Abstract
Context: An ethnobotanical survey was completed in a remote village and surrounding country of Xinjiang, where most Uyghur medicinal plants could be collected. This work clarifies and increases ethnobotanical data.
Objectives: We surveyed and organized aromatic medicinal plants that are commonly used in clinical settings to provide a significant reference for studying new medical activities.
Materials and methods: In the survey, informants who have traditional knowledge on aromatic Uyghur medicinal plants were interviewed between March 2014 and September 2014. Aromatic medicinal plant species and pertinent information were collected. Some therapeutic methods and modes of preparation of traditional aromatic medicinal plants were found.
Results: A total of 86 aromatic medicinal plant species belonging to 36 families were included in our study. We identified 34 plant species introduced from different regions such as Europe, India and Mediterranean areas. Fruits and whole plants were the most commonly used parts of plant, and most aromatic medicinal plants could be applied as medicine and food. We assigned the medicinal plants a use value (UV). Knowing the UV of species is useful in determining the use reliability and pharmacological features of related plants.
Conclusions: Xinjiang is an area in which indigenous aromatic medicinal plants are diversely used and has therefore established a sound dimensional medical healthcare treatment system. Some aromatic Uyghur medicinal plants are on the verge of extinction. Hence, further strategies for the conservation of these aromatic medicinal plants should be prioritized.
Introduction
China is a unified multi-ethnic country, where ethnic medicine is the official unified name for the traditional medicines of Chinese ethnic minorities because of the barriers produced by the different medical systems, language, culture and species characteristics. Research based on ethnic medicinal resources is rare (Li et al. Citation2006). In some ethnic minority areas, the production technology of traditional ethnic medicine and clinically common and key ethnic medicinal prescriptions is facing the risk of severe loss, without being passed on to the next generation (Vandebroek & Balick Citation2012).
Uyghur medicine is the scientific summary and the synthesis of the wisdom of the Uyghur people, who have been hard-working in the long-term practice of production to fight diseases. Therefore, Uyghur medicine has a complete theoretical system, involving rich practical experience and a unique method of diagnosis and treatment, representing a treasure among Chinese traditional medicine.
Uyghur medicine originated from Hetian, located in Xinjiang, and has a long history (Jiang & Nie Citation2015). There are more than 1000 Uyghur medicinal plants on record, among which, approximately 450 are most commonly used. Most Uyghur medicines are made from plants. The Uyghur people are skilled at using aromatic drugs, which commonly involve roses, lavender (Gonçalves & Romano Citation2013; Mendoza et al. Citation2014), lip vanilla, safflower, coriander, chicory, clove (Dalai et al. Citation2014), cardamom (Bajaj et al. Citation1993) and long pepper (Tian et al. Citation2012; Ding et al. Citation2014). An aromatic plant is a plant that contains a high content of aromatic substances (essential oils or resin) that can be used as a medicine or spice. These plants are both highly useful and of high value. The aromatic medicinal species included in this report were selected according to two books, on Chinese Aromatic Plants and Uyghur Medicine. There are many aromatic plants included in records on processing and utilization in the ancient literature of China. People have often used aromatic plants for flavouring, healthcare, in wine and cosmetics, as moth repellents and refreshing substances, and for cleaning air.
Uyghur medicine is the object of this article, therefore, herbal monographs from the literature, research data, standards and regulations and physical specimens were collected, mainly to obtain information about aromatic plant varieties (Shang et al. Citation2012). Information about the species used in Uyghur medicines and their distribution, clinical efficacy and applied resources (preparations) was collected and reorganized, supporting the analysis, application (Auerbach et al. Citation2012), sharing, use and protection of Uyghur herbal resources (Zheng et al. Citation2006; Fred-Jaiyesimi et al. Citation2015).
Materials and methods
Study area
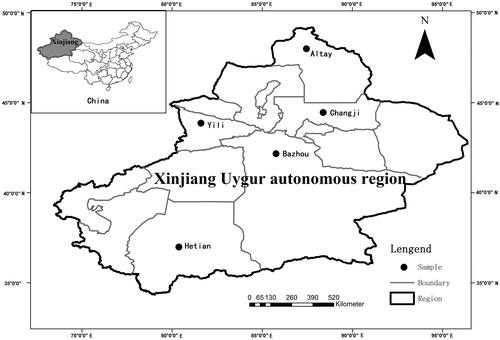
Xinjiang Uyghur autonomous region lies in the northwest of China and is located in the centre of Eurasia. Its area is 166 km2, which covers 1/6 of the total area of China. Xinjiang Uyghur autonomous region has a population of more than 16.9 million, of which more than 7.906 million are Uyghur nationality. An obvious feature of the terrain is the ‘three mountain clip two basins’ (Liu et al. Citation2014) (). Xinjiang is characterized by its dry climate, with the main features of sufficient sunshine and deficient rainfall. The area is far from the ocean and is surrounded by mountains, which is not only reflected in reduced moisture in the area, but also in the difference in the rainfall distribution. The Tianshan Mountains prevent cold air from flowing to the south, thus, constituting the climate demarcation line that separates the temperate zone in the north from the warm temperate zone in the south. The annual average temperature in southern Xinjiang ranges from 10 to 13 °C, whereas it is below 10 °C in the north. The average rainfall is only 45 mm, and rainfall in the north is much greater than in the south (YIN et al. Citation2011). Another characteristic of the area is the great discrepancy of temperature between day and night; generally, the temperature increases rapidly during the day, whereas it drops at night. People in northern Xinjiang are vulnerable to rheumatism because of the cold weather, whereas people in the south commonly suffer from liver disease, gastrointestinal disease, cardiovascular disease (Cámara-Leret et al. Citation2014), vitiligo and psoriasis, which can be attributed to their eating habits (giving priority to meat) and its dryness and temperature range.
The food consumed in Xinjiang is quite rich, the cooking material mainly contains meat (mutton, beef and horsemeat), dairy products and cooked wheaten food. The method of cooking them is based on roasting, stewing, steaming and so forth. For example, the popular ethnic food in Xinjiang includes mutton kebabs, kao quanyang, zhuafan, nang, etc. The people in Xinjiang basically eat meaty food. Moreover, people eating meat and grilled food are more susceptible to chronic disease. Additionally, women in a dry climate are more likely to develop various sorts of gynaecopathy. Uyghur medicine has gradually developed in areas where the above diseases have frequently occurred for quite some time.
Field interview methods
We carried out semi-structured ethnobotanical interviews with individual natives residing in the study area in the Uyghur region between March 2014 and September 2014. A total of 200 individuals (101 men, 99 women) were interviewed in five districts, including Altay, Changji, Yili, Bazhou and Hetian. In each district, we interviewed four counties. Bazhou, Hetian are relatively large area in the south of Xinjiang. The areas are multi-ethnic areas; therefore the research on the ethnic medicine has certain representative, Altay and Yili, in the north and northwest of Xinjiang, respectively. The main nationality are Uyghur and Kazak, they have a certain understanding of the research of the ethnic medicine. Changji in the east of Xinjiang, it can be representative of the people in the east of Xinjiang on the ethnic medicine research. These five areas in Xinjiang are very representative of the region.
Interviews were conducted in bazaars, houses and parks. We confirm that the field studies did not involve endangered or protected species. Additionally, no specific permissions were required for these locations because all of the locations were public, not private. After explaining the objective of our study, we asked detailed questions related to the medicinal uses of plants (Wang et al. Citation2013). People who demonstrated knowledge of plants were interviewed at least twice (Polat et al. Citation2013). The obtained information was compared with other areas and local counties to verify its accuracy. The interviewees ranged in age from 35 to 95 years, most of whom were elders. We transcribed all interviews and deposited the recordings with the Medicinal Resources Census Project Team of China (Chen et al. Citation2014).
The participants provided their verbal informed consent to participate in this study. During the survey, after explaining the objective of our study, the interviewees provided us with detailed answers to questions related to the medicinal uses of plants. We subsequently transcribed all the interviews and deposited the recordings in our storehouse. All the information on aromatic Uyghur medicinal plants was recorded in tables produced by the Resource Census Project Team of China. Written consent was collected and analyzed by the authors, and the authors used another method to express the main meaning of the participants’ consent. Therefore, all of the written consents are listed in . Of course, the Medical Ethics Committees of Xinjiang Medical University approved this consent procedure.
Table 1. Plant species used for medicinal purposes in Xinjiang, China.
The interview questions were aimed at understanding the traditional uses of medicinal plants, including local plant names, ailments for which the plants were used, the parts of the plants used, and methods of preparation and administration. We accompanied the interviewees into the field to collect specimens of the plants to which they were referred. We also deposited the plant materials collected in our study with the Medicinal Resources Census Project Team of China ().
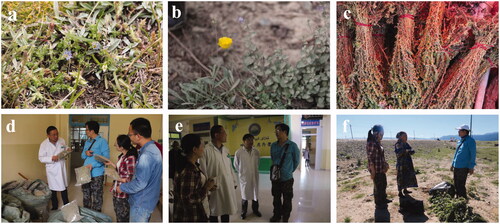
Figure 2. Aromatic Uyghur medicinal plants and field interview. (a) Gentiana scabra Bunge, (b) Papaver somniferum L., (c) commercially available Artemisia rupestris L., (d) field interview about Ziziphora clinopodioides Lam., (e) field interview about aromatic Uyghur medicinal plants in ethnological hospital, (f) field interview about Melissa officinalis L. with retired ethnological doctor.
Voucher specimen collection
To exemplify and protect the aromatic medicinal plants obtained in Xinjiang to the best extent possible, we collected voucher specimens between March and September 2014. Voucher specimens were collected and prepared under the directions of herbalists and local people, who have much experience with these aromatic Uyghur medicinal plants. The plants were identified by a research team specialized in Uyghur medicinal resources, consist of several pharmaceutical professors and several graduate students from Xinjiang Medicine University, and specimens were deposited in the Traditional Chinese Medicine Voucher Herbarium of Xinjiang Medicine University. All data were collected in a database.
Data analysis
The use value (UV), a quantitative index that indicates the relative importance of locally known species, was also calculated according to the following formula: UV = U/N, where U is the number of reported uses cited by each informant for a given species, and N refers to the total number of reports in which UV refers to the UV of a species. UVs are high when there are many reported uses for a plant, thereby indicating that the plants are actively used by local people, whereas when there are few reports related to a plant’s use, the UV approaches zero (0) (Boakye et al. Citation2015). Therefore, knowing the UV of a species may be useful in determining the reliability of the use and pharmacological features of related plants.
Results and discussion
Families and medicinal plants
A total of 86 aromatic medicinal species belonging to 36 families were included in the present study (). About 12 medicinal species belonged to Lamiaceae, which was the family with the highest percentage (13.95%) of medicinal species used by the Uyghur people, followed by Apiaceae and Rosaceae (11.63%) with 10 species, and Compositae (9.30%) with 8 species. These four families account for 46.51% of the total number of aromatic medicinal species identified. The remaining 46 species belongs to 8 other families with less than six species each, while only one species was obtained for approximately 20 families ().
Table 2. Frequency of plant species by family used for medicinal purposes in the study area.
In the analysis conducted in this study, many species collected in Xinjiang were observed to be used medicinally and were easily accessed (Liu & Shawuti Citation1985; Liu Citation1999).
Plant parts and mode of preparation
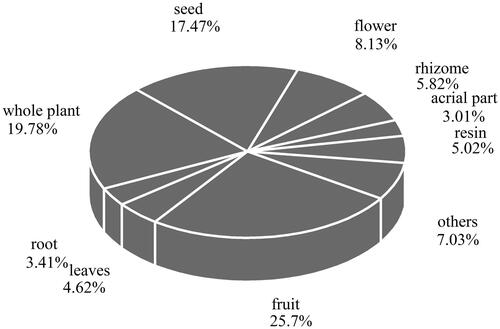
Fruits (22 species) were the most commonly used parts of the plants, followed by the whole plant (17 species), seeds (15 species) and flowers (7 species), respectively (). Additionally, for 13 species, two or more parts are used in the treatment and curing of diseases, with different parts employed for different effects. For example, the root of Ephedra presents a hidroschesis function to treat the night sweats caused by pulmonary tuberculosis and weakness of the body, while the herbaceous stem, which is also used for sweating, is applied to cure colds, coughs, bronchial asthma and malaria. Based on the above findings, we can safely draw the conclusion that different parts of the plants exhibit different functions. We must clarify the function of every part before it can be used to cure diseases (Song et al. Citation2005).
Figure 3. Frequency of aromatic Uygur medicinal plants parts used by the village people of Xinjiang.
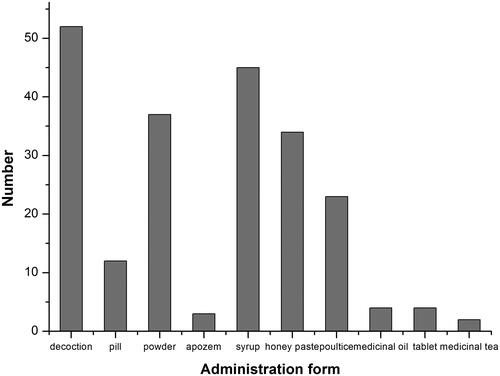
The results of our survey demonstrated that decoction was the most common mode of preparing aromatic medicinal plants, accounting for 61.72% of the recorded preparations, followed by syrups (47.66%), powders (45.31%), honey pastes (35.16%), poultices (28.13%) and pills (16.41%) (). Therefore, there are several methods for the preparation of aromatic medicinal plants (Liu et al. Citation1993). However, different methods present different efficiencies, and the most appropriate preparation method should be chosen.
Disorders treated
Based on this survey, the collected aromatic plants are widely used in local traditional Chinese medicine, specifically in Uyghur medicine, to treat gastropathy, liver complaints, parasites and dysentery. Commonly, doctors combine two or more aromatic medicinal plants to treat a particular ailment. In this survey, most of the identified aromatic medicinal plants can be employed as both medicine and food. The local population uses these plants daily to maintain good health in the long-term (Halmurat et al. Citation2011, King et al. Citation2015). Some aromatic medicinal plants can be made into healthcare products, such as herbal teas, medicinal liquors and essential oils, which contribute to health in therapies or prevention. In addition, a few of the aromatic plants can be developed into insecticides against parasites. Furthermore, some farmers cultivate aromatic vegetables with certain characteristics that are conducive to supplying the body with necessary nutrients and particular trace elements.
In our survey, plants such as lavender, saffron crocus and mint were found to be commonly used. Lavender essential oil made from lavender plants is good for nervous system disease, paralysis, amnesia, melancholia and arthralgia. Meanwhile it has anti-inflammatory and anti-bacterial functions. The lavender essential oil treatment balanced the inflammatory signaling induced by S. aureus by repressing the principal pro-inflammatory cytokines and their receptors and inducing the heme oxygenase-1 gene transcription. The essential oil can stimulate the human innate macrophage response to a bacterium, which is responsible for one of the most important nosocomial infection (Giovannini et al. Citation2016). Saffron crocus is a kind of common, traditional precious herb among local aromatic medicinal plants. Saffron crocus have anti-oxidant, analgesic, anti-inflammatory, anti-diabetic and several other properties. The kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside from saffron crocus treatment increased the level of total protein and prevented the carbon tetrachloride-induced increases in serum aspartate aminotransferase, serum alkaline phosphatase and hepatic malondialdehyde levels. And, it has protective effects against acute carbon tetrachloride-induced oxidative liver damage (Wang et al. Citation2015). Mint presents a wide range of uses; its basic pharmacology involves anti-pyretic and anti-sweating effects. Mint is both a medicinal and culinary herb, employed in mint condiments, spices, teas and so on. There were many aromatic plants identified during this survey that present unique characteristics and play specific roles in the medical community.
Intake of aromatic medicinal plants
According to the results of our study, the most common methods of application are oral and external, accounting for 72.2% of applications, while 23 of the aromatic plants can be used as oral medicines (26.8%), whereas only one plant, Nerium indicum Mill., was reported to be employed only as an externally applied drug (Qian et al. Citation2005). Under some circumstances, oral and external treatments can better cure disease.
Additional description of introduced aromatic medicinal materials
Families and plant parts
In the present study, some of the medicinal plants we investigated were not native materials. We identified 34 introduced plants, belonging to 24 families, coming from different regions, such as surrounding areas of Europe and the Mediterranean (Souza et al. Citation2014). Zingiberaceae was the family accounting for the greatest percentage of introduced medicinal materials (25.00%), followed by Rutaceae (20.83%), Lamiaceae (16.67%), Rosaceae (12.50%). Fruits (22.73%) are the most widely used part of the plant, followed by the whole plant (15.91%), roots (13.64%) and seeds (6.82%).
Remedy of aromatic plants, administration form and route
Aromatic plants are vital as remedies and in the economic development of Xinjiang. Introduced plants can be used to treat diseases such as colds, gastric diseases and asthma. The most important form of administration of these plants is decoction, similar to findings for native medicinal plants, while the oral administration route is used for every plant. Compared with the native plant species employed in Xinjiang, some introduced plants present specific functions in local use (Di Novella et al. Citation2013).
Conclusions
This study first recorded use information on aromatic plants employed in traditional Uyghur medicine in Xinjiang, demonstrating that Xinjiang possesses various raw medicinal herbs. A total of 86 kinds of aromatic plants used by local people belonging to 36 genera were identified, and these plants are still commonly used in daily life. To evaluate the value of the medicinal plants in the target region, the UV was employed in a quantitative analysis. Many plants are used to relieve coughs, eliminate phlegm in treating cardiovascular diseases, colds, haemorrhoids, constipation, stomach diseases, diabetes, urinary diseases, respiratory conditions and throat disease. Therefore, Xinjiang is an area where indigenous medicinal plants present diverse uses, and a sound dimensional medical healthcare treatment system has been developed in this region.
However, some of the traditional Uyghur medicines used in this region still lack physiotherapeutic evidence. Hence, analysis of the chemical constituents and pharmacological activities of certain Uyghur medicines are necessary to explore the potential of Uyghur medicinal plants. This study also provides protection for the local medicinal plant group. Some Uyghur medicinal plants are on the verge of extinction because of frequent natural disasters and the development of urbanization, and the UV of these plants therefore cannot be presented. Thus, the development of further strategies for the conservation of these medicinal plants should be of priority.
Acknowledgements
We present our sincere gratitude for the enthusiasm of the local people interviewed in this study. Not only did they offer us a considerable amount of information about Uyghur medicine, but they also helped us in the protection of plants. We appreciate that they helped the present research to be completed in a satisfactory way, contributing to laying the foundation for further studies in the Uyghur region of Xinjiang.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Ayatollahi H, Javan AO, Khajedaluee M, Shahroodian M, Hosseinzadeh H. 2014. Effect of Crocus sativus L.(saffron) on coagulation and anticoagulation systems in healthy volunteers. Phytother Res. 28:539–543.
- Auerbach BJ, Reynolds SJ, Lamorde M, Merry C, Kukunda-Byobona C, Ocama P, Semeere AS, Ndyanabo A, Boaz I, Kiggundu V. 2012. Traditional herbal medicine use associated with liver fibrosis in rural Rakai, Uganda. PLoS ONE. 7:1–8.
- Bagheri H, Manap MYBA, Solati Z. 2014. Antioxidant activity of Piper nigrum L. essential oil extracted by supercritical CO2 extraction and hydro-distillation. Talanta. 121:220–228.
- Bai L, Guo S, Liu Q, Cui X, Zhang X, Zhang L, Yang X, Hou M, Ho CT, Bai N. 2016. Characterization of nine polyphenols in fruits of Malus pumila Mill by high-performance liquid chromatography. J Food Drug Anal. 24:293–298.
- Bajaj YPS, Reghunath BR, Gopalakrishnan PK. 1993. Elettaria cardamomum Maton (Cardamom): aromatic compounds, in vitro culture studies, and clonal propagation. Biotech Agr Forestry. 21:32–147.
- Bhuiyan MNI, Begum J, Bhuiyan MNH. 2008. Analysis of essential oil of eaglewood tree (Aquilaria agallocha Roxb.) by gas chromatography mass spectrometry. J Pharmacol. 4:24?28.
- Boakye MK, Pietersen DW, Kotzé A, Dalton DL, Jansen R. 2015. Knowledge and uses of African pangolins as a source of traditional medicine in Ghana. PLoS One. 10:1–14.
- Cámara-Leret R, Paniagua-Zambrana N, Balslev H, Macía MJ. 2014. Ethnobotanical knowledge is vastly under-documented in northwestern South America. PLoS One. 9:1–8.
- Chan SC, Chang YS, Wang JP, Chen SC, Kuo SC. 1998. Three new flavonoids and antiallergic, anti-inflammatory constituents from the heartwood of Dalbergia odorifera. Planta Med. 64:153–158.
- Chen G, Zhang J, Guo Y. 2004. Analysis of volatile components of fresh Perilla frutescens (L.) Britt. var. acuta (Thunb.) Kudo by headspace GC/MS. J Essent Oil Res. 16:435–436.
- Chen L, Yin H, Lan Z, Ma S, Zhang C, Yang Z, Li P, Lin B. 2011. Anti-hyperuricemic and nephroprotective effects of Smilax china L. J Ethnopharmacol. 135:399–405.
- Chen W, Lewith G, Wang LQ, Ren J, Xiong WJ, Lu F, Liu JP. 2014. Chinese proprietary herbal medicine listed in 'China National Essential Drug List' for common cold: a systematic literature review. PLoS One. 9:e110560.
- Dalai M, Bhadra KS, Chaudhary SK, Bandyopadhyay A, Mukherjee PK. 2014. Anti-cholinesterase activity of the standardized extract of Syzygium aromaticum L. Pharmacog Mag. 10:276–282.
- Dey P, Chaudhuri TK. 2013. Pharmacological aspects of Nerium indicum Mill: a comprehensive review. Pharmacogn Rev. 8:156–162.
- Dhima K, Vasilakoglou I, Garane V, Ritzoulis C, Lianopoulou V, Panou-Philotheou E. 2010. Competitiveness and essential oil phytotoxicity of seven annual aromatic plants. Weed Sci. 58:457–465.
- Di Novella R, Di Novella N, De Martino L, Mancini E, De Feo V. 2013. Traditional plant use in the National Park of Cilento and Vallo di Diano, Campania, Southern, Italy. J Ethnopharmacol. 145:328–342.
- Ding W, Yang T, Liu F, Tian S. 2014. Effect of different growth stages of Ziziphora clinopodioides Lam. on its chemical composition. Pharmacog Mag. 10:1–5.
- do Nascimento Silva MK., De Alencar Carvalho VR., Ferreira Matias EF. 2016. Chemical Profile of Essential oil of Ocimum gratissimum L. and Evaluation of Antibacterial and Drug Resistance-modifying Activity by Gaseous Contact Method. Pharmacogn J. 8:4–9.
- Dũng NX, Cu LD, Thái NH, Moi LD, Van Hac L, Leclercq PA. 1996. Constituents of the leaf and flower oils of Agastache rugosa (Fisch. et Mey) O. Kuntze from Vietnam. J Essent Oil Res. 8:135–138.
- Ebrahimabadi EH, Ghoreishi SM, Masoum S, Ebrahimabadi AH. 2016. Combination of GC/FID/Mass spectrometry fingerprints and multivariate calibration techniques for recognition of antimicrobial constituents of Myrtus communis L. essential oil. J Chromatogr B. 1008:50–57.
- Farzaei MH, Bahramsoltani R, Abbasabadi Z, Rahimi R. 2015. A comprehensive review on phytochemical and pharmacological aspects of Elaeagnus angustifolia L. J Pharm Pharmacol. 67:1467–1480.
- Fred-Jaiyesimi A, Ajibesin KK, Tolulope O, Gbemisola O. 2015. Ethnobotanical studies of folklore phytocosmetics of South West Nigeria. Pharm Biol. 53:313–318.
- Ge JF, Wang TY, Zhao B, Lv X, Jin Y, Peng L, Yu S, Li J. 2009. Anti-inflammatory effect of triterpenoic acids of Eriobotrya japonica (Thunb.) Lindl. leaf on rat model of chronic bronchitis. Am J Chin Med. 37:309–321.
- Ghanemi A, Boubertakh B. 2015. Shorter and sturdier bridges between traditional Chinese medicines and modern pharmacology. Saudi Pharm J. 23:330–332.
- Giovannini D, Gismondi A, Basso A, Canuti L, Braglia R, Canini A, Mariani F, Cappelli G. 2016. Lavandula angustifolia Mill. essential oil exerts antibacterial and anti-inflammatory effect in macrophage mediated immune response to staphylococcus aureus. Immunol Invest. 45:11–28.
- Gonçalves S, Romano A. 2013. In vitro culture of lavenders (Lavandula spp.) and the production of secondary metabolites. Biotechnol Adv. 31:166–174.
- Gong H, Zhang BK, Yan M, Fang PF, Li HD, Hu CP, Yang Y, Cao P, Jiang P, Fan XR. 2015. A protective mechanism of licorice (Glycyrrhiza uralensis): isoliquiritigenin stimulates detoxification system via Nrf2 activation. J Ethnopharmacol. 162:134–139.
- Govindarajan M, Sivakumar R, Rajeswary M, Yogalakshmi K. 2013. Chemical composition and larvicidal activity of essential oil from Ocimum basilicum (L.) against Culex tritaeniorhynchus, Aedes albopictus and Anopheles subpictus (Diptera: Culicidae). Exp Parasitol. 134:7–11.
- Goyal S, Gupta N, Chatterjee S. 2016. Investigating therapeutic potential of Trigonella foenum-graecum L. as our defense mechanism against several human diseases. J toxicol. 2016:1–10.
- Halmurat U, Nurmuhammat A, Liu WX. 2011. Greek medicine, Islamic medicine, Chinese traditional medicine and Uyghur medicine. Xinjiang: Xinjiang Medical University Publishing House; p. 490–495.
- Han W, Xu JD, Wei FX, Zheng YD, Ma JZ, Xu XD, Wei ZG, Wang W, Zhang YC. 2015. Prokinetic activity of Prunus Persica (L.) Batsch flowers extract and its possible mechanism of action in rats. BioMed Res Int. 2015:1–10.
- Hajlaoui H, Mighri H, Aouni M, Gharsallah N, Kadri A. 2016. Chemical composition and in vitro evaluation of antioxidant, antimicrobial, cytotoxicity and anti-acetylcholinesterase properties of Tunisian Origanum majorana L. essential oil. Microb Pathog. 95:86–94.
- Harzallah A, Bhouri AM, Amri Z, Soltana H, Hammami M. 2016. Phytochemical content and antioxidant activity of different fruit parts juices of three figs (Ficus carica L.) varieties grown in Tunisia. Ind Crops Prod. 83:255–267.
- Heeba GH, Abd-Elghany MI. 2010. Effect of combined administration of ginger (Zingiber officinale Roscoe) and atorvastatin on the liver of rats. Phytomedicine. 17:1076–1081.
- Huang SH, Agrawal DC, Wu FS, Tsay HS. (2014). In vitro propagation of Gentiana scabra Bunge–an important medicinal plant in the Chinese system of medicines. Bot Stud. 55:1–7.
- Ji TF, Yang JB, Song WX, Wang AG, Su YL, Yuan L. 2007. Studies on chemical constituents of Artemisia rupestris (II). Zhongguo Zhongyao Zazhi. 32:1187–1189.
- Jiang Y, Nie WJ. 2015. Chemical properties in fruits of mulberry species from the Xinjiang province of China. Food Chem. 174:460–466.
- Jirovetz L, Wlcek K, Buchbauer G, Stoilova I, Atanasova T, Stoyanova A, Krastanov A, Schmidt E. 2009. Chemical composition, olfactory evaluation and antioxidant effects of essential oil from Mentha canadensis. Nat Prod Commun. 4:1011–1016.
- King SC, Snow J, Meiselmanc HL, Sainsburyd J, Carre BT, McCaffertya D, Serranoa D, Gillettea M, Millarda L, Lia Q. 2015. Development of a questionnaire to measure consumer wellness associated with foods: the WellSense ProfileTM. Food Quality and Preference. 39:82–94.
- Ladan Moghadam AR. 2016. Chemical Composition and Antioxidant Activity Cuminum cyminum L. Essential Oils. Int J Food Prop. 19:438–442.
- Lata S, Saxena KK, Bhasin V, Saxena RS, Kumar A, Srivastava VK. 1991. Beneficial effects of Allium sativum, Allium cepa and Commiphora mukul on experimental hyperlipidemia and atherosclerosis-a comparative evaluation. J Postgrad Med. 37:132–135.
- Lee HH, Ahn JH, Kwon AR, Lee ES, Kwak JH, Min YH. 2014. Chemical composition and antimicrobial activity of the essential oil of apricot seed. Phytother Res. 28:1867–1872.
- Li C, Son HJ, Huang C, Lee SK, Lohakare J, Wang MH. 2010. Comparison of Crataegus pinnatifida Bunge var. typica Schneider and C. pinnatifida Bunge fruits for antioxidant, anti-α-glucosidase, and anti-inflammatory activities. Food Sci Biotechnol. 19:769–775.
- Li S, Long C, Liu F, Lee S, Guo Q, Li R, Liu Y. 2006. Herbs for medicinal baths among the traditional Yao communities of China. J Ethnopharmacol. 108:59–67.
- Li X, Wang Y, Zhu J, Xiao Q. 2011. Essential oil composition analysis of three cultivars seeds of Resina ferulae from Xinjiang, China. Pharmacogn mag. 7:116–120.
- Li GY, Zheng YX, Sun FZ, Huang J, Lou MM, Gu JK, Wang JH. 2015. In silico analysis and experimental validation of active compounds from Cichorium intybus L. ameliorating liver injury. Int J Mol Sci. 16:22190–22204.
- Lima EBC, Sousa CNS, Meneses LN, Ximenes NC, Júnior S, Vasconcelos GS, Lima NBC, Patrocínio MCA, Macedo D, Vasconcelos SMM. 2015. Cocos nucifera (L.)(Arecaceae): a phytochemical and pharmacological review. Braz J Med Biol Res. 48:953–964.
- Liu T, Zhao J, Ma L, Ding Y, Su D. 2012. Hepatoprotective effects of total triterpenoids and total flavonoids from Vitis vinifera L against immunological liver injury in mice. J Evid Based Complement Altern Med. 2012:1–8.
- Liu W, Yin D, Liu J, Li N. 2014. Genetic diversity and structure of Sinopodophyllum hexandrum (Royle) Ying in the Qinling Mountains, China. PLoS One. 9:10-e110500.
- Liu YM. 1999. Pharmacography of Uyghur (Volume Two), Xinjiang: Xinjiang Science & Technology & Hygiene Publishing House; p. 1–195.
- Liu YM, Cheng LY, Sun DJ, Wu YX, Wu SC, Chen XY, Zhang YF, Ma XY, Wang KY, Bi ZX, et al. (1993). The standard of medicinal material of Uighur (Volume One). Xinjiang: Xinjiang Science & Technology & Hygiene Publishing House. p. 1–160.
- Liu YM, Sha Wu Ti Yi Ke Mu. 1985. Pharmacography of Uyghur, Xinjiang: Xinjiang People’s Publishing House; p. 1–124.
- Maimaitiyiming D, Hu G, Aikemu A, Hui SW, Zhang X. 2014. The treatment of Uygur medicine Dracocephalum moldavica L on chronic mountain sickness rat model. Pharmacogn Mag. 10:477–482.
- Mendoza PI, Muñoz BJ, Arrillaga I, Segura J. 2014. Deoxyxylulose 5-phosphate reductoisomerase is not a rate-determining enzyme for essential oil production in spike lavender. J Plant Physiology. 171:1564–1570.
- Menichini F, Loizzo MR, Bonesi M, Conforti F, De Luca D, Statti GA, de Cindio B, Menichini F, Tundis R. 2011. Phytochemical profile, antioxidant, anti-inflammatory and hypoglycemic potential of hydroalcoholic extracts from Citrus medica L. cv Diamante flowers, leaves and fruits at two maturity stages. Food Chem Toxicol. 49:1549–1555.
- Muruzović MŽ, Mladenović KG, Stefanović OD, Vasić SM, Čomić LR. 2016. Extracts of Agrimonia eupatoria L. as sources of biologically active compounds and evaluation of their antioxidant, antimicrobial, and antibiofilm activities. J Food Drug Anal. 24:539–547.
- Nigam MC, Nigam IC, Handa KL, Levi L. 1965. Essential oils and their constituents XXVIII. Examination of oil of cardamom by gas chromatography. J Pharm Sci. 54:799–801.
- Olatunji OJ, Chen H, Zhou Y. 2015. Anti-ulcerogenic properties of Lycium chinense Mill extracts against ethanol-induced acute gastric lesion in animal models and its active constituents. Molecules. 20:22553–22564.
- Ooi LS, Li Y, Kam SL, Wang H, Wong EY, Ooi VE. 2006. Antimicrobial activities of cinnamon oil and cinnamaldehyde from the Chinese medicinal herb Cinnamomum cassia Blume. Am J Chin Med. 34:511–522.
- Parveen Z, Nawaz S, Siddique S, Shahzad K. 2013. Composition and antimicrobial activity of the essential oil from leaves of Curcuma longa L. Kasur variety. Ind J Pharm Sci. 75:117–122.
- Pastorova I, De Koster CG, Boon JJ. 1997. Analytical study of free and ester bound benzoic and cinnamic acids of gum benzoin resins by GC–MS and HPLC-frit FAB-MS. Phytochem Anal. 8:63–73.
- Paul BD, Dreka C, Knight ES, Smith ML. 1996. Gas chromatographic/mass spectrometric detection of narcotine, papaverine, and thebaine in seeds of Papaver somniferum. Planta Med. 62:544–547.
- Pei Y, Wang S, Wang W, Zhou Y, Zhao H, Jia L. 2014. Isolation and structure-activity relationship of the antioxidant chemical constituents from the flowers of Rosa chinensis Jacq. Int J Food Properties. 17:38–44.
- Phaechamud T, Toprasri P, Chinpaisal C. 2009. Antioxidant activity Areca catechu extracts in human hepatocarcinoma HepG2 cell lines. Pharm Biol. 47:242–247.
- Polat R, Cakilcioglu U, Satıl F. 2013. Traditional uses of medicinal plants in Solhan (Bingöl-Turkey). J Ethnopharmacol. 148:951–963.
- Prakash B, Mishra PK, Kedia A, Dubey NK. 2014. Antifungal, antiaflatoxin and antioxidant potential of chemically characterized Boswellia carterii Birdw essential oil and its in vivo practical applicability in preservation of Piper nigrum L. fruits. LWT-Food Sci Technol. 56:240–247.
- Qian XZ, Huang JS, Chen HF, Ji ZP, Guo ZH, Wu JP, Tu TJ, Shi MX, Zhao XW, Feng G, et al. 2005. Chinese medical encyclopedia (Uyghur Traditional Medicine). Shanghai: Shanghai Scientific and Technical Publishers. p. 177–308.
- Rahim H, Khan MA, Sadiq A, Khan S, Chishti KA, Rahman IU. 2015. Comparative studies of binding potential of Prunus armeniaca and Prunus domestica gums in tablets formulations. Pak J Pharm Sci. 28:909–914.
- Rahimi R, Ardekani MRS. 2013. Medicinal properties of Foeniculum vulgare Mill. in traditional Iranian medicine and modern phytotherapy. Chin J Integr Med. 19:73–79.
- Rajesh Kumar J. 2013. Volatile composition and antimicrobial activity of the essential oil of Artemisia absinthium growing in Western Ghats region of North West Karnataka, India. Pharm Biol. 51:888–892.
- Ratheesh M, Shyni GL, Sindhu G, Helen A. 2011. Inhibitory effect of Ruta graveolens L. on oxidative damage, inflammation and aortic pathology in hypercholesteromic rats. Exp Toxicol Pathol. 63:285–290.
- Rokbeni N, M'Rabet Y, Dziri S, Chaabane H, Jemli M, Fernandez X, Boulila A. 2013. Variation of the chemical composition and antimicrobial activity of the essential oils of natural populations of Tunisian Daucus carota L. (Apiaceae). Chem Biodivers. 10:2278–2290.
- Rong R, Cui MY, Zhang QL, Zhang MY, Yu YM, Zhou XY, Yu ZG, Zhao YL. 2016. Anesthetic constituents of Zanthoxylum bungeanum Maxim. pharmacokinetic study. J Sep Sci. 14:2728–2735.
- Ru W, Wang D, Xu Y, He X, Sun YE, Qian L, Zhou X, Qin Y. 2015. Chemical constituents and bioactivities of Panax ginseng (CA Mey.). Drug Discov Ther. 9:23–32.
- Sadati SN, Ardekani MRS, Ebadi N, Yakhchali M, Dana AR, Masoomi F, Khanavi M, Ramezany F. 2016. Review of scientific evidence of medicinal convoy plants in traditional Persian medicine. Pharmacogn Rev. 10:33.
- Sahebkar A, Iranshahi M. 2011. Volatile constituents of the genus Ferula (Apiaceae): a review. J Essent Oil Bear Pl. 14:504–531.
- Samojlik I, Mijatović V, Petković S, Škrbić B, Božin B. 2012. The influence of essential oil of aniseed (Pimpinella anisum L.) on drug effects on the central nervous system. Fitoterapia. 83:1466–73.
- Seo CS, Lim HS, Jeong SJ, Shin HK. 2015. Anti-allergic effects of sesquiterpene lactones from the root of Aucklandia lappa Decne. Mol Med Rep. 12:7789–7795.
- Settanni L, Randazzo W, Palazzolo E, Moschetti M, Aleo A, Guarrasi V, Mammina C, San Biagio PL, Marra FP, Moschetti G, Germanà MA. 2014. Seasonal variations of antimicrobial activity and chemical composition of essential oils extracted from three Citrus limon L. Burm. cultivars. Nat Prod Res. 28:383–391.
- Shakeri A, Sahebkar A, Javadi B. 2016. Melissa officinalis L: a review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 188:204–228.
- Shang X, Tao C, Miao X, Wang D, Wang Y, Yang Y, Pan H. 2012. Ethno-veterinary survey of medicinal plants in Ruoergai region, Sichuan province, China. J Ethnopharmacol. 142:390–400.
- Shin JS, Ryu S, Jang DS, et al. (2016). Amomum tsao-ko fruit extract suppresses lipopolysaccharide-induced inducible nitric oxide synthase by inducing heme oxygenase-1 in macrophages and in septic mice. Int J Exp Pathol. 96:395–405.
- Song LR, Hu L, Hong X, Wang JH, Yin L, Liu XH, Xu HQ, Li Y, Chen RS, Hang AW, et al. (2005). Chinese material medica (Uyghur Medicine Volume), Shanghai: Shanghai Scientific and Technical Publishers. p. 45–409.
- Souza RKD, da Silva MAP, de Menezes IRA, Ribeiro DA, Bezerra LR, de Almeida Souza MM. 2014. Ethnopharmacology of medicinal plants of carrasco, northeastern Brazil. J Ethnopharmacol. 157:99–104.
- Tian J, Ban X, Zeng H, He J, Chen Y, Wang Y. 2012. The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PloS One. 7:e30147.
- Tian S, Yu Q, Wang D, Upur H. 2012. Development of a rapid resolution liquid chromatography-diode array detector method for the determination of three compounds in Ziziphora clinopodioides Lam from different origins of Xinjiang. Pharmacog Mag. 8:280–284.
- Vandebroek I, Balick MJ. 2012. Globalization and loss of plant knowledge: challenging the paradigm. PLoS One. 7:5–e37643.
- Tolkachev ON, Abizov EA, Abizova EV, Mal’tsev SD. 2008. Phytochemical study of the bark of some plants of the Elaeagnaceae family as a natural source of β-carboline indole alkaloids. Pharm Chem J. 42:630–632.
- Vohora SB, Shah SA, Dandiya PC. 1990. Central nervous system studies on an ethanol extract of Acorus calamus rhizomes. J Ethanopharmacol. 28:53–62.
- Wu-bao W, Han-kui W, Hang B, Akber Aisa H. 2005. Flavonoids in Sabina vulgaris antoine. Chem Nat Compd. 41:473–474.
- Wang QS, Gao T, Cui YL, Gao LN, Jiang HL. 2014. Comparative studies of paeoniflorin and albiflorin from Paeonia lactiflora on anti-inflammatory activities. Pharm Biol. 52:1189–1195.
- Wang T, Guo R, Zhou G, Zhou X, Kou Z, Sui F, Li C, Tang L, Wang Z. 2016. Traditional uses, botany, phytochemistry, pharmacology and toxicology of Panax notoginseng (Burk.) FH Chen: a review. J Ethnopharmacol. 188:234–258.
- Wang W, Wang H, Zhang Y, Zu Y. 2013. In vitro antioxidant and antimicrobial activity of anthotaxy extracts from Dendranthema morifolium (Ramat.) Tzvel. and Chrysanthemum indicum L. J Med Plants Res. 7:2657–2661.
- Wang Y, Tang C, Zhang H. 2015. Hepatoprotective effects of kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside from Carthamus tinctorius L. on CCl4-induced oxidative liver injury in mice. J Food Drug Anal. 23:310–317.
- Wang YM, Ren AX, Xiao YH, Pan CX, Cui SM, Yu MG, Ma LX, Bai Y, Han FY, He JM, et al. 2013. Medicinal and aromatic plants. Beijing: Science Publishing House; p. 16–302.
- Wenqiang G, Shufen L, Ruixiang Y, Yanfeng H. 2006. Comparison of composition and antifungal activity of Artemisia argyi Levl. et Vant inflorescence essential oil extracted by hydrodistillation and supercritical carbon dioxide. Nat Prod Res. 20:992–998.
- Yin Q, Xu XJ, Hu GM, Liang XH, Han L. 2011. The analysis of electrolyte and the relative factors of hypertension among Uyghur people in Hetian, Xinjiang. Int J Cardiol. 152:S39.
- Yildiz H. 2016. Chemical composition, antimicrobial, and antioxidant activities of essential oil and ethanol extract of Coriandrum sativum L. leaves from Turkey. Int J Food Prop. 19:1593–1603.
- Yoshizawa C, Kitade M, Mikage M. 2004. Herbological studies on Chinese crude drug Ma-huang. Part 1-On the botanical origin of Ma-huang in ancient China and the origin of Japanese Ma-huang. Yakushigaku Zasshi. 40:107–116.
- Youdim KA, Deans SG. 1999. Dietary supplementation of thyme (Thymus vulgaris L.) essential oil during the lifetime of the rat: its effects on the antioxidant status in liver, kidney and heart tissues. Mech Ageing Dev. 109:163–175.
- Zhang LL, Tian K, Tang ZH, Chen XJ, Bian ZX, Wang YT, Lu JJ. 2016. Phytochemistry and pharmacology of Carthamus tinctorius L. Am J Chin Med. 44:197–226.
- Zhang W, Zhang Y, Yuan X, Sun E. 2015. Determination of Volatile Compounds of Illicium verum Hook. f. Using simultaneous distillation-extraction and solid phase microextraction coupled with gas chromatography-mass spectrometry. Trop J Pharm Res. 14:1879–1884.
- Zarai Z, Chobba IB, Mansour RB, Békir A, Gharsallah N, Kadri A. 2012. Essential oil of the leaves of Ricinus communis L.: in vitro cytotoxicity and antimicrobial properties. Lipids Health Dis. 11:102.
- Zheng Y, Xie Z, Jiang L, Shimizu H, Drake S. 2006. Changes in holdridge life zone diversity in the Xinjiang Uyghur Autonomous Region (XUAR) of China over the past 40 years. J Arid Environ. 66:113–126.
- Zia-Ul-Haq M, Shahid SA, Ahmad S, Qayum M, Khan I. 2012. Antioxidant potential of various parts of Ferula assafoetida L. J Med Plants Res. 6:3254–3258.