Abstract
Context: Piqueria trinervia Cav. (Asteraceae) is a plant species with a long history in traditional medicine to cure diarrhoea and other digestive disorders.
Objective: The study investigates the antigiardial activity of piquerol, trinervinol, red oil and two fractions (F1 and F2) from P. trinervia.
Materials and methods: P. trinervia was collected in the Ajusco in Mexico City. Aerial parts were ground and mixed with water to obtain the extract, which was treated with dichloromethane to isolate piquerol and trinervinol (P & T). Remnants were the red oil, fractions 1 and 2 (RO, F1 & F2). Trophozoites of Giardia intestinalis were treated with P, T, RO, F1 and F2 at different concentrations (0.78–200 μg/mL) for 48 h. Antigiardial activity was measured using the methylene blue reduction, and the cytotoxicity assayed on human fibroblasts and Vero cells by reduction of tetrazolium salts.
Results: Trinervinol and piquerol showed antigiardial activity with an IC50 = 2.03 and 2.42 μg/mL, and IC90 = 13.03 and 8.74 μg/mL, respectively. The concentrations of trinervinol (CC50 = 590 μg/mL) and piquerol (CC50 = 501 μg/mL) were not cytotoxic to human fibroblasts.
Conclusions: Compounds from P. trinervia showed antigiardial activity; to enhance this activity, piquerol and trinervinol can be chemically modified.
Keywords:
Introduction
Infectious diseases have been the cornerstone of human evolution. Before the development of modern hygiene practices, vaccines and antibiotics, the life expectancy was approximately 20 years, whereas today it is nearly 80 years (Siddle & Quintana-Murci Citation2014). Since 1940, more than 335 infectious diseases have emerged, which are predominantly zoonoses (Jones et al. Citation2008). Giardiasis is a re-emergent infectious disease that infects mammals, including humans (Thompson Citation2000). The disease can be asymptomatic or present abdominal pain, diarrhoea, weight loss and nutrient malabsorption (Adam Citation2001; Martínez-Gordillo et al. Citation2014). Giardia intestinalis (Syn. G. lamblia, G. duodenalis) impairs children’s physical and mental development (Berkman et al. Citation2002; Eppig et al. Citation2010). The World Health Organization (WHO) established that inhabitants of underdeveloped countries in Latin America, Asia and Africa experience 200 million infections yearly (Comité OMS d'Experts Citation1998). The metronidazole, tinidazole, albendazole, mebendazole, quinacrine, furazolidone and nitazoxanide are drugs against Giardia. However, these compounds produce undesirable secondary effects, such as hyporexia, nausea, abdominal discomfort, vertigo, mutagenic and carcinogenic effects (Ali & Nozaki Citation2007; Rufino-González et al. Citation2012). Additionally, the evolution of Giardia could give rise to resistant strains (Sangster et al. Citation2002) and strains with the ability to live within the duodenal epithelium (Reynoso-Robles et al. Citation2015). Therefore, it is imperative to search for new strategies for the treatment of giardiasis. Hence, we examined ethno-botanic and traditional medicines, and we found that Piqueria trinervia Cav. (Asteraceae) is a wild herbaceous plant with a wide distribution in the warm, dry and mild climates of Mexico (Rzedowski & Rzedowski Citation2005). It is usually called ‘hierba de San Nicolás’ or ‘hierba de tabardillo’. This plant has been used since the 16th century as an antipyretic and an antimalarial as well as to treat abdominal pain (Bejar et al. Citation2000). Since 1970, different terpenes were isolated, such as piquerol A, piquerol B and carquejilo acetate (Romo et al. Citation1970). Biologically, piquerol has allelopathic activity by preventing seed germination (González-Parra et al. Citation1981). It was also used against snails, vectors of Fasciola and Schistosoma (Cruz-Reyes et al. Citation1989), gravid female ticks (González-Parra et al. Citation1991), Trypanosoma cruzi (Castro et al. Citation1992) and pathogenic bacteria (Ruiz-Esparza et al. Citation2007). Trinervinol has activity against fungi that parasitized plants (Saad et al. Citation2000). In this work, it is reported the in vitro antigiardial activity of compounds from P. trinervia.
Materials
Biological material
Piqueria trinervia is a perennial herb that grows commonly in open areas of the pine oak forest of the mountains throughout Mexico and Central America.
The collection of P. trinervia was carried out in November 2010 in the Ajusco Mountain zone at 19° 15.042' N and 99° 14.623' W, of Mexico City, Mexico. Dr Jimenez deposited the vegetal material in the National Herbarium located at the Biology Institute of the National Autonomous University of Mexico. Dr Robert Bye identified the vegetal samples. The voucher specimen code number is MEXU1003. The G. intestinalis trophozoites were from the WB isolate GL50803 genotype A.
Fractionation and constituents
The plants were dried at ambient temperature then aerial parts were crushed and pulverized to increase the contact surface. We obtained the piquerol and trinervinol as described elsewhere (Romo et al. Citation1970). The pulverized materials (760 g) was mixed with Millipore quality water for 24 h. Next, the materials was treated with dichloromethane following the procedures outlined by Ruiz-Esparza et al. (Citation2007). The remaining solutions were designated as red oil (RO), fractions 1 and 2 (F1 and F2). Then the mentioned fractions were analysed in a preparative thin layer chromatography (TLC) plates and eluted with 1:1 hexane–ethyl acetate (EtOAc). The pattern of the bands was revealed with 1% cerium sulphate and by exposed to ultraviolet light (data not shown). The components of RO and F1 and F2 were identified by gas chromatography coupled to mass spectrometry (Joel GC-MS Mate II system, Shimadzu, Kyoto, Japan), equipped with an HP5-5Ms fused-silica capillary column (30 m × 0.25 mm). The GC oven temperature was programmed to increase at a rate of 8 °C min−1 until reaching 305 °C and held for 3 min.
The samples were dissolved in methanol and carried in helium. The electron impact technique (70 eV) was used to obtain the mass spectrum of the compounds in the fractions. The mass spectrum was continuously acquired from Total Ion Current (T.I.C.).
The GC-MS spectral data were digitalized, with the Mass Spectrum Digitizer program from the National Institute of Standards and Technology (NIST). The search performed in the MassBank of the Institute of Research and Development of Bioinformatics from the Japan Science, and Technology Agency and the Molecular Physiology of Plants Data Base Max Planck Institute (GMD_20111121_MDN35_ALK_MSP.txt).
Bioassays
Giardia intestinalis (WB) trophozoites grew to confluence in a TYI-S-33 media, supplemented with bile and foetal calf serum. The culture tubes were chilled in an ice bath for 15 min to harvest Giardia trophozoites. The detached trophozoites were concentrated, transferred to Eppendorf tubes and washed three times with phosphate-buffered solution (PBS) pH 7.20. The size of the population was calculated using a Neubauer chamber and all assays were performed with 2 × 104 Giardia trophozoites. The compounds to test were dissolved in dimethyl sulfoxide (DMSO at 0.4%). Serial dilutions, from 200 to 0.78 μg/mL, were made with TYI-S-33 media and placed in 96-well culture plates to a final volume of 300 μL. Controls were untreated trophozoites, exposed to metronidazole and DMSO. The plates were incubated at 37 °C for 48 h, in an atmosphere containing 5% CO2 and 80% humidity. All manipulations were performed in a sterile environment in a vertical laminar flow cabinet. To assess viability the solution was removed by inversion (decantation), trophozoites were fixed with methanol and stained with methylene blue 0.1% for 15 min. The trophozoites were incubated for 30 min with 0.1 M hydrochloric acid at room temperature to extract the dye products. The concentration was determined at 650 nm (Busatti & Gomez Citation2007; Houngkong et al. Citation2011).
Toxicity test on Vero cells and human fibroblasts
The piquerol, trinervinol, fraction 2 and metronidazole were dissolved in DMSO, and performed toxicity assays at concentrations from 6.2 to 800 μg mL−1 on Vero cells [(ATCC: CCL-81), from the green monkey kidney (Cercopithecus aethiops)] and primary cultures of human fibroblasts. Vero cells or human fibroblasts grew in 25 mL tissue culture flasks with DMEM media (supplemented with 1X antibiotics-antimycotics, 1X l-glutamine and 10% foetal bovine serum) at 37 °C and 5% CO2. The cells grew up to confluence and then harvested, washed and counted in a Neubauer chamber. About 100 μL of medium containing 1.0 × 105 Vero cells or human fibroblasts were seeded in a 96-well plate and incubated for 24 h, afterward; exposed to growing concentrations of the compounds (6.2–800 μg/mL) in 100 μL DMEM medium. The assay was repeated three times in triplicate. The negative controls were unexposed Vero cells, human fibroblasts and cells exposed to 0.2% DMSO. All experiments were incubated 48 h at 37 °C with 5% CO2. The cellular viability was assessed by the reduction of MTT-tetrazolium salts (3[4,5-dimethylthiazol-2-y1]-2,5-diphenyl-tetrazolium bromide) to formazan as described elsewhere (Ponce-Macotela et al. Citation1994) with several modifications. Briefly, the treated cells (Vero or human fibroblasts) were concentrated by centrifugation; then we added 10 μL of MTT (5 mg/mL). Afterward, incubated at 37 °C for 4 h, and the formazan was solubilized overnight in 0.01 N HCl with 10% SDS in a final volume of 100 μL. The concentration of formazan was measured in a Uniskan spectrophotometer (Labsystem, Manchester, UK) at 570 nm.
Statistics
All experiments were performed in a blinded fashion and repeated three times in triplicate. The trophozoites mortality and concentration was carried out, ANOVA and Tukey tests were conducted to compare P. trinervia compounds activity. Probit analysis was performed to calculate the IC50, IC90 and CC50 (concentration to reduce cell viability by 50%) at 95% confidence. The analysis was performed using the SPSS software (V 17.9) and JMP (V 9.0).
Results
Fractions and constituents
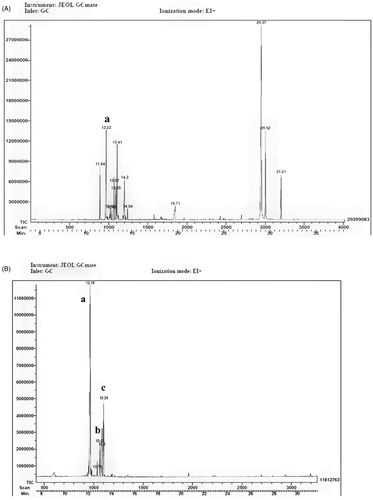
The yield from the vegetal material was piquerol 1.52 g (0.2%), trinervinol 0.76 g (0.1) and red oil 8.36 g (1.1%). The TLC showed several bands, the RO, F1 and F2 samples were complex mixes (Figure 1 Supplementary material). Patterns with 13 and 5 peaks for F1 and F2 were identified, the retention times were between 5 and 35 min. , which corresponds to F-1 and displays 13 compounds, shows that piquerol had a retention time of 12.22 min. In , in total five peaks were detected. Several compounds were identified based on their retention times and mass spectra using the database of the National Institute Standard and Technology (NIST) library software as follows: piquerol, 12.18; piquerinol, 13.03; and carquejol 13.35 min. The other peaks are probably piquerol isomers and other compounds, such as those identified by Ruiz-Esparza et al. (Citation2007).
Figure 1. GC/MS spectrum of the chemical constituents of F1 and F2 from Piqueria trinervia. (A) Fraction 1. The peak at 12.22 corresponds to piquerol. (B) Fraction 2. The peaks correspond to (a) piquerol, (b) piquerinol and (c) parquejol with retention times of 12.18, 13.03 and 13.35 min, respectively.
Bioassay
The summarized the susceptibility of Giardia trophozoites to pure compounds and fractions from P. trinervia. The Probit analysis showed that trinervinol had the best antigiardial activity (IC50 = 2.03 μg/mL), followed of piquerol (IC50 = 2.42 μg/mL). A comparison between piquerol and trinervinol did not show statistical significance ().
Table 1. Fractions from Piqueria trinervia with antigiardiasic activity.
Table 2. Probit test of compounds from Piqueria trinervia.
Toxicity
Determination of the cytotoxicity of P. trinervia compounds against mammal cells could be a significant step to advance in its use as antiparasitic. Trinervinol activity against Vero cells and human fibroblasts were negligible; CC50 was 278 and 590 μg/mL, respectively (). The cytotoxicity of metronidazole on human fibroblasts was 452 μg/mL.
Table 3. Cytotoxic analysis of compounds from Piqueria trinervia on Vero cells and human fibroblasts.
Discussion
Both plants and animals have co-evolved in a complex web of interactions. Plants are the primary producers of the alimentary network and under selective pressure from grass-eaters. In response, plants evolved and developed a defence system based on secondary metabolites that protect them from herbivores, and also to facing infections from parasites, fungus, bacteria and viruses (Großkinsky et al. Citation2012; Jeandet Citation2015). Empirical observations since the first human societies were the touchstone to develop a pharmacology based on plant products; P. trinervia is a plant with a long ethnobotanical history. The new world societies used Piqueria as antipyretic and to treat abdominal pain, documented in the Florentine codex (Bejar et al. Citation2000). Chemical studies on this plant began in the second half of the twentieth century.
Today antimicrobial resistance is becoming a worldwide problem owing to the paucity of research and development of new antibiotics, and because the indiscriminate usage of antibiotics is selecting resistant strains (O’Neill Citation2016). The main difficulty is in bacteria. However, there is evidence of resistance in protozoa, helminths, virus and cancer cells. The history had shown that natural products are a source for the discovery of antibiotics. The antimicrobial resistance is prompting to looking back to the investigation of natural products (Brown & Wright Citation2016).
Here, were presented evidence that P. trinervia has, at least, two promising compounds: trinervinol and piquerol, which were able to kill G. intestinalis. Since 2006, Ruíz-Esparza demonstrated antibacterial activity at concentrations ranging in mg mL−1. The piquerol dose (IC50 = 2.42 μg/mL) used against Giardia was 50 times lower than those reported against epimastigotes of Trypanosoma cruzi (Castro et al. Citation1992). Additionally, it is necessary to annotate that the scaffold of pure compounds can be chemically modified, as was performed by Jiménez-Estrada et al. (Citation1996). The piquerol is the compound more studied than trinervinol. However, data showing IC50 = 2.03 μg/mL on G. intestinalis, suggest that trinervinol must be investigated and modified to enhance its antigiardial activity. Reports as antifungal compound showed activity at 250 mg/mL on Phoma macdonaldii (Saad et al. Citation2000) a very high concentration in comparison with the actual IC50 = 2.03 μg/mL on G. intestinalis.
The F1 and F2 were also able to eliminate Giardia trophozoites because these fractions had piquerol, piquerinol, carquejol, piquerol isomers and other compounds not yet biologically characterized ().
Additionally, the pure compounds trinervinol and piquerol showed low toxicity on human fibroblasts with CC50 = 590 and 510 μg/mL, respectively, a value similar to metronidazole.
Conclusions
The antigiardial activity of compounds from P. trinervia was demonstrated. Trinervinol and piquerol showed little toxicity on fibroblasts. In the era of antimicrobial resistance, the natural products can be a source of antibiotic substances. There are pending tasks that would enhance our results, such as chemically modifying purified metabolites and finding the targets of these molecules on G. intestinalis trophozoites.
Mario_Mart_nez-Gordillo_et_al_supplemental_content.zip
Download Zip (983.7 KB)Disclosure statement
We declare that there are no conflicts of interests.
References
- Adam R. 2001. Biology of Giardia lamblia. Clin Microbiol Rev. 14:447–475.
- Ali V, Nozaki T. 2007. Current therapeutics, their problems, and sulfur-containing-amino-acid metabolism as a novel target against infections by “amitochondriate” protozoan parasites. Clin Microbiol Rev. 20:164–187.
- Bejar E, Reyes-Chilpa R, Jiménez-Estrada M. 2000. Bioactive compounds from selected plants used in XVI century Mexican traditional medicine. In: Rhaman AU, editor. Studies in natural products chemistry. Amsterdam: Elsevier; p. 799–844.
- Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. 2002. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet 359:564–571.
- Brown ED, Wright GD. 2016. Antibacterial drug discovery in the resistance era. Nature 529:336–343.
- Busatti HGNO, Gomez MA. 2007. A simple colourimetric method to determine anti-giardial activity of drugs. Parasitol Res. 101:819–821.
- Castro C, Jiménez-Estrada M, González-Parra M. 1992. Inhibitory effect of piquerol A on the growth of epimastigotes of Trypanosoma cruzi. Planta Med. 58:281–282.
- Comité OMS d'Experts. 1998. Importance de parasitoses intestinalis en santé publique. Bull Org Mond Santé. 66:23–34.
- Cruz-Reyes A, Chavarín C, Campos-Arias MP, Taboada J, Jiménez-Estrada M. 1989. Actividad molusquicida del piquerol A aislado de Piqueria trinervia (Compositae) sobre ocho especies de caracoles pulmonados. Mem Inst Oswaldo Cruz. 84:35–40.
- Eppig C, Fincher CL, Thornhill R. 2010. Parasite prevalence and the worldwide distribution of cognitive ability. Proc Biol Sci. 277:3801–3808.
- González-Parra M, Anaya AL, Espinoza F, Jiménez-Estrada M, Castillo R. 1981. Allelopathic potential of Piqueria trinervia (Compositae) and piquerols A and B. J Chem Ecol. 7:509–515.
- González-Parra M, Chávez-Peña D, Jiménez-Estrada M, Ramos-Mundo C. 1991. Acaricidal potential of piquerols A and B against Boophilus microplus. Pest Sci. 33:73–80.
- Großkinsky DK, van der Graaff E, Roitsch T. 2012. Phytoalexin transgenics in crop protection-fairy tale with a happy end? Plant Sci. 195:54–70.
- Houngkong K, Sawangjaroen N, Phongpaichit S. 2011. A colorimetric method for the evaluation of anti-giardial drugs in vitro. Exp Parasitol. 127:600–603.
- Jeandet P. 2015. Phytoalexins: current progress and future prospects. Molecules 20:2770–2774.
- Jiménez-Estrada M, Navarro A, Flores MV, Reyes-Chilpa R, Hernández B, Anaya AL, Lotina-Hennsen B. 1996. Transformation of terpenepiquerol A to hydroquinone and phenolic derivatives effect of these compounds on weeds. J Agric Food Chem. 44:2839–2841.
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451:990–994.
- Martínez-Gordillo MN, González-Maciel A, Reynoso-Robles R, Montijo-Barrios E, Ponce-Macotela M. 2014. Intraepithelial Giardia intestinalis: a case report and literature review. Medicine 93:e277.
- O’Neill J. 2016. The review on antimicrobial resistance. Tackling drug-resistant infection globally: final report and recommendations. London: HM Government and Wellcome trust.
- Ponce-Macotela M, Navarro-alegría I, Martínez-Gordillo MN, Álvarez-Chacón R. 1994. Efecto antigiardiásico in vitro de 14 plantas. Rev Invest Clín. 46:343–347.
- Reynso-Robles R, Ponce-Macotela M, Rosas-López LE, Ramos-Morales A, Martínez-Gordillo MN, González-Maciel A. 2015. The invasive potential of Giardia intestinalis in an in vivo model. Sci Rep. 5:15168.
- Romo J, Romo-deVivar A, Quijano L, Ríos T, Díaz E. 1970. Los componentes terpenoides de Piqueria trinervia Cav. Rev Lat Quim. 1:72–81.
- Rufino-González Y, Ponce-Macotela M, González-Maciel A, Reynoso-Robles R, Jiménez-Estrada M, Sánchez-Contreras A, Martínez-Gordillo MN. 2012. In vitro activity of the F-6 fraction of oregano against Giardia intestinalis. Parasitology 139:434–440.
- Ruiz-Esparza R, Bye T, Meckes M, Torres-López J, Jiménez-Estrada M. 2007. Antibacterial activity of Piqueria trinervia a Mexican medicinal plant used to treat diarrhea. Pharm Biol. 45:446–452.
- Rzedowski GC, Rzedowski J. 2005. Flora fanerógamica del Valle de México. 2da Ed, 1a reimp. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Pátzcuaro (Michoacán), 1406 pp.
- Saad VI, Díaz E, Chávez I, Reyes-Chilpa R, Rubio A, Jiménez-Estrada M. 2000. Antifungal monotherpene production in elicited cell suspension cultures of Piqueria trinervia. Phytochemistry 55:51–57.
- Sangster N, Batterham P, Chapman HD, Duraisingh M, Jambre LL, Shirley M, Upcroft J, Upcroft P. 2002. Resistance to antiparasitic drugs: the role of molecular diagnosis. Int J Parasitol. 32:637–653.
- Siddle KJ, Quintana-Murci L. 2014. The red queen's long race: human adaptation to pathogen pressure. Curr Opin Genet Dev. 29:31–38.
- Thompson RCA. 2000. Giardiasis as a re-emerging infectious disease and its zoonotic potential. Int J Parasitol. 30:1259–1212.
