?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Context: Nothing could be found in the literature concerning Cinnamomum glanduliferum (Wall) Meissn (Lauraceae) bark (CG) in Egypt.
Objective: To investigate CG volatile oil chemically and its anti-inflammatory and gastroprotective effects.
Materials and methods: Essential oils were investigated by GC-MS. Leaves oil was assessed at doses of 250, 500 and 1000 mg/kg for its anti-inflammatory effect against carrageenan-induced rat oedema model. Serum inflammation markers were measured. The gastro-protective effect of the same doses of the volatile oil was also tested in ethanol-induced non-ulcerative gastritis model in rats. Stomach oxidative stress markers were examined following 1 h after intragastric ethanol administration.
Results: Twenty-five and 20 compounds were identified from leaf and branch oils, respectively (98.85 and 99.13%). The major ones were: eucalyptol (59.44%; 55.74%), sabinene (14.99%; 7.12%), α-terpineol (6.44%; 9.81%), α-pinene (5.27%; 4.71%). Following 4 h of treatment leaves volatile oil at doses of 250, 500 and 1000 mg/kg significantly reduced paw volume to 94, 82 and 69%, respectively. The same doses significantly reduced COX-2 activity to 73.8, 50.7 and 21.4 nmol/min/mL, respectively. A significant reduction of PGE2 concentration was observed (2.95 ± 0.2, 2.45 ± 0.15 and 1.75 ± 0.015 pg/mL). CG oil exhibited a significant modulatory effect on ethanol-induced gastritis in rats as the level of NO reduced to 32, 37 and 41 μM nitrate/g and also a significant inhibition of lipid peroxidation was observed via reduction of MDA concentration (1.15, 1.11 and 1.04 nmol/g).
Conclusion: CG volatile oil exhibited an anti-inflammatory effect and protected against ethanol-induced non-ulcerative gastritis.
Introduction
Benefits of essential oil stem from their reported medicinal uses as antioxidant, antimicrobial and anti-inflammatory properties (Eldahshan Citation2015; Salleh et al. Citation2015; Eldahshan & Halim Citation2016; Omri Hichri et al. Citation2016). Cinnamomum (Lauraceae) is a genus of evergreen shrubs and trees. It contains over 250 species, distributed in tropical and subtropical regions of America, Asia, Oceania, and Australasia (Krikorian Citation1992; Kirtikar & Basu Citation2000). Some Leaves of Cinnamomum species worldwide have been subjected to volatile oil analysis, where their volatile oil constituents revealed different compositional patterns. For example, Cinnamomum tamala T. Nees & Eberm. from India, the major components in hydrodistilled oil are trans-sabinene hydrate, (Z)-β-ocimene, and germacrene A (Mir et al. Citation2004). Several chemotypes of Cinnamomum tamala volatile oil in Uttarakhand showed that linalool, 1,8-cineole, cinnamyl acetate, E-cinnamaldehyde and eugenol were in major quantities (Agrawal et al. Citation2012).
The volatile oil of Cinnamomum zeylanicum Blume (Lamiaceae) leaves of Pantnagar contains linalool, E-cinnamaldehyde and E-cinnamyl acetate. While the essential oil of C. zeylanicum, in South Indian, showed dominance of eugenol (Agrawal et al. Citation2012). Indian C. zeylanicum buds, revealed that α-bergamotene and α-copaene are the major constitutes (Jayaprakasha et al. Citation2002).
Analysis of essential oil of Cinnamomum camphora (L.) J. Presl, from Uttarakhand, revealed the presence of a camphor as a single major constituent (Agrawal et al. Citation2012). Meanwhile, GC analysis of essential oil of Cinnamomum camphora cultivated at Medicinal Plant Resources and Development Centre (MRDC) at Uttarakhand, India, revealed the presence of a camphor as a single major constituent (Agrawal et al. Citation2012). The major components present in the Fijian C. verum J. Presl leaf volatile oils were eugenol, (E)-caryophyllene and linalool in the Indian sample (Patel et al. Citation2007). On the other hand, major compound in Cinnamomum pauciflorum Nees, leaves essential oil in northeast India was (E)-cinnamaldehyde (Nath et al. Citation2006).
Cinnamomum glanduliferum is large-sized evergreen tree species native to the tropical Himalayan regions of India and Nepal. In northern India, It occurs naturally in both the hills and plains up to an altitude of 1200 m and is known as ‘Gondsoroi tree.’ Almost all parts of the plants are aromatic (Finnemore Citation1926). In India, 2 chemotypes C. glanduliferum leaves were identified; the 1st could be characterized by the dominance of (E)-nerolidol, followed by caryophyllene oxide (Baruah & Nath Citation2006). The 2nd chemotype oils of C. glanduliferum, contains cineole as the major component (Chowdhury Citation1999). The leaves were used as carminative and stimulant (Pullaiah Citation2006).
Carrageenan-induced oedema model was used in this study to evaluate the potential anti-inflammatory effect of CG oil. Oedema due to carrageenan injection is presumed to be through inflammatory mediators that increase vascular permeability and/or increase blood flow (Ialenti et al. Citation1992). On the other hand, ethanol-induced gastric ulcer and gastritis is a widely used experimental model for evaluation of gastroprotective activity. Ethanol is well known as a damaging agent to the stomach that acts by a direct necrotizing action, which in turn decreases bicarbonate secretion and mucus production. Thus, the ethanol-induced gastric damage may be related to the generation of reactive species, reduced cell proliferation, and an exacerbated inflammatory response (Amaral et al. Citation2013).
In a previous study, C. glanduliferum bark volatile oil in Egypt was subjected to GC-MS analysis (Taha & Eldahshan Citation2017) but leaf and stem have not been studied for yet. Thus our aim in this study was phyto-investigation of its volatile oil (leaves and green branches) as well as the anti-inflammatory and gastroprotective effects of the volatile oil in leaves.
Materials and methods
Plant material
Cinnamomum glanduliferum, leaves and green branches, were collected from Al-Zohria Garden, Cairo, Egypt in February 2014. The species was authenticated by Dr. Usama K. Abdel Hameed, Department of Botany, Faculty of Science, Ain Shams University, Cairo, Egypt. A voucher specimen (voucher specimen number; PHG-P-CG-152) is deposited at Pharmacognosy Department, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt.
Isolation of volatile oils
The C. glanduliferum plant material was chopped into small pieces. The essential oil was isolated from each part by hydro-distillation (5 h) using a Clevenger-type all glass apparatus. The oil was transferred to a screw-capped glass vial, dried over anhydrous sodium sulphate and stored at 4 °C in the dark.
Analysis of volatile oils by GC and GC-MS
GC analysis was carried out using a GC HP 5890 Hewlett Packard equipped with FID and HP-5 fused silica capillary column (30 m × 0.25 mm i.d., film thickness 0.25 μm). Sample volume; 0.03 μL. Oven temperature was programed from 60 °C to 240 °C at 3 °C/min; injector temperature, 250 °C; detector temperature, 280 °C; carrier gas, helium (1.0 mL/min); automatic sample injection, 0.02 μL of the oil; split: 1/70. The relative proportions of the essential oil constituents were expressed as percentages obtained by peak area normalization. GC–MS analysis was performed on a Perkin-Elmer quadrupole MS system (Model 5) coupled with the GC HP 5972, equipped with a HP-5 capillary column. Oven temperature was programed from 45 °C to 240 °C at 3 °C/min; injector temperature, 250 °C; carrier gas, helium (0.5 mL/min); automatic sample injection, 0.02 μL of the oil; split: 1/70. The MS operating parameters were: interface temperature: 300 °C, ion source temperature: 200 °C, EI mode: 70 eV, scan range: 41–400 amu.
Compounds identification
Mass spectra of the individual GC peaks were identified by a computer search of the commercial libraries (WILEY, NIST). The identification was further confirmed by the calculation of the retention indices (RI) relative to (C6-C22) n-alkanes (Adams Citation2007).
Chemicals
Indomethacin was purchased from Sigma-Aldrich (St. Louis, MO). Inflammatory-grade carrageenan was purchased from FMC (Rockland, ME). Ethanol was obtained from El-Nasr chemical Co., Egypt. PGE2 ELISA (Abnova Co., Walnut, CA) and COX-2 activity colorimetric assay kits were purchased from Cayman Co. (Ann Arbor, MI). All other chemicals used were of analytical grade.
Animals
In this study, 60 male Wistar albino rats, with a mean weight of 150–170 g were obtained from the animal house of the National Central Institute; Dokki, Cairo, Egypt and allowed to acclimatize to their environment for 1 week before the experiment. The rats were housed in stainless-steel cages (eight animals per cage) and kept on 12 h light/dark cycle and constant environmental conditions. The rats were allowed to free access to water and food (fed on standard pellet). All efforts were made to minimize animal pain or suffering during experimentation. The study was conducted according to regulations of the ethics committee of the National Research Centre which gave its consent in accordance with the National Regulations on Animal Welfare and Institutional Animal Ethical Committee.
Determination of median lethal dose (LD50)
Five groups of six rats each received volatile oil in doses ranging from 1 to 4 g/kg body weight. The toxic symptoms, mortality rate, and postmortem findings in each group were recorded 24 h after administration. The LD50 of the tested extract was calculated according to the following formula:
where, Dm means the largest dose that kills all animals; z means the mean of dead animals between two successive groups; d means the constant factor between two successive doses; N means the number of animals in each group; ∑ means the sum of z × d. One fifth, one tenth and one twentieth of the maximum dose (5 g/kg body weight) of the plant extract that did not cause mortalities or toxic symptoms in rats were chosen to be used for the biological investigation throughout the study.
Study groups of acute anti-inflammatory test
Animals were divided into five groups (six rats each). The first group served as control and received normal saline. The second group was administered with indomethacin (10 mg/kg p.o.) as the standard anti-inflammatory drug. The third, fourth and fifth groups received the volatile oil at doses of 250, 500 and 1000 mg/kg body weight p.o., respectively. One hour after the oral administration of the extract, all the animals were injected with 0.1 mL of 1% (v/v) carrageenan solution in saline subcutaneously at the sub-planter area of the right hind paw. The paw volume of each rat was measured using planimeter before carrageenan injection and then followed by hourly measurement up to 4 h post carrageenan administration. The percent change in paw volume compared to base line measurement was taken as the criteria of comparison and was calculated as follows; where, Vo is the paw volume before carrageenan injection (mL); Vt is the paw volume at t hour after carrageenan injection (mL):
Percent of inhibition of paw oedema was also calculated as follows; where, Ec is the oedema of control group; Et is the oedema of volatile oil-treated group:
Examination of serum COX-2 and PGE2 level
COX-2 was assessed using a readymade colorimetric kit (Cayman co., Ann Arbor, MI) and the results were expressed as nmole/min/mL. PGE2 was also measured by ELISA kit (Abnova Co., Walnut, CA), and the results were expressed as pg/mL.
Induction of gastritis in rats
Animals were divided into five groups (six rats each). One group received saline as control; the second group received famotidine (50 mg/kg) and the three remaining groups received the volatile oil at doses of 250, 500 and 1000 mg/kg body weight p.o., respectively. One hour later, gastric lesion was induced in rats by intragastric administration of 1 mL ethanol (99% v/v) to rats that had been fasted for 18 h with access to water ad lib in a slight modification to the method described by Al-Shabanah (Citation1997).
Rats were sacrificed one hour after ethanol administration by cervical dislocation after being lightly anesthetized with ether. Stomach of experimental rats was excised, washed with saline, and were used for histopathological examination and biochemical studies.
Gastritis-histopathological evaluation
The collected stomach specimens were immersed directly into 10% neutral buffered formalin for 4 weeks. The formalin-preserved samples were continuously transferred to freshly prepared fixative every week. Fixed samples were briefly rinsed into 70% ethanol for 24 h, then dehydrated through a graded series of ethanol (75, 80, 90, 95%, absolute alcohol I, II and III) at 4 h intervals, cleared in three changes of xylene (2 h each), then embedded in paraffin wax (melting point 60 °C) forming paraffin blocks. The specimens were serially sectioned at 5–7 μm thickness. The prepared sections were stained using Harris heamatoxylin and Eosin (H & E). The severity of histopathological alteration in stomach samples of different experimental rat groups were scored by an independent observer.
Evaluation of nitric oxide content
Nitric oxide produced in gastric tissue samples was evaluated spectrophotometrically using the method of Miranda et al. (Citation2001). Total NO tissue content was calculated based on a standard curve constructed using sodium nitrate and was expressed as μM nitrate/g tissue.
Assessment of lipid peroxidation
Stomach samples were stored immediately at −20 °C until analysis. Tissue samples were homogenized in 1 mL of 10 mmol/L Tris-HCl buffer of pH 7.1 and homogenate was used for further biochemical analysis. The stomach lipid peroxidation was evaluated by measurement of gastric MDA content according to Mihara and Uchiyama (Citation1978).
Statistical analysis
All data are expressed as mean ± standard error of the mean (S.E.M.) of six rats per experimental group. Statistical analysis was performed using Instat 3.06 statistical software package. Parametric oneway analysis of variance (ANOVA) followed by Tukey–Kramer Multiple Comparisons Test was used to compare the mean values of quantitative variables among the groups. The minimal level of significance was identified at p < 0.05.
Results
Chemical composition of essential oils
revealed qualitative similarities between both oils but they are quantitatively different. The yield of leaves oil was 1.00% v/w while that of branches was 0.1% v/w. Twenty-five compounds were identified from leaves oil (98.85% of the total detected components). The major constituents of the oil were eucalyptol (59.44%) followed by sabinene (14.99%), α-terpineol (6.44%), α-pinene (5.27%), β-pinene (3.75%). Terpine-4-ol and α-humulene were present in considerable amount. trans-Sabinene hydrate was not detected in branches. Some sesquiterpenes were present in low amounts in leaves oil and not detected in the oil of branches as β-elemene, germacrene D, germacrene B, spathulenol and globulol.
Table 1. Essential oil composition of Cinnamomum glanduliferum leaf and green branches.
Oil of branches is pale yellow in colour. Twenty peaks were identified which represent 99.13% of the total detected constituents. Eucalyptol represents also the major peak (55.74%) followed by α-terpineol (9.81%), sabinene (7.12%), terpine-4-ol (5.75%) and α-pinene (4.71%), β-pinene (3.09%) and γ-terpinene (2.88%). Other components as α-terpinene, limonene, p-cymene and α-humulene were present in <2%. The oil of leaves was characterized by presence of higher amount of eucalyptol, sabinene and α-pinene than those in the branches while the latter is characterized by the presence of higher amount of α-terpineol, 4-terpineol and γ-terpinene than leaves.
Rat paw oedema model
Intraplantar injection of 0.1 mL of 1% carrageenan successfully established the oedema experimental model resulting in significant increase in the mean volume of the challenged paw compared to that of the untreated paws (159% of the untreated paws following 4 h post carrageenan administration, ). Pretreatment of rats with volatile oil at different doses of 250, 500 and 1000 mg/kg significantly inhibited the carrageenan-induced increase in the oedema volume of the paws after 1, 2, 3 and 4 h (). Following 4 h of carrageenan challenge, doses of 250, 500 and 1000 mg/kg significantly reduced the paw volume to 94, 82 and 69% change, respectively. However, indomethacin-treated group showed more significant anti-oedema effect (31.5% of the challenged paws).
Figure 1. Time and dose-dependent effects of volatile oil in carregenin-induced rat oedema model. Each value represents mean % change of paw oedema volume ± SEM (n = 6). Statistical analysis was carried out by One-way ANOVA followed by Tukey post hoc test. a: Statistical significance as compared to the control. b: Statistical significance as compared to the Dose 250 treated group. c: Statistical significance as compared to the indomethacin treated group.
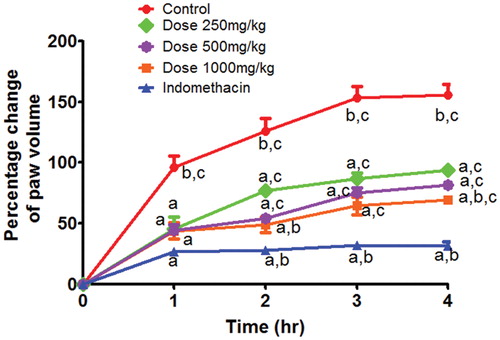
Table 2. Effects of ascending doses of volatile oil (250, 500 and1000 mg/kg, p.o.) on carrageenan-induced rat paw oedema model.
Inflammatory markers
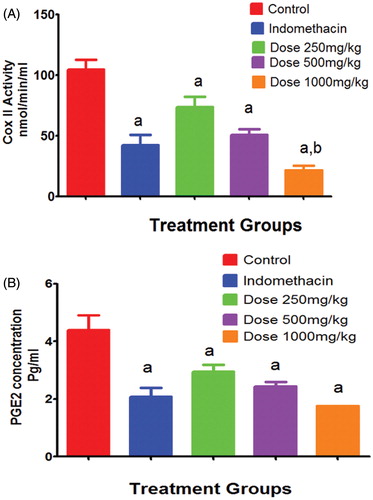
Regarding the serum inflammatory markers, indomethacin treatment after 4 h of carrageenan injection significantly reduced COX-2 activity (42.4 ± 8.5 nmol/min/mL) compared to that untreated animals in group I (104.6 ± 8.3 nmol/min/mL). Volatile oil application in doses of 250, 500 and 1000 mg/kg showed significant reduction of COX-2 activity by 1.4, 2.1 and 4.9 folds to reach 73.8, 50.7and 21.4 nmol/min/mL, respectively, compared to group I (). On the other hand, indomethacin treated animals in group II resulted in mean PGE2 concentration of 2.1 ± 0.3 pg/mL in inflammatory exudates compared to group I of carrageenan challenge that resulted in 4.4 ± 0.5 (). Animals receiving volatile oil showed significant reduction of the PGE2 concentration by 1.5, 1.8 and 2.5 folds (to reach 2.95 ± 0.2, 2.45 ± 0.15 and 1.75 ± 0.015 pg/mL) for the doses of 250, 500 and 1000 mg/kg, respectively).
Histopathological study of the gastritis model
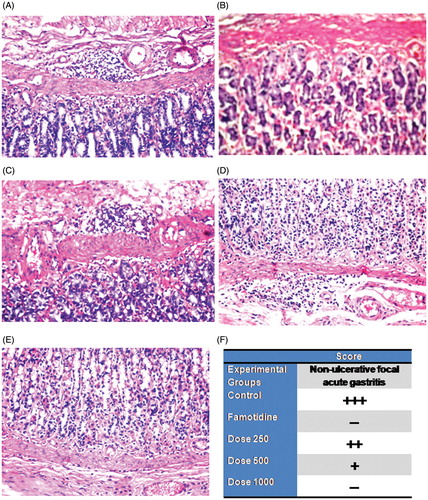
Focal inflammatory cells infiltration with dilatation in the blood vessels was detected in the submucosa of the control group (). There was no histopathological alteration and the normal histological structure of the mucosa, submucosa, muscularis and serosa were recorded in the famotidine group (). The submucosa showed focal inflammatory cells infiltration in the group treated with dose 250 mg/kg (). Focal few inflammatory cells infiltration with dilatation in the blood vessels were recorded in the submucosa in the group treated with dose 500 mg/kg (). There were very few inflammatory cells infiltration in the base of the mucosa in the group treated with dose 1000 mg/kg (). Scoring of the gastric lesion (non-ulcerative focal acute gastritis) was compared and described in the different experimental groups ().
Figure 3. Photomicrographs of stomach sections of different treatment groups stained by H&E. A: Control group (vehicle treated) showing focal inflammatory cells infiltration with dilated blood vessels in submucosa (×40). B: Famotidine treated group showing normal submucosa (×40). C: Dose 250 mg/kg treated group showing focal inflammatory cells infiltration in submucosa (×40). D: Dose 500 mg/kg treated group showing focal inflammatory cells infiltration with dilated blood vessels in submucosa (×40). E: Dose 1000 mg/kg treated group showing focal inflammatory cells infiltration in base of mucosa (×40). F: Scoring the severity of the histopathological alterations (Focal inflammatory cells infiltration with dilated blood vessels in submucosa) in stomach of different experimental groups. +++: Severe histopathological alteration. ++: moderate histopathological alteration. +: mild histopathological alteration. –: nil histopathological alteration.
Oxidative stress markers
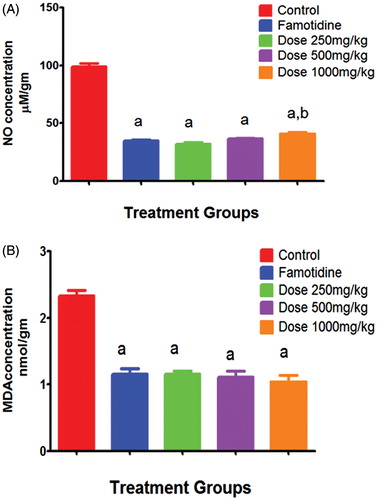
Regarding the tissue oxidative stress markers, famotidine treatment 1 h before ethanol intragastric administration reduced NO concentration (34.9 ± 0.7 μM nitrate/g) compared to that of untreated animals in group I (98.9 ± 2.6 μM nitrate/g). Volatile oil adminstration in doses of 250, 500 and 1000 mg/kg showed significant reduction of NO concentration by 3.1, 2.7 and 2.4 folds to reach 32, 37 and 41 μM nitrate/g respectively compared to group I (). On the other hand, famotidine treated animals in group II resulted in mean MDA concentration of 1.15 ± 0.08 nmol/g in inflammatory exudates as compared to group I of ethanol challenge that resulted in 2.3 ± 0.08 (). Animals receiving volatile oil showed significant reduction of the MDA concentration by 2, 2.1 and 2.2 folds (to reach 1.15, 1.11 and 1.04 nmol/g) for the doses of 250, 500 and 1000 mg/kg, respectively).
Discussion
The present study shows a comparison of the composition of the volatile oil of leaves and green branches of C. glanduliferum as well as evaluation of the potential anti-inflammatory activity of volatile oil of leaves (higher yield) in carrageenan-induced rat oedema model. Oedema due to carrageenan injection is presumed to be through inflammatory mediators that increase vascular permeability and/or increase blood flow (Ialenti et al. Citation1992). Thus, the effect of volatile oil may be attributed to the effect of the volatile oil on the inflammatory mediators and also on the pathway of prostaglandins synthesis.
CG volatile oil caused a time-dependent reduction of carrageenan-induced rat paw oedema. Following 4 h of treatment, volatile oil of leaves at doses 250, 500 and 1000 mg/kg caused a significant inhibition of rat paw oedema volume by 40%, 48% and 56%, respectively, in comparison to the control group. Such conclusion was further confirmed by assessing COX-2 activity and serum PGE2 level in the same model. Prostaglandins have been long recognized as a major mediator of inflammation. They are arachidonic acid metabolites synthesized by COX-1 and COX-2 isozymes (Herschman Citation1996). Our results indicate that the volatile oil caused statistically significant reduction of COX-2 activity and serum PGE2 concentration. This finding clearly underlines the anti-inflammatory effect of the tested volatile oil, where the effect of volatile oil may be attributed to the influence of the volatile oil on the inflammatory mediators and also on the pathway of prostaglandins synthesis.
The major components of leaves volatile oil is 1,8-cineole (59.44%) which is known as eucalyptol or cajeputol. It consists of 2 isoprene units (C10) which is structurally related in to human isoprenoid as tocopherols (C20) and steroid hormones (C30). Our results agree with Singh et al. (Citation2014), where the major component identified from CG leaves grown in India was 1,8-cineole (41.42%).
Many reports provided evidences that eucalyptol possesses anti-inflammatory activity, gastroprotective and ulcer healing effect. Thus, it could inhibit carrageenan oedema, increased capillary permeability and granuloma formation (Santos & Rao Citation2000). Moreover, it exhibited a steroid-like suppression of arachidonic acid metabolism and cytokine production in vitro (Juergens et al. Citation1998a, Citation1998b). The chemical relationship between eucalyptol and glucocorticosteroids (human isoprenoid; C20), explains that there is a common mechanism of anti-inflammatory mediator suppression for their anti-inflammatory effect and this was obvious in its ability to inhibit the formation of inflammatory mediators as cytokines (TNFa, IL1-b), leukotriene B4, thromboxane B2 and prostaglandin E2 (Juergens et al. Citation1998a, Citation1998b).
On the other hand, the present results show that CG volatile oil could protect against gastric lesions and gastritis induced by ethanol administration. This was confirmed by histopathological investigation as well as reduction of NO & MDA tissue levels. This effect could be attributed to the presence of eucalyptol in high percent. Eucalyptol itself is an important gastroprotective agent. The suggested mechanisms of action are explained through cytoprotective mechanism (causing an increase in gastric mucus), antioxidant activity (preventing depletion of sulfhydryl groups and reducing levels of lipid peroxidation and myeloperoxidase activity in the gastric mucosa) and finally healing ability (Rocha Caldas et al. Citation2015).
Santos and Rao reported that 1,8-cineole prevented ethanol-induced gastric injury in rats where the antioxidant and lipoxygenase inhibitory actions are of prime importance in affording gastroprotection against ethanol injury in the rat (Santos & Rao Citation2001). Other essential oils whose main constituent is eucalyptol as Hyptis martiusii Benth (Lamiaceae) exibited gastroprotective effect in various gastric lesion models in rats. The mechanism was through an antisecretory activity mediated by the histamine H2 and gastrin CCK2 receptors, hence reduces levels of lipid peroxidation and also increase the healing of chronic ulcers promoting significant regeneration of the gastric mucosa (Caldas et al. Citation2014).
Furthermore, C. glanduliferum oil contains also other anti-inflammatory constituents in considerable amounts. Citrus reticulate Blanco (Rutaceae) and Zornia diphylla (L.) Pers (Fabaceae) volatile oils showed anti-inflammatory effect as sabinene is the major compound in both (34.75% and 43.1%) (kim et al. Citation2013; ArunKumar et al. Citation2014). Terpinene-4-ol exhibited anti-inflammatory activity in vitro and in vivo (Ninomiya et al. Citation2013). α-Pinene also exerts a role in the management of inflammatory process (De Cassia da Silveira e Sa et al. Citation2013). α-Pinene (50.8%) and cineole (20.3%) are the major components of Hyptis spicigera Lam. (Lamiaceae) essential oil which exhibited antiulcerogenic and gastroprotective actions in the gastric mucus production induced by PGE2 levels. A healing activity was produced with 87% reduction in ulcerative lesion area. It increased COX-2 (75%) and EGF (115%) expression in gastric mucosa so increase the healing of acetic acid-induced gastric lesions (Takayama et al. Citation2011). α-Terpineol exhibited gastroprotective activity against ethanol-induced ulcers. It showed gastroprotective activity which does not involve a decrease in the gastric acid secretion or an increase in the synthesis of endogenous prostaglandin (Souza et al. Citation2011).
In conclusion, for the first time, volatile oil of C. glanduliferum leaves was shown to possess a potent anti-inflammatory activity as well as gastroprotective effect at the tested doses (250, 500 and 1000 mg/kg). This is evidenced by reducing paw oedema, COX-2 activity and PGE2 content. Furthermore, in ethanol-induced animal model, as evidenced by histopathologic examination volatile oil exhibited gastroprotective effect and also managed to reduce NO content and MDA levels in the gastric homogenate. These anti-inflammatory and gastroprotective effects are possibly attributed to the synergistic effect of special components in the oil specially eucalyptol (high percent), and others as sabinene and α-pinene. Cinnamomum glanduliferum could also be used as a good source of 1,8-cineole for medical purpose.
Disclosure statement
All authors declare that they have no competing financial or personal interest or any kind of conflict of interest relevant to this study.
References
- Adams RP. 2007. Identification of essential oil components by gas chromatography/mass spectrometry. Illinois, USA: Allured Publishing Corporation.
- Agrawal R, Pant AK, Prakash O. 2012. Chemistry of phytopotentials: health, energy and environmental perspectives: Chemical composition and biological activities of essential oils of Cinnamomum tamala, Cinnamomum zeylanicum and Cinnamomum camphora growing in Uttarakhand. Berlin Heidelberg: Springer. p. 87–92.
- Al-Shabanah OA. 1997. Effect of evening primrose oil on gastric ulceration and secretion induced by various ulcerogenic and necrotizing agents in rats. Food Chem Toxicol. 35:769–775.
- Amaral GP, de Carvalho NR, Barcelos RP, Dobrachinski F, Portella Rde L, da Silva MH, et al. 2013. Protective action of ethanolic extract of Rosmarinus officinalis L. in gastric ulcer prevention induced by ethanol in rats. Food Chem Toxicol. 55:48–55.
- ArunKumar R, Nair SA, Rameshkumar KB, Subramoniam A. 2014. The essential oil constituents of Zornia diphylla (L.) Pers, and anti-inflammatory and antimicrobial activities of the oil. Rec Nat Prod. 8:385–393.
- Baruah AR, Nath SC. 2006. Leaf essential oils of Cinnamomum glanduliferum (Wall) Meissn and Cinnamomum glaucescens (Nees) Meissn. J Essent Oil Res. 18:200–202.
- Caldas GF, Oliveira AR, Araujo AV, Quixabeira DC, Silva-Neto Jda C, Costa-Silva JH, et al. 2014. Gastroprotective and ulcer healing effects of essential oil of Hyptis martiusii Benth. (Lamiaceae). PLoS One. 9:e84400.
- Chowdhury AR. 1999. Essential oil from Cinnamomum glanduliferum (Wal.) Wees. Indian Perfum. 43:64–66.
- De Cassia da Silveira e Sa R, Andrade LN, de Sousa DP. 2013. A review on anti-inflammatory activity of monoterpenes. Molecules. 18:1227–1254.
- Eldahshan OA. 2015. Comparison of chemical and antimicrobial studies of Egyptian mandarin leaves and green branches volatile oils. European J Med Plants. 5:248–254.
- Eldahshan OA, Halim FA. 2016. Comparison of the composition and antimicrobial activities of the essential oils of green branches and leaves of Egyptian navel orange (Citrus sinensis (L.) OSBECK var. malesy. Chem Biodivers. 13:1–5.
- Finnemore H. 1926. The essential oils. London: Ernest Benn Ltd.
- Herschman HR. 1996. Prostaglandin synthase 2. Biochim Biophys Acta. 5:125–140.
- Ialenti A, Ianaro A, Moncada S, Di Rosa M. 1992. Modulation of acute inflammation by endogenous nitric oxide. Eur J Pharmacol. 11:177–182.
- Jayaprakasha GK, Rao LJ, Sakariah KK. 2002. Chemical composition of volatile oil from Cinnamomum zeylanicum buds. Z Naturforsch, C J Biosci. 57:990–993.
- Juergens UR, Stober M, Schmidt-Schilling L, Kleuver T, Vetter H. 1998a. Anti-inflammatory effects of euclyptol (1.8-cineole) in bronchial asthma: inhibition of arachidonic acid metabolism in human blood monocytes ex vivo. Eur J Med Res. 17:407–412.
- Juergens UR, Stober M, Vetter H. 1998b. Inhibition of cytokine production and arachidonic acid metabolism by eucalyptol (1.8-cineole) in human blood monocytes in vitro. Eur J Med Res. 17:508–510.
- Kim MJ, Yang KW, Kim SS, Park SM, Park KJ, kim KS, young n, choi YH, Cho KH, Lee 1 NH, Hyun 1 CG. 2013. Chemical composition and anti-inflammatory effects of essential oil from hallabong flower. Excli J 12:933–942.
- Kirtikar KR, Basu BD. 2000. Indian medicinal plants. Lalitmohan Basu: Allahabad, pp. 2147.
- Krikorian AD. 1992. Wealth of India: raw materials. vol II. New Delhi, India: Publication and Information Directorate, CSIR.
- Mihara M, Uchiyama M. 1978. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 86:271–278.
- Mir SR, Ali M, Kapoor R. 2004. Chemical composition of essential oil of Cinnamomum tamala Nees et Eberm. leaves. Flavour Fragr J. 19:112–114.
- Miranda KM, Espey MG, Wink DA. 2001. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 5:62–71.
- Nath SC, Baruah AR, Kanjilal PB. 2006. Chemical composition of the leaf essential oil of Cinnamomum pauciflorum nees. Flavour Fragr J. 21:531–533.
- Ninomiya K, Hayama K, Ishijima SA, Maruyama N, Irie H, Kurihara J, Abe S. 2013. Suppression of inflammatory reactions by terpinen-4-ol, a main constituent of tea tree oil, in a murine model of oral candidiasis and its suppressive activity to cytokine production of macrophages in vitro. Biol Pharm Bull. 36:838–844.
- Omri Hichri A, Mosbah H, Majouli K, Besbes Hlila M, Ben Jannet H, Flamini G, Aouni M, Selmi B. 2016. Chemical composition and biological activities of Eruca vesicaria subsp. longirostris essential oils. Pharm Biol. 54:2236–2243.
- Patel K, Ali S, Sotheeswaran S, Dufour JP. 2007. Composition of the leaf essential oil of Cinnamomum verum (Lauraceae) from Fiji Islands Kirti. Jeop. 10:374–377.
- Pullaiah T. 2006. Encyclopedia of world medicinal plants. vol I. New Delhi: Daya Books.
- Rocha Caldas GF, Oliveira AR, Araújo AV, Lafayette SS, Albuquerque GS, Silva-Neto Jda C, Costa-Silva JH, Ferreira F, Costa JG, Wanderley AG. 2015. Gastroprotective mechanisms of the monoterpene 1,8-cineole (eucalyptol). PLoS One. 10:e0134558.
- Salleh WM, Kammil MF, Ahmad F, Sirat HM. 2015. Antioxidant and anti-inflammatory activities of essential oil and extracts of Piper miniatum. Nat Prod Commun. 10:2005–2008.
- Santos FA, Rao VSN. 2000. Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother Res. 14:240–244.
- Santos FA, Rao VSN. 2001. 1,8-Cineole, a food flavoring agent, prevents ethanol-induced gastric injury in rats. Dig Dis Sci. 46:331–337.
- Singh C, Singh S, Pande C, Tewari G, Kharkwal GC. 2014. Chemical Composition of the Leaves Essential Oil from Cinnamomum glanduliferum (Wall) Meissn from Uttarakhand, India. jeobp. 17:927–930.
- Souza R, Cardoso M, Menezes C, Silva J, De Sousa D, Batista J. 2011. Gastroprotective activity of alpha-terpineol in two experimental models of gastric ulcer in rats. Daru. 19:277–281.
- Taha AS, Eldahshan OA. 2017. Chemical characteristics, antimicrobial and cytotoxic activities of the essential oil of Egyptian Cinnamomum glanduliferum Bark. Chem Biodivers. [Epub ahead of print]. doi: 10.1002/cbdv.201600443
- Takayama C, de-Faria FM, de Almeida AC, Valim-Araujo Dde A, Rehen CS, Dunder RJ, et al. 2011. Gastroprotective and ulcer healing effects of essential oil from Hyptis spicigera Lam. (Lamiaceae). J Ethnopharmacol. 26:147–155.