a density of 1 × 105 cells/0.5 mL, and then treated with the comp for 1 h before stimulation with CpG DNA. Supernatants were harvested 18 h after stimulation. Concentrations of murine IL-12 p40, IL-6 and TNF-α in the culture supernatants were measured by enzyme linked immunosorbent assay (ELISA) (BD PharMingen, San Jose, CA).
Cell viability assay
The cell viability was measured by standard procedure of 3-(4,5-dimethyl-2,5 thiazolyl)-2,5 diphenyltetrazolium bromide (MTT) assay (Chae et al. Citation2013).
Western blot analysis
Bone marrow-derived dendritic cells (BMDCs) were dispensed to 35 mm culture dishes at a concentration of 2 × 106 cells/2 mL and incubated for 1 h at 37 °C. The cells were pretreated with or without comp for 1 h before treatment with CpG DNA at the indicated time points. The cells were collected and, then, lysed in lysis buffer (PRO-PREP lysis buffer, iNtRON Biotechnology, South Korea). A protein sample (30 μg) was subjected to electrophoresis in 10% SDS-polyacrylamide gels and transferred to a polyvinylidene fluoride membrane (Bio-Rad, Hercules, CA). The membrane was incubated with 1/1000-diluted rabbit polyclonal antibodies that specifically recognize phospho-ERK1/2, phospho-p38, phospho-JNK1/2, phosphor-IκBα (Cell Signaling Technology, Danvers, MA), and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA). After washing, the membrane was incubated with a horseradish peroxidase-linked goat anti-rabbit IgG (Cell Signaling Technology), and immunoactive bands were detected as previously described (Koo et al. Citation2012).
Luciferase assay
For AP-1 and NF-κB reporter assays, HEK293T cells were plated in 24-well plates and grown overnight as described previously (Manzoor et al. Citation2013a, Citation2013b). Cells were transfected using Fugene 6 (Roche, Indianapolis, IN) with a AP-1 or NF-κB reporter gene, pRLnull (Promega, Madison, WI) and pcDNA3 (empty vector) or TLR9-encoding pcDNA3 kindly provided by Prof. R. Medzhitov (Yale University, New Haven, CT). After incubation of 24 h, cells were pretreated with comp for 1 h before stimulation with CpG (1 μM). After further incubation for 18 h, cells were lysed in a passive lysis buffer (Promega), and firefly luciferase vs. Renilla activities were measured using a Dual Luciferase Reporter assay system (Promega, Madison, WI).
Statistical analysis
All experiments were performed at least three times, and the data are presented as the mean ± standard deviation (SD) of three independent experiments. One-way ANOVA was used for comparison between the treated and the control groups. p < 0.05 was considered statistically significant.
Results
Effect of comp on the cell viability of BMDCs
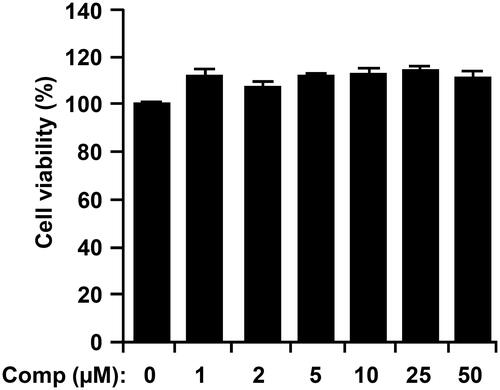
The chemical structure of comp isolated from U. pertusa is shown in . To assess the possible cytotoxicity of comp on BMDCs, cell viability was determined by using MTT assay. The results demonstrate that cell viability was not affected by comp at the indicated doses ().
Figure 2. Effects of comp on cell viability of bone marrow-derived dendritic cells (BMDCs). BMDCs were treated with comp (1–50 μM) for 18 h and viability was measured using MTT assay. Results shown are the mean ± SD of an experiment done in triplicate and are representative of three separate experiments. Comp, 3-hydroxy-4,7-megastigmadien-9-one.
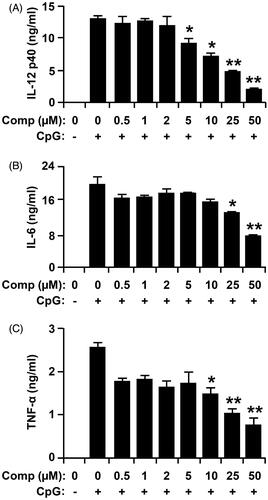
Inhibitory effect of comp on pro-inflammatory cytokine production in CpG DNA-stimulated dendritic cells
Mature and activated DCs produce high amounts of pro-inflammatory cytokines, such as IL-12, TNF-α and IL-6 (Wei et al. Citation2015). To explore the anti-inflammatory activity, comp was evaluated for inhibition of IL-12 p40, IL-6 and TNF-α production in CpG DNA-stimulated bone marrow derived DCs. DCs treated with comp alone showed no production of pro-inflammatory cytokines (data not shown). Stimulation of BMDCs with CpG DNA caused significant increase in the production of IL-12 p40, IL-6 and TNF-α (). Comp pretreatment exhibited strong inhibition of IL-12 p40, IL-6 and TNF-α production in the CpG-stimulated BMDCs ().
Figure 3. Inhibitory effects of comp on pro-inflammatory cytokine production in CpG DNA-stimulated bone marrow-derived dendritic cells (BMDCs). BMDCs were treated with comp at the indicated doses for 1 h before stimulation with CpG DNA (1 μM). Enzyme-linked immunosorbent assay (ELISA) was used to measure the concentrations of murine IL-12 p40 (A), IL-6 (B) and TNF-α (C) in the culture supernatants. Data are representative of three independent experiments. Comp, 3-hydroxy-4,7-megastigmadien-9-one. *p < 0.05, **p < 0.01 vs. comp-untreated cells in the presence of CpG DNA.
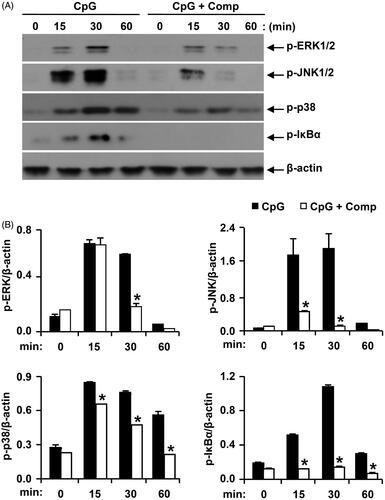
Effects of comp on the phosphorylation of MAPK and IκBα by CpG DNA-stimulated BMDCs
In response to extracellular signals, the three major MAPKs including p38 kinase, c-jun N-terminal kinase (JNK) and extracellular signal-regulated kinase get activated through phosphorylation leading to the increase gene expression of certain pro-inflammatory cytokines like IL-12, IL-6 and TNF-α (Intayoung et al. Citation2016). Therefore, through western blot analysis, we evaluated the effects on MAPK phosphorylation and NF-κB activation in CpG DNA-stimulated BMDCs, with and without comp treatment (). All three MAPKs get phosphorylated in BMDCs stimulated with CpG DNA. p38, JNK1/2 and ERK1/2 phosphorylation was detected between 15 and 30 min of CpG DNA-stimulation. ERK1/2 and JNK1/2 phosphorylation returned to baseline levels within 60 min of CpG DNA-stimulation. Comp pretreatment in the presence of CpG DNA showed strong inhibition of phosphorylation of ERK1/2, JNK1/2 and p38 (). Activation of NF-κB requires phosphorylation and subsequent degradation of IκB, which allowed nuclear translocation of NF-κB leading to the expression of certain pro-inflammatory cytokine genes (Tsumagari et al. Citation2015). Activation of NF-κB pathway was analysed indirectly through phosphorylation of IκBα. CpG DNA stimulation induced IκBα phosphorylation within 15 min get to maximum level at 30 min and return to baseline at 60 min of stimulation (). Comp pretreatment completely inhibited phosphorylation of IκBα in CpG DNA-stimulated BMDCs (). Taken together these results demonstrate that comp can inhibit CpG DNA-stimulated phosphorylation of ERK1/2, JNK1/2, p38 and IκBα and consequently inhibited MAPK and NF-κB pathways.
Figure 4. Effects of comp on the phosphorylation of MAPK and IκBα by CpG DNA-stimulated BMDCs. (A) Cells were pretreated with or without comp (50 μM) for 1 h before stimulation with CpG DNA (1 μM). Total cell lysate was obtained at the indicated time intervals. Western blot analysis was performed on the cell lysate to assess phosphorylation of ERK, JNK, p38 and IκBα. β-Actin was taken as the loading control. Data are representative of three independent experiments. (B) Phosphorylation of ERK, JNK, p38 and IκBα protein expression was quantified using scanning densitometry, and the band intensities were normalized by that of β-actin. Comp: 3-hydroxy-4,7-megastigmadien-9-one. *p < 0.05 vs. comp-untreated cells in the presence of CpG DNA.
Comp treatment inhibited AP-1 reporter activity in HEK293T cells
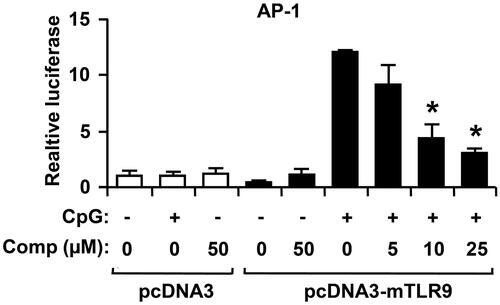
AP-1 is activated by MAPK signalling pathway, which up regulates the transcription of gene encoding pro-inflammatory cytokines (Lee & Yang Citation2013; Saba et al. Citation2015). To investigate whether the comp had an inhibitory effect on CpG DNA-stimulated AP-1 transcriptional activity, an AP-1 reporter gene assay was conducted (). The HEK293T cells transfected with empty pcDNA3 showed little AP-1-dependent luciferase activity on CpG DNA-stimulation. On the contrary, the HEK293T cells transfected with pcDNA3-mTLR9 exhibited robust AP-1-dependent luciferase activity on CpG DNA-stimulation. However, comp pretreatment exhibited strong inhibition of AP-1-dependent luciferase activity in HEK293T cells transfected with pcDNA3-mTLR9 (). Thus, the data suggest that comp has an inhibitory effect on TLR9-dependent AP-1 activation on CpG DNA stimulation.
Figure 5. Effects of comp on AP-1 reporter activity in HEK293T cells. HEK293T cells were transfected with empty vector (pcDNA3) or a TLR9-expressing plasmid (pcDNA3-mTLR9) and then treated with comp for 1 h before stimulation with CpG DNA (1 μM). Cell lysates were prepared, luciferase activity was assayed by the dual luciferase reporter assay and the results were expressed as relative luciferase. Data are representative of three independent experiments. Comp, 3-hydroxy-4,7-megastigmadien-9-one. *p < 0.05 vs. comp-untreated cells in the presence of CpG DNA.
Comp treatment inhibited NF-κB reporter activity in HEK293T cells
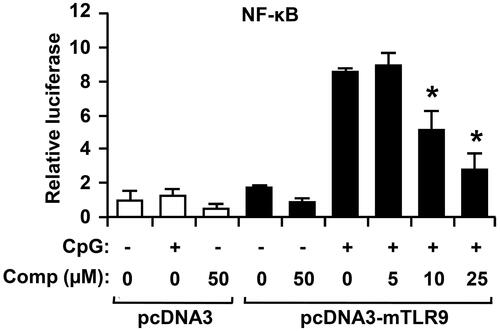
NF-κB is a family of transcription factors that has a key role in regulation of inflammation and immune response (Pacifico & Leonardi Citation2010). To determine whether comp inhibits CpG DNA-stimulated NF-κB transcriptional activity, an NF-κB reporter gene assay was preformed (). The HEK293T cells transfected with pcDNA3-mTLR9 exhibited robust NF-κB-dependent luciferase activity on CpG DNA stimulation. However, comp pretreatment exhibited strong inhibition of NF-κB-dependent luciferase activity in HEK293T cells transfected with pcDNA3-mTLR9 (). Thus, these data demonstrate that comp has inhibitory effect on TLR9-dependent NF-κB activation.
Figure 6. Effects of comp on NF-κB reporter activity in HECK293T cells. HEK293T cells were transfected with empty vector (pcDNA) or a TLR9-expressing plasmid (pcDNA3-mTLR9) and then treated with comp for 1 h before stimulation with CpG DNA (1 μM). Cell lysates were prepared, luciferase activity was assayed by the dual luciferase reporter assay and the results were expressed as relative luciferase. Data are representative of three independent experiments. Comp, 3-hydroxy-4,7-megastigmadien-9-one. *p < 0.05 vs. comp-untreated cells in the presence of CpG DNA.
Discussion
In this study, the anti-inflammatory potential of comp, a constituent of U. pertusa was examined. To the best of our knowledge, this is the first study to ascertain that comp, isolated from U. pertusa has the potential for medicinal solicitation in inflammation-associated diseases. A large body of evidence indicates that inflammation is major risk factor in the development of various human diseases like chronic asthma, rheumatoid arthritis, inflammatory bowel disease (IBD), multiple sclerosis and psoriasis. Therefore, inhibiting inflammation is critical to prevent or control various diseases (Debnath et al. Citation2013). The agent that reduced pro-inflammatory mediator and cytokines level is regarded as an effective therapeutic strategy for relieving a chronic inflammatory disease.
The pro-inflammatory cytokine IL-12 is expressed predominantly by activated dendritic and phagocytic cells, and is stimulated by pathogens, TLR ligands and CD40L (Teng et al. Citation2010). IL-12 p40 have important immunoregulatory activities and is a key cytokine in Th1-mediated autoimmune responses (Chae et al. Citation2013). Pretreatment of comp strongly inhibited IL-12 p40 production in CpG DNA-stimulated DCs. IL-6 is a multi-functional cytokine, secreted by many cell types and is transcriptionally induced by many stimuli. Overproduction of IL-6 is associated with tumour growth and autoimmune inflammatory diseases. Significant therapeutic effect of anti-IL-6 receptor antibody on various inflammatory diseases like rheumatoid arthritis, shows that overproduction of IL-6 has a major role in the pathogenesis of these ailments (Masuda et al. Citation2013). In this study, comp exhibited inhibition of IL-6 production in CpG DNA-stimulated BMDCs. TNF-α is a pro-inflammatory cytokine and its overproduction exacerbates different inflammatory conditions including sepsis and rheumatoid arthritis (Cho et al. Citation2015). In this study, comp showed a significant inhibitory effect on the production of TNF-α in CpG DNA-stimulated BMDCs, suggesting that it may have the potential to ameliorate TNF-α-associated diseases. Hence, the strong inhibitory properties of comp against IL-12 p40, IL-6 and TNF-α production warrant further studies concerning potential in future anti-inflammatory application.
ERK, p38 kinase and c-jun-N-terminal kinase (JNK) get activated through phosphorylation in response to extracellular signals and ultimately leads to modulation of gene expression in the nucleus (Manzoor & Koh Citation2012). Phosphorylation of IκB tag it for proteosomal degradation resulting into release and nuclear translocation of NF-κB and hence activation of NF-κB pathway (Tsumagari et al. Citation2015). In this study, we found that comp inhibited the phosphorylation and hence activation of all three MAPK kinases including ERK1/2, JNK1/2 and p38 in CpG DNA-stimulated BMDCs. Similarly comp also inhibited NF-κB pathway activation by suppressing the phosphorylation of IκBα in CpG DNA-stimulated BMDCs. Stimulation of TLR9 by bacterial DNA (Koh Citation2011; Masuda et al. Citation2013) activates AP-1 and NF- κB transcription factors leading to production of inflammatory cytokines (Debnath et al. Citation2013; Manzoor et al. Citation2014b). In this study, we found that comp down-regulated AP-1 and NF-κB in HEK293T cells.
In summary, our study demonstrates that comp significantly suppressed the level of pro-inflammatory cytokines. It has also been demonstrated that comp strongly inhibited MAPK and NF-кB pathways. The anti-inflammatory effect of comp was further confirmed by inhibition of AP-1 and NF-кB reporter gene activity. Taken together, comp-mediated anti-inflammatory activity represents a potential therapeutic use of the comp for inflammatory diseases.
Conclusions
In conclusion, our data suggest that this compound can act as potent anti-inflammatory drug. We found that comp had an inhibitory effect on the production of pro-inflammatory cytokines by down-regulating TLR9-dependent AP-1 and NF-κB activation. These results suggest that comp might be beneficial in the treatment of inflammation and autoimmune-associated diseases. Therefore, further studies are required on the detailed mode of action and in vivo efficacy of comp.
Disclosure statement
The authors report no declaration of interest.
Funding
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (MEST) [NRF-C1ABA001-2011-0021037].
References
- Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell. 124:783–801.
- Chae D, Manzoor Z, Kim SC, Kim S, Oh TH, Yoo ES, Kang HK, Hyun JW, Lee NH, Ko MH, et al. 2013 . Apo-9'-fucoxanthinone, isolated from Sargassum muticum, inhibits CpG-induced inflammatory response by attenuating the mitogen-activated protein kinase pathway. Mar Drugs. 11:3272–3287.
- Cho KH, Kim DC, Yoon CS, Ko WM, Lee SJ, Sohn JH, Jang JH, Ahn JS, Kim YC, Oh H. 2015. Anti-neuroinflammatory effects of citreohybridonol involving TLR4-MyD88-mediated inhibition of NF-кB and MAPK signaling pathways in lipopolysaccharide-stimulated BV2 cells. Neurochem Int. 15:30079–30086.
- Debnath T, Kim DH, Lim BO. 2013. Natural products as a source of anti-inflammatory agents associated with inflammatory bowel disease. Molecules. 18:7253–7270.
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. 2004. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 118:285–296.
- Honstettre A, Ghigo E, Moynault A, Capo C, Toman R, Akira S, Takeuchi O, Lepidi H, Raoult D, Mege JL. 2004. Lipopolysaccharide from Coxiella burnetii is involved in bacterial phagocytosis, filamentous actin reorganization, and inflammatory responses through toll-like receptor 4. J Immunol. 172:3695–3703.
- Hossen MJ, Kim SC, Son YJ, Baek KS, Kim E, Yang WS, Jeong D, Park JG, Kim HG, Chung WJ, et al. 2015. AP-1-targeting anti-inflammatory activity of the methanolic extract of Persicaria chinensis. Evid Based Complement Alternat Med. 2015:608126.
- Intayoung P, Limtrakul P, Yodkeeree S. 2016 . Antiinflammatory activities of crebanine by inhibition of NF-κB and AP-1 activation through suppressing MAPKs and Akt signaling in LPS-induced RAW264.7 Macrophages. Biol Pharm Bull. 39:54–161.
- Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN. 2013 . CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 24:631–644.
- Khan MN, Choi JS, Lee MC, Kim E, Nam TJ, Fujii H, Hong YK. 2008 . Anti-inflammatory activities of methanol extracts from various seaweed species. J Environ Biol. 29:465–469.
- Koh YS. 2011. Nucleic acid recognition and signaling by toll-like receptor 9: compartment-dependent regulation. J Bacteriol Virol. 41:131–132.
- Koo JE, Hong HJ, Mathema VB, Kang HK, Hyun JW, Kim GY, Kim YR, Maeng YH, Hyun CL, Chang WY, et al. 2012. Inhibitory effects of Carpinus tschonoskii leaves extract on CpG-stimulated pro-inflammatory cytokine production in murine bone marrow-derived macrophages and dendritic cells. In Vitro Cell Dev Biol Anim. 48:197–202.
- Lee IT, Yang CM. 2013 . Inflammatory signalings involved in airway and pulmonary diseases. Mediators Inflamm. 2013:791231.
- Ma J, Bang BR, Lu J, Eun SY, Otsuka M, Croft M, Tobias P, Han J, Takeuchi O, Akira S, et al. 2013. The TNF family member 4-1BBL sustains inflammation by interacting with TLR signaling components during late-phase activation. Sci Signal. 6:87–99.
- Machida K, Kikauchit M. 1996. Norisoprenoids from Viburnum dilatatum. Phytochemistry. 5:1333–1336.
- Manzoor Z, Kim S, Chae D, Yoo ES, Kang HK, Hyun JW, Lee NH, Suh IS, Koh YS. 2013a. Sea lettuce (Ulva fasciata) extract has an inhibitory effect on pro-inflammatory cytokine production in CpG-stimulated bone marrow-derived macrophages and dendritic cells. Food Sci Biotechnol. 22:781–786.
- Manzoor Z, Koh YS. 2012. Mitogen-activated protein kinases in inflammation. J Bacteriol Virol. 42:189–195.
- Manzoor Z, Koo JE, Koh YS. 2014a. Mitogen-activated protein kinase signaling in inflammation-related carcinogenesis. J Bacteriol Virol. 44:297–304.
- Manzoor Z, Mathema VB, Chae D, Kang HK, Yoo ES, Jeon YJ, Koh YS. 2013b. Octaphlorethol A inhibits the CpG-iduced inflammatory response by attenuating the mitogen-activated protein kinase and NF-κB pathways. Biosci Biotechnol Biochem. 77:1970–1972.
- Manzoor Z, Mathema VB, Chae D, Yoo ES, Kang HK, Hyun JW, Lee NH, Ko MH, Koh YS. 2014b. Extracts of the seaweed Sargassum macrocarpum inhibit the CpG-Induced inflammatory response by attenuating the NF-κB pathway. Food Sci Biotechnol. 23:293–297.
- Masuda K, Ripley B, Nishimura R, Mino T, Takeuchi O, Shioi G, Kiyonari H, Kishimoto T. 2013. Arid5a controls IL-6 mRNA stability, which contributes to elevation of IL-6 level in vivo. Proc Natl Acad Sci USA. 110:9409–9414.
- Pacifico F, Leonardi A. 2010. Role of NF-kappaB in thyroid cancer. Mol Cell Endocrinol. 321:29–35.
- Pengzhan Y, Ning L, Xiguang L, Gefei Z, Quanbin Z, Pengcheng L. 2003. Antihyperlipidemic effects of different molecular weight sulfated polysaccharides from Ulva pertusa (Chlorophyta). Pharmacol Res. 48:543–549.
- Qi H, Liu X, Zhang J, Duan Y, Wang X, Zhang Q. 2012 . Synthesis and antihyperlipidemic activity of acetylated derivative of ulvan from Ulva pertusa. Int J Biol Macromol. 50:270–272.
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. 2004 . Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 118:229–241.
- Saba E, Jeon BR, Jeong DH, Lee K, Goo YK, Kwak D, Kim S, Roh SS, Kim SD, Nah SY, et al. 2015. A novel Korean red ginseng compound gintonin inhibited inflammation by MAPK and NF-κB pathways and recovered the levels of mir-34a and mir-93 in RAW 264.7 cells. Evid Based Complement Alternat Med. 2015:624132.
- Shi J, Cheng C, Zhao H, Jing J, Gong N, Lu W. 2013 . In vivo anti-radiation activities of the Ulva pertusa polysaccharides and polysaccharide-iron(III) complex. Int J Biol Macromol. 60:341–346.
- Shi Q, Cheng L, Liu Z, Hu K, Ran J, Ge D, Fu J. 2015. The p38 MAPK inhibitor SB203580 differentially modulates LPS-induced interleukin 6 expression in macrophages. Cent Eur J Immunol. 40:276–282.
- Song L, Chen X, Liu X, Zhang F, Hu L, Yue Y, Li K, Li P. 2016. Characterization and comparison of the structural features, immune-modulatory and anti-avian influenza virus activities conferred by three algal sulfated polysaccharides. Mar Drugs. 14:1–17.
- Takeuchi O, Akira S. 2010. Pattern recognition receptors and inflammation. Cell. 140:805–820.
- Teng MW, Andrews DM, McLaughlin N, Scheidt B, Ngiow SF, Moller A, Hill GR, Iwakura Y, Oft M, Smyth MJ. 2010. IL-23 suppresses innate immune response independently of IL-17A during carcinogenesis and metastasis. Proc Natl Acad Sci USA. 107:8328–8333.
- Tsumagari K, Elmageed ZY, Sholl AB, Friedlander P, Abdraboh M, Xing M, Boulares AH, Kandil E. 2015. Simultaneous suppression of the MAP kinase and NF-κB pathways provides a robust therapeutic potential for thyroid cancer. Cancer Lett. 368:46–53.
- Uronis JM, Muhlbauer M, Herfarth HH, Rubinas TC, Jones GS, Jobin C. 2009 . Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS One. 4:e6026
- Wei F, Xu S, Jia X, Sun X, Yang X, Wei W, Chang Y. 2015. BAFF and its receptors involved in the inflammation progress in adjuvant induced arthritis rats. Int Immunopharmacol. 31:1–8.
- Yuk JM, Jo EK. 2011. Toll-like receptors and innate immunity. J Bacteriol Virol. 41:225–235.
- Zaki MH, Vogel P, Malireddi RK, Body-Malapel M, Anand PK, Bertin J, Green DR, Lamkanfi M, Kanneganti TD. 2011 . The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell. 20:649–660.