Abstract
Context: Tanshinone IIA (Tan IIA) is a constituent of Danshen Salvia miltiorrhiza Bunge (Lamiaceae); however, its antifatigue activity remains unclear.
Objective: To study the antifatigue properties of Tan IIA and its underlying mechanisms.
Materials and methods: In program I, three mouse groups were separately subjected to three gavages with 0, 1 and 6 mg/kg Tan IIA and forced swimming test (FST) weekly for 8 weeks; in program II, one gavage with 0, 2 and 10 mg/kg Tan IIA was administered plus FST weekly for 4 weeks. Serum glucose, lactate, superoxide dismutase (SOD), malondialdehyde (MDA) and blood urea nitrogen (BUN) were determined after final FST.
Results: Tan IIA significantly prolonged swimming durations in program I but not in program II. Swimming times were 3208 ± 1054 and 2443 ± 1054 s for the 1 and 6 mg/kg treatments and 856 ± 292 s for the vehicle control. The two doses significantly reduced serum glucose levels (40.3 ± 8.5 and 60.0 1 ± 11.8 mg/kg) and lactate levels (61.3 ± 27.5 and 68.8 ± 8.5 mg/kg) in treated mice compared with those in control mice (137.5 ± 38.6 mg/kg and 122.7 ± 18.2 mg/kg, respectively). However, no significant differences were observed regarding SOD, MDA or BUN levels.
Discussion and conclusions: Tan IIA has antifatigue activity and is associated with reductions in serum glucose and lactate levels. Further studies should assess muscle hypertrophy and efficient aerobic glycolysis caused by Tan IIA. Tan IIA has potential as a pharmacological agent for fatigue resistance.
Introduction
It is well known that regular exercise prevents obesity and hypertension and reduces the risk of major illnesses, such as cardiovascular disease, type 2 diabetes and cancers (Blair et al. Citation2001; Crespo et al. Citation2002; Oguma et al. Citation2002; Bassuk and Manson Citation2005; Roberts and Barnard Citation2005). Despite these advantages, strenuous exercise causes skeletal muscle fatigue, a state of reversible physical exhaustion, and, hence, decreases exercise performance (Allen et al. Citation2008). Fatigue is, in part, attributed to the accumulation of lactic acid initiated by anaerobic metabolism of glucose. Therefore, high-intensity exercise is accompanied by a decrease in blood glucose levels to subphysiological concentrations and an increase in lactic acid. The latter causes lactic acidosis, leading to symptoms, such as septic or septic, cardiogenic, obstructive and haemorrhagic shock; trauma; asthma; and liver dysfunction (Andersen et al. Citation2013). In addition, ammonia, a byproduct of protein metabolism, becomes significantly elevated with intensive or prolonged exercise when ATP supply is insufficient. Ammonia toxicity can have serious central nervous system effects, which affect continuing coordinated activity (Banister and Cameron Citation1990). Ammonia is transformed into urea and is transported to the kidneys through the blood. Therefore, exhausting exercise-induced fatigue is marked by an elevation in blood urea nitrogen (BUN). Conclusively, fatigue causes damage to various organs; therefore, studies have increasingly focused on strategies to minimize fatigue.
It is well established that exercise is closely accompanied by oxidative stress responses (Coombes et al. Citation2002; Kennedy et al. Citation2005). Numerous studies in rodents and humans have confirmed that elevated levels of reactive oxygen species (ROS) in skeletal muscle result from muscle contractions during exhaustive exercise (Dillard et al. Citation1978; Davies et al. Citation1982). High levels of ROS promote contractile dysfunction resulting in muscle atrophy and fatigue. Some dietary antioxidants, such as tea catechins and red mould rice, decrease exercise-induced oxidative stress and improve fatigue resistance (Wang et al. Citation2006; Trapp et al. Citation2010).
The forced swimming test (FST) has emerged as a representative animal model for evaluating antifatigue activity of a wide variety of food or plant components (Choi et al. Citation2012; Venu Prasad and Khanum Citation2012; Xu et al. Citation2013; Hao et al. Citation2014; Huang et al. Citation2015). Tanshinone IIA (Tan IIA) is a major diterpene quinone component of a Salvia miltiorrhiza Bunge (Lamiaceae) (Danshen) hydrophobic extract. Danshen is a popular traditional phytomedicine widely used in East Asian countries for treating cardiovascular diseases and enhancing blood circulation. Tan IIA has multiple demonstrated effects. Besides being an anticoagulant (Wu et al. Citation2012) with an anti-inflammatory effect (Fan et al. Citation2009), it has also been reported to have a blood pressure lowering effect as a result of increased vasodilation (Chan et al. Citation2011). Vasodilation benefits the transportation of oxygen and nutrition (Mairbaurl Citation2013), which provides more energy to muscles during exercise. Vasodilation further improves the elimination of catabolic wastes, such as lactic acid and ammonia, which cause muscle fatigue and failure. Moreover, Tan IIA displays antioxidant activity against ROS (Fu et al. Citation2007); however, its role in reducing fatigue has not been confirmed. Hence, this study was conducted to assess the role of Tan IIA in reducing fatigue.
Materials and methods
Materials
High-performance liquid chromatography-grade Tan IIA (purity, 98%) was purchased from Kesure Biotechnology Co. (Kunming, PR China) and malondialdehyde (MDA) was purchased from Sigma-Aldrich Co. (Milwaukee, WI).
Experimental animals
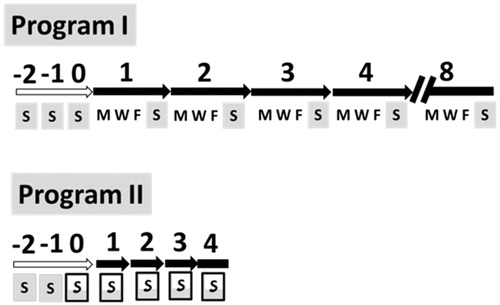
NMRI strain mice were maintained in the Animal Center of Tzu Chi University and used in accordance with IACUC guidelines. To avoid fighting among males, only female mice were subjected to antifatigue studies. Mice about 3 weeks of age and weighing 10–12 g were forced to swim three times to get accustomed to swimming. Based on their swimming performance record, the mice were separated into six groups of six mice each. The Tan IIA antifatigue experiment was carried out in two programs as follows. Program I was a course of eight consecutive weeks using three groups of mice. An oral gavage with Tan IIA at doses of 0, 1 and 6 mg/kg body weight (bw) was administered on Monday, Wednesday and Friday, and the FST was executed on Sunday. Program II was a course lasting four consecutive weeks using the other three groups of mice. An oral gavage with Tan IIA at doses of 0, 2 and 10 mg/kg bw was administered separately on Sunday of each week. After 30 min, a subsequent FST was conducted. The vehicle was administered to the control groups as 0 mg/kg bw dose for the two programs ().
Figure 1. Programs I and II of the antifatigue experiments. S, M, W and F are abbreviations for Sunday, Monday, Wednesday and Friday, respectively. Mice were fed tanshinone IIA (Tan IIA) on these weekdays. The full grey box refers to the forced swimming test (FST) on Sunday without Tan IIA and the half grey framed box refers to administering Tan IIA 30 min prior to the FST. Numbers above arrows or bars show the week numbers. White arrows indicate the accustomed swimming on three succeeding Sundays, whereas solid arrows or bars specify the real experiments. In program I, mice were given 0, 1 and 6 mg/kg body weight (bw) of Tan IIA via oral gavage on the three weekdays, followed by the FST the following Sunday. In program II, mice were given 0, 2 and 10 mg/kg Tan IIA via oral gavage, followed by FST on each Sunday. Serum was collected from each mouse at the end of last FST for quantitative determination of glucose, lactate, super oxide dismutase (SOD), malondialdehyde (MDA) and blood urea nitrogen (BUN).
Forced swimming test
The mice were fasted for 24 h before the FST. The test was conducted as described previously (Lee et al. Citation2011; Can et al. Citation2012). Briefly, the mice were individually placed into a 30 cm diameter glass jar containing 25 cm deep water maintained at 25.0 ± 1 °C. A lead wire (3% bw) was attached to each mouse’s tail. Swimming time was defined as the time from entering the water through floating, struggling and moving until near-drowning due to exhaustion. Exhaustion was determined by swimming failure, i.e., mouse immersed in water for 5 min without raising their head up to the surface of the water to breathe. Forced swimming capacity was measured by the swimming time record. After the measurement, the mice were picked up from the jar, wrapped with a paper towel to dry, and returned to their cages for the next round of testing.
Whole blood collection
Following the final FST, whole blood samples were drawn into syringes from the hearts of the mice and transferred into heparinized tubes. The samples were kept standing for 2 h at 4 °C, and serum was obtained by centrifugation at 5000 rpm for 15 min.
Serum lactate
Serum lactate concentrations were measured using the EDGE™ hand-held lactate analyser (Woodley Equipment Co., Lancashire, UK) according to the manufacturer’s instructions. Serum was siphoned using an enclosed strip, and lactate was detected using a biosensor in the kit specific for blood lactate concentration.
Serum glucose
Serum glucose concentrations were measured using the Accu Chek Performa meter (Roche Diagnostics, Indianapolis, IN) (Tack et al. Citation2012), according to the manufacturer’s instructions. Serum was siphoned using a strip and glucose was detected using the biosensor specific for blood glucose concentration.
Serum superoxide dismutase (SOD) activity
SOD activity was determined using a commercially available colorimetric 16190 SOD determination kit from Sigma-Aldrich Co. (Milwaukee, WI) for total antioxidant capacity of Danshen, according to the manufacturer’s instructions. This assay is based on the conversion reaction of WST-1 tetrazolium salt to WST-1 formazan by SOD. A sample solution (20 μL) was added to each well of test and Blank 2, and 20 μL of double distilled water was added to the wells of Blank 1 and Blank 3. The well contents were then mixed with 200 μL of WST-1 working solution. A 20 μL aliquot of dilution buffer was added for Blank 2 and Blank 3. Afterwards, 20 μL of WST-1 tetrazolium salt was added to each sample well and Blank 1. The plate was incubated at 37 °C for 20 min, and WST-1 formation was determined at a wavelength of 450 nm using a microplate-photometer (Multiskan Spectrum; Thermo Fisher Corp., Waltham, MA) to determine the optical density (OD) value. SOD activity was calculated using the following equation: SOD activity = {[(A Blank 1 − A Blank 3) − (A sample − A Blank 2)]/(A Blank 1 − A Blank 3)} × 100.
Serum malondialdehyde activity
MDA content was determined using the thiobarbituric acid method. An equal volume of 0.67% thiobarbituric acid reagent was added to each sample supernatant and boiled for 60 min at 90 °C. After cooling to room temperature, the sample was mixed with 445 μL of n-butanol, vortexed, and centrifuged at 4 °C and 12,000 rpm for 5 min. The absorbance of each supernatant was measured at 532 nm. MDA content was calculated based on the MDA standard curve.
Blood urea nitrogen
Serum BUN level was determined using a commercially available colorimetric assay kit from Sigma-Aldrich Co. (Milwaukee, WI) following the manufacturer’s instructions. The determination is based on a coupled enzyme assay. Each sample in a 96-well plate was mixed with 50 µL of a reaction mix containing urea assay buffer, peroxidase substrate, enzyme mix, developer, and the converting enzyme from the kit. The reaction time was 60 min at 37 °C in the dark, and the OD was measured at 570 nm. The standard curve was generated using a quality controlled urea standard and measured in the same way. BUN content was calculated based on the standard curve.
Statistical analysis
Results are expressed as the mean ± standard deviation (SD). Comparisons between groups were made using Student’s t-test, and a p value <0.05 or p < 0.01 was considered significant.
Results and discussion
Tan IIA increases forced swimming capacities in program I
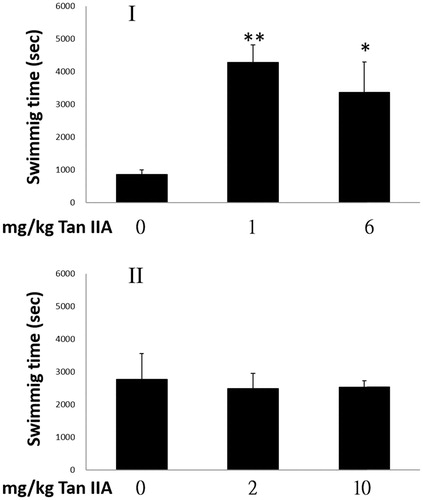
Both test and control mice were assessed for their antifatigue potential using FST. The results are shown in . The periods of swimming time were prolonged 5.0- and 3.9-fold, based on the ratios of 3208 ± 1054 s for the low (1 mg/kg) and 2443 ± 1054 s for the high (6 mg/kg) Tan IIA gavage doses, respectively, to 856 ± 292 s of the control group. These results indicate that the antifatigue activity at the higher dose was not superior to that of the lower dose. In contrast, program II did not show the antifatigue property, as swimming time was not prolonged. Judging from the significant prolongation of forced swimming time, the swimming capacities of mice were improved by Tan IIA in program I but not in program II.
Figure 2. Effects of tanshinone IIA (Tan IIA) on exhaustive swimming time of mice in programs I and II. Three groups of mice (n = 6) were separately gavaged three times weekly with 0, 1 and 6 mg/kg body weight (bw) Tan IIA and subjected to the forced swimming test (FST) for 8 weeks in program I, once-weekly with 0, 2 and 10 mg/kg bw Tan IIA and subjected to a FST for 4 weeks in program II. Durations are presented as mean ± SD in each group. *p < 0.05 and **p < 0.01 when swimming times were compared separately with the vehicle control group of each program.
Tan IIA reduces levels of blood glucose in program I
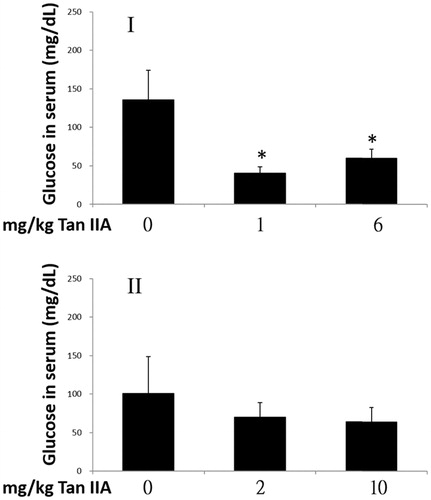
Blood glucose acts as the primary source of energy for exercise. As shown in , when mice completed the exercise in program I, blood glucose concentrations had decreased significantly by 30% and 44% in groups treated with 1 and 6 mg/kg bw Tan IIA, respectively, compared with those in the vehicle control group. However, no significant differences were observed in program II. These results suggest that the mice with enhanced forced swimming capacity consumed more glucose.
Figure 3. Effects of tanshinone IIA (Tan IIA) on serum glucose levels in programs I and II. Three groups of mice (n = 6) were separately gavaged three times weekly with 0, 1 and 6 mg/kg Tan IIA and subjected to the forced swimming test (FST) for 8 weeks in program I and once-weekly with 0, 2 and 10 mg/kg Tan IIA and subjected to the FST for 4 weeks in program II. Serum was collected to measure glucose after the final test. Data of glucose levels in mg/dL are represented as mean ± standard deviation in each group. *p < 0.05 when glucose levels are separately compared with the vehicle control group.
Tan IIA reduces serum lactate concentration in program I but increases it in program II
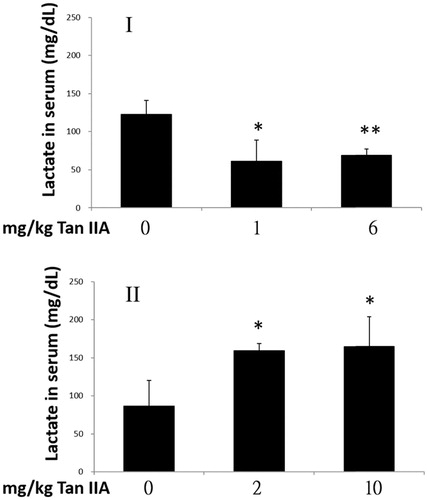
During intense or endurance exercise, the amount of ATP used increases compared with a steady state, which is then converted to ADP and Pi for use in muscle contraction. However, this breakdown releases a large quantity of protons, which cause cell acidosis, a major contributor to physical fatigue. Both protons and ATP are released during anaerobic glycolysis of glucose to pyruvate. The former can be depleted by further transformation of pyruvate into lactate, whereas the latter, together with other sources of ATP, continues to generate protons after breaking down (Robergs et al. Citation2004). Although lactate is not the direct cause of acidosis, the consistency of increased lactate production and cellular acidosis is a valid indirect biomarker for measuring the degree of muscle fatigue (Wang et al. Citation2006; Yu et al. Citation2008). As shown in , blood lactate levels of mice in exercise program I decreased significantly to 50% and 56% in response to 1 and 6 mg/kg doses of Tan IIA, respectively, compared with those in the vehicle control group. However, serum lactate levels increased significantly by 1.8- and 1.9-fold in the 2 and 10 mg/kg bw Tan IIA groups compared to those observed in program II. These results show that Tan IIA, when administered as per program I, effectively delayed the onset of fatigue, largely due to aerobic rather than an anaerobic glycolytic mechanism.
Figure 4. Effects of tanshinone IIA (Tan IIA) on serum lactate levels in programs I and II. Three groups of mice (n = 6) were separately gavaged three times weekly with 0, 1 and 6 mg/kg of Tan IIA and subjected to a forced swimming test (FST) for 8 weeks in program I and once weekly with 0, 2 and 10 mg/kg Tan IIA and subjected to a FST for 4 weeks in program II. Serum was collected to measure lactate after the final test. Data of lactate levels in mg/dL are represented as mean ± SD in each group. *p < 0.05 and **p < 0.01 when the lactate level is separately compared with the vehicle control group.
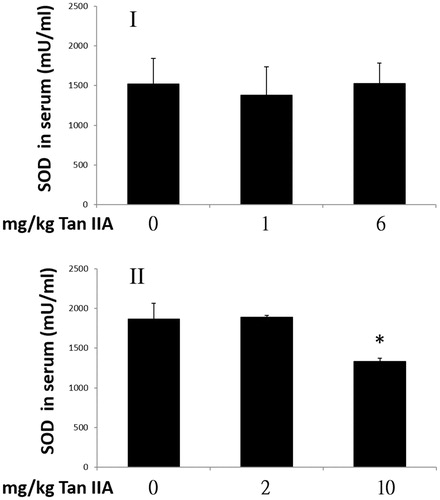
Influence of tan IIA on superoxide dismutase activity
Exhaustive exercise is accompanied by increased ROS production, and internal antioxidant enzymes protect tissues from the damage of overproduction of oxidation products during exercise. SOD protects cells from antioxidant reactions by catalysing the dismutation of superoxide radicals to O2 and H2O2. Thus, we assessed if Tan IIA activated SOD and contributed to prolonged exercise time to exhaustion; however, no significant increase in SOD activity was observed in program I. In contrast, SOD activity decreases significantly by 69% of that observed in program II at a Tan IIA concentration of 10 mg/kg, which did not influence swimming time (). These results indicate that the change in SOD might not confer the effect of Tan IIA.
Figure 5. Effects of tanshinone IIA (Tan IIA) on serum super oxide dismutase (SOD) activities in programs I and II. Three groups of mice (n = 6) were separately gavaged three times weekly with 0, 1 and 6 mg/kg Tan IIA and subjected to the forced swimming test (FST) for 8 weeks in program I and once-weekly with 0, 2 and 10 mg/kg Tan IIA and subjected to a FST for 4 weeks in program II. Serum was collected for SOD detection after the final test. Data of SOD levels in mU/mL are represented as mean ± SD in each group. *p < 0.05 when SOD levels were separately compared with the vehicle control group.
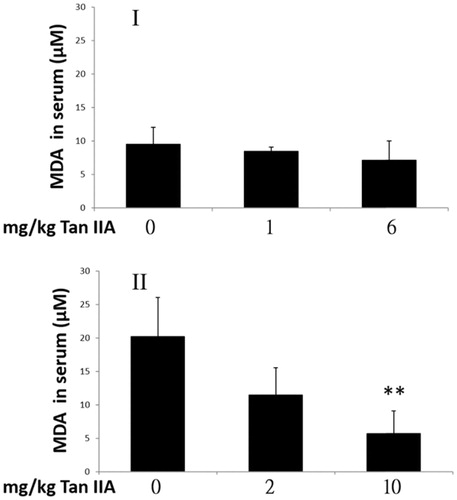
Influence of tan IIA on serum malondialdehyde concentrations
MDA is a major end-product of lipid peroxidation (Ding et al. Citation2011), which increases in liver and muscle during intense physical exercise (Wang et al. Citation2006). MDA is a valid biomarker of oxidative stress arising from membrane lipid peroxidation attack by free radicals. In a previous study, Tan IIA was shown to inhibit the lipid peroxidation effect marked by reduced MDA formation (Cao et al. Citation1996); however, the results of the present study indicate that the serum MDA levels were not significantly different from controls in either of the Tan IIA-treated groups of program I. However, MDA levels decreased by 29% in the 10 mg/kg bw Tan IIA dose group in program II (). It was unclear whether MDA levels in the Tan IIA-treated groups were elevated with extended swimming time in program I. Despite that MDA levels decreased in response to 10 mg/kg Tan IIA in program II; it was insufficient to extend swimming time.
Figure 6. Effects of tanshinone IIA (Tan IIA) on serum malondialdehyde (MDA) levels in programs I and II. Three groups of mice (n = 6) were separately gavaged three times weekly with 0, 1 and 6 mg/kg Tan IIA and subjected to the forced swimming test (FST) for 8 weeks in program I and once-weekly with 0, 2 and 10 mg/kg Tan IIA and subjected to the FST for 4 weeks in program II. Serum was collected for MDA detection after the final test. Data of MDA levels in µM are represented as mean ± SD in each group. **p < 0.01 when the MDA levels are compared with the vehicle control group.
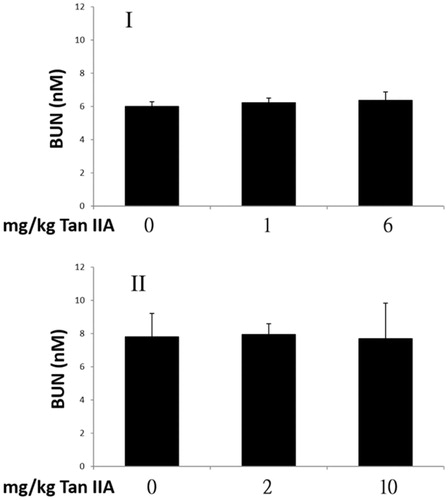
Influence of tan IIA on blood urea nitrogen concentrations
Urea is a waste product produced in the liver from protein metabolism. A positive correlation between BUN concentration and the degree of exercise tolerance is well documented (Warburton et al. Citation2002; Tang et al. Citation2008). The BUN levels are shown in . BUN concentrations in Tan IIA-treated mice were not significantly different from those of controls after the last FST in both programs. These results suggest that Tan IIA did not affect the BUN level; however, they do not exclude the possibility that BUN level increased along with prolonged swimming capacity but were reduced by Tan IIA, especially in program I.
Figure 7. Effects of tanshinone IIA (Tan IIA) on blood urea nitrogen (BUN) levels in programs I and II. Three groups of mice (n = 6) were separately gavaged thrice-weekly with 0, 1 and 6 mg/kg Tan IIA and subjected to the forced swimming test (FST) for 8 weeks in program I and once-weekly with 0, 2 and 10 mg/kg Tan IIA and subjected to a FST for 4 weeks in program II. Serum was collected for BUN detection after the final test. Data of BUN levels in nM are presented as mean ± SD in each group. No significant difference was found in BUN levels when separately compared with the vehicle control group (p > 0.05).
Speculated mechanisms for the antifatigue effect of tan IIA
The less supportive results of these serum biochemical parameters, such as SOD, MDA and BUN, suggest the involvement of another corresponding antifatigue mechanism, e.g., muscle hypertrophy. Tan IIA has been reported to selectively induce oestrogen receptor β (ERβ)-related myotube hypertrophy in vitro through an anabolic effect (Zhao et al. Citation2015). ERβ is involved in skeletal muscle hypertrophy, which is beneficial for muscle building and exercise tolerance and is inducible by the phytoecdysteroid ecdysterone (Parr et al. Citation2014). Exercise endurance was not significantly extended before the end of week 8 (data not shown). Muscle hypertrophy may shape endurance exercise performance for extended periods of time. Therefore, it is worth studying whether muscle hypertrophy is a mechanism for the antifatigue activity of Tan IIA.
Aerobic glycolysis is an effective means of converting glucose to pyruvate when oxygen supply is limited, especially during intense and short exercise. To prevent the acidosis caused by protons, pyruvate is transformed to lactate by lactate dehydrogenase. However, more oxygenated blood is presumably transported into muscle with the help of Tan IIA, which promotes the tendency for aerobic glycolysis from pyruvate, produces more ATP, and reduces the accumulation of lactate as we found in the present study (). Moreover, efficient aerobic glycolysis prevents the accumulation of protons caused by anaerobic glycolysis and renders less fatigue, in turn, prolonging exercise duration which consumes more glucose, as the results indicate ().
Conclusions
In the present study, we assessed the antifatigue activity of Tan IIA by measuring exhaustive swimming time and the serum levels of indicators related to physical fatigue. Our animal experimental results show that Tan IIA has an antifatigue function, as evidenced by extending the exhaustive swimming time as much as 5.0-fold. The antifatigue activity of Tan IIA appears to be closely associated with serum glucose depletion and lactate deprivation but poorly related to ROS scavenging activity, as well decreased BUN and MDA. Taken together, our results suggest that Tan IIA could be potentially used as a pharmacological agent to prevent fatigue.
Acknowledgments
We thank Dr. Jye-Siung Fang for continuous encouragement and Tzu-Tung Yu for technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Allen DG, Lamb GD, Westerblad H. 2008. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 88:287–332.
- Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. 2013. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 88:1127–1140.
- Banister EW, Cameron BJ. 1990. Exercise-induced hyperammonemia: peripheral and central effects. Int J Sports Med. 11:S129–S142.
- Bassuk SS, Manson JE. 2005. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol. 99:1193–1204.
- Blair SN, Cheng Y, Holder JS. 2001. Is physical activity or physical fitness more important in defining health benefits? Med Sci Sports Exerc. 33:S379–S399. discussion S419–S320.
- Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD. 2012. The mouse forced swim test. J Vis Exp. 59:e3638.
- Cao EH, Liu XQ, Wang JJ, Xu NF. 1996. Effect of natural antioxidant tanshinone II-A on DNA damage by lipid peroxidation in liver cells. Free Radic Biol Med. 20:801–806.
- Chan P, Liu IM, Li YX, Yu WJ, Cheng JT. 2011. Antihypertension induced by tanshinone IIA isolated from the roots of Salvia miltiorrhiza. Evid Based Complement Alternat Med. 2011:392627.
- Choi EH, Kang JI, Cho JY, Lee SH, Kim TS, Yeo IH, Chun HS. 2012. Supplementation of standardized lipid-soluble extract from maca (Lepidium meyenii) increases swimming endurance capacity in rats. J Funct Foods. 4:568–573.
- Coombes JS, Rowell B, Dodd SL, Demirel HA, Naito H, Shanely RA, Powers SK. 2002. Effects of vitamin E deficiency on fatigue and muscle contractile properties. Eur J Appl Physiol. 87:272–277.
- Crespo CJ, Palmieri MR, Perdomo RP, McGee DL, Smit E, Sempos CT, Lee IM, Sorlie PD. 2002. The relationship of physical activity and body weight with all-cause mortality: results from the Puerto Rico Heart Health Program. Ann Epidemiol. 12:543–552.
- Davies KJ, Quintanilha AT, Brooks GA, Packer L. 1982. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 107:1198–1205.
- Dillard CJ, Litov RE, Savin WM, Dumelin EE, Tappel AL. 1978. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J Appl Physiol Respir Environ Exerc Physiol. 45:927–932.
- Ding J-F, Li Y-Y, Xu J-J, Su X-R, Gao X, Yue F-P. 2011. Study on effect of jellyfish collagen hydrolysate on anti-fatigue and anti-oxidation. Food Hydrocolloids. 25:1350–1353.
- Fan GW, Gao XM, Wang H, Zhu Y, Zhang J, Hu LM, Su YF, Kang LY, Zhang BL. 2009. The anti-inflammatory activities of Tanshinone IIA, an active component of TCM, are mediated by estrogen receptor activation and inhibition of iNOS. J Steroid Biochem Mol Biol. 113:275–280.
- Fu J, Huang H, Liu J, Pi R, Chen J, Liu P. 2007. Tanshinone IIA protects cardiac myocytes against oxidative stress-triggered damage and apoptosis. Eur J Pharmacol. 568:213–221.
- Hao G, Zhang C, Cao W, Hao J. 2014. Effects of intragastric administration of five oyster components on endurance exercise performance in mice. Pharm Biol. 52:723–728.
- Huang WC, Chiu WC, Chuang HL, Tang DW, Lee ZM, Wei L, Chen FA, Huang CC. 2015. Effect of curcumin supplementation on physiological fatigue and physical performance in mice. Nutrients. 7:905–921.
- Kennedy G, Spence VA, McLaren M, Hill A, Underwood C, Belch JJ. 2005. Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radic Biol Med. 39:584–589.
- Lee JC, Kao JY, Kuo DH, Liao CF, Huang CH, Fan LL, Way TD. 2011. Antifatigue and antioxidant activity of alcoholic extract from Saussurea involucrata. J Tradit Complement Med. 1:64–68.
- Mairbaurl H. 2013. Red blood cells in sports: effects of exercise and training on oxygen supply by red blood cells. Front Physiol. 4:332.
- Oguma Y, Sesso HD, Paffenbarger RS, Jr, Lee IM. 2002. Physical activity and all cause mortality in women: a review of the evidence. Br J Sports Med. 36:162–172.
- Parr MK, Zhao P, Haupt O, Ngueu ST, Hengevoss J, Fritzemeier KH, Piechotta M, Schlorer N, Muhn P, Zheng WY. 2014. Estrogen receptor beta is involved in skeletal muscle hypertrophy induced by the phytoecdysteroid ecdysterone. Mol Nutr Food Res. 58:1861–1872.
- Roberts CK, Barnard RJ. 2005. Effects of exercise and diet on chronic disease. J Appl Physiol. 98:3–30.
- Robergs RA, Ghiasvand F, Parker D. 2004. Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol. 287:R502–R516.
- Tack C, Pohlmeier H, Behnke T, Schmid V, Grenningloh M, Forst T, Pfutzner A. 2012. Accuracy evaluation of five blood glucose monitoring systems obtained from the pharmacy: a European multicenter study with 453 subjects. Diabetes Technol Ther. 14:330–337.
- Tang W, Zhang Y, Gao J, Ding X, Gao S. 2008. The anti-fatigue effect of 20(R)-ginsenoside Rg3 in mice by intranasally administration. Biol Pharm Bull. 31:2024–2027.
- Trapp D, Knez W, Sinclair W. 2010. Could a vegetarian diet reduce exercise-induced oxidative stress? A review of the literature. J Sports Sci. 28:1261–1268.
- Venu Prasad MP, Khanum F. 2012. Antifatigue activity of ethanolic extract of Ocimum sanctum in rats. Res J Med Plant. 6:37–46.
- Wang JJ, Shieh MJ, Kuo SL, Lee CL, Pan TM. 2006. Effect of red mold rice on antifatigue and exercise-related changes in lipid peroxidation in endurance exercise. Appl Microbiol Biotechnol. 70:247–253.
- Warburton DE, Welsh RC, Haykowsky MJ, Taylor DA, Humen DP. 2002. Biochemical changes as a result of prolonged strenuous exercise. Br J Sports Med. 36:301–303.
- Wu LC, Lin X, Sun H. 2012. Tanshinone IIA protects rabbits against LPS-induced disseminated intravascular coagulation (DIC). Acta Pharmacol Sin. 33:1254–1259.
- Xu C, Lv J, Lo YM, Cui SW, Hu X, Fan M. 2013. Effects of oat β-glucan on endurance exercise and its anti-fatigue properties in trained rats. Carbohydr Polym. 92:1159–1165.
- Yu B, Lu Z-X, Bie X-M, Lu F-X, Huang X-Q. 2008. Scavenging and anti-fatigue activity of fermented defatted soybean peptides. Eur Food Res Technol. 226:415–421.
- Zhao P, Soukup ST, Hegevoss J, Ngueu S, Kulling SE, Diel P. 2015. Anabolic effect of the traditional Chinese medicine compound tanshinone IIA on myotube hypertrophy is mediated by estrogen receptor. Planta Med. 81:578–585.
