Abstract
Context: Hippophae rhamnoides L. (Elaeagnaceae), commonly known as seabuckthorn (SBT), is known for its medicinal and nutritional properties.
Objective: Evaluation of in vivo adjuvant activity of SBT leaf extract (SBTE) with inactivated rabies virus antigen (Rb).
Materials and methods: Swiss albino mice were immunized with aqueous-alcoholic SBTE (100 mg/kg body weight) or algel (aluminium hydroxide gel) with or without Rb (5% v/v). After priming, booster was administered on day 14. Rabies virus neutralizing antibody (RVNA) titers were estimated by rapid fluorescent focus inhibition test in sera samples collected on days 7, 14, 21, 28 and 35. Effect of adjuvant administration on cytotoxic T lymphocytes (CTLs), memory T cells, plasma and CD11c+ cells was studied by flow cytometry. In vitro hemolysis was assayed in human RBC.
Results: RVNA titers were significantly enhanced (p < 0.05) after booster administration in mice immunized with SBTE + Rb as compared to the controls. In combination, SBTE, algel and Rb, enhanced the RVNA titers. CTLs significantly increased (p < 0.05) in SBTE + Rb immunized mice. Memory T cells and plasma cells were 27.9 and 15.9%, respectively, in SBTE + Rb immunized mice as compared to that of 20.3 and 11.3%, respectively, in Rb immunized group. SBTE + Rb enhanced peritoneal CD11c+ cells (25.8%) as compared to 9.4% cells in Rb immunized mice, showed 3.2-fold increment in LPS induced IL-1β. No RBC hemolysis was observed with SBTE.
Conclusions: This study demonstrates the potential adjuvant activity of SBTE with Rb by increasing RVNA titers and CTL response.
Introduction
Vaccination results in the generation of a robust immune response against an administered antigen to provide protection in terms of both magnitude and duration (Petrovsky and Aguilar Citation2004). The new generation vaccines containing purified antigens are safer to use but generally induce weaker immune response (Arakawa Citation2011). Consequently, purified subunit vaccines require the addition of exogenous adjuvant to enhance the immune response to the antigens following immunization (Philippa et al. Citation2009).
Vaccine adjuvants represent a variegated class of compounds such as mineral salts, microbial products, emulsions, microparticles and liposomes. Dozens of adjuvants have demonstrated their efficacy in preclinical and clinical studies. Nevertheless, only a few, such as aluminium-based salts, the squalene-in-water emulsion, MF59 and monophosphoryl lipid A (MPL) have been approved for human use (Lambrecht et al. Citation2009). In fact, aluminium salts are at the moment the most widely used clinical adjuvants for both human and veterinary vaccines, however, alum has the potential to cause sterile abscesses, eosinophilia and myofascitis (Petrovsky and Aguilar Citation2004).
Adjuvant activity has been demonstrated in numerous natural products through serendipity and by trial and error. Xie et al. (Citation2008) demonstrated that saponins from the root of Platycodon grandiflorum A. DC. (Campanulaceae) increased antigen specific antibody and cellular response against ovalbumin in mice. Advax™ adjuvant derived from inulin was evaluated with influenza vaccine to enhance immunogenicity and protection in mice (Okubo et al. Citation2012). A phytol based adjuvant showing its safety and efficacy in eliciting both humoral and cell-mediated immune responses has been reported by Lim et al. (Citation2006). The most widely used saponin based adjuvants are Quil A and QS-21, derived from the bark of Quillaja saponaria M. (Quillajaceae), which have also been evaluated in numerous clinical trials. Their unique ability to activate both the Th1 immune response and the production of cytotoxic T-lymphocytes (CTLs) against exogenous antigens makes them ideal for use in subunit vaccines and vaccines directed against intracellular pathogens as well as for therapeutic cancer vaccines. However, Quillaja saponins have serious limitations such as high toxicity, hemolytic effect and instability in aqueous phase, which restrict their use as adjuvant in vaccination (Sun et al. Citation2009). Thus, one of the major challenges in adjuvant research is to gain efficacy while minimizing toxicity.
Seabuckthorn (SBT), Hippophae rhamnoides L. (Elaeagnaceae), is a wild shrub used in different parts of the world for its medicinal and nutritional properties (Gupta et al. Citation2006). Many reports have documented the immunomodulatory, anti-inflammatory and antioxidant potential of SBT (Jain et al. Citation2008; Mishra et al. Citation2008, Citation2011; Suryakumar and Gupta 2011; Jayashankar et al. Citation2012). The adjuvant activity of SBT extracts has not been explored so far. Here, we studied the adjuvant activity of SBT extract (SBTE) derived from leaves of SBT with rabies virus antigen. Our study demonstrates that SBTE has the potential to elicit both humoral and cell-mediated immune response against rabies virus antigen by increasing RVNA titers and CTL population. It also enhanced memory T cells, plasma cells and CD11c+ cell population.
Materials and methods
Collection of plant material and preparation of SBTE
Leaves of SBT were collected from hill regions of Western Himalayas, India, in the month of September 2010. After ethno-botanical identification by Dr. O.P. Chaurasia, at Defence Institute of High Altitude Research, DRDO, Leh, India, leaves were washed thoroughly in distilled water and shade dried in clean dust free conditions for extraction procedure. Dried leaves were powdered and extracted with 70% ethanol overnight at room temperature (25 ± 2 °C). The supernatant was stored and the residue was re-extracted with 70% ethanol and the process was repeated four times. Collected supernatants were pooled and vacuum dried using Buchi Rotavapor R-124 (Buchi Labortechnik AG, Postfach, CH-9230 Flawil/Schweiz, Switzerland) at 35–40 °C. The dried extract was stored at 4 °C. A stock solution of the extract in aqueous-ethanol solvent was prepared for immunization in mice.
HPLC profile of SBTE
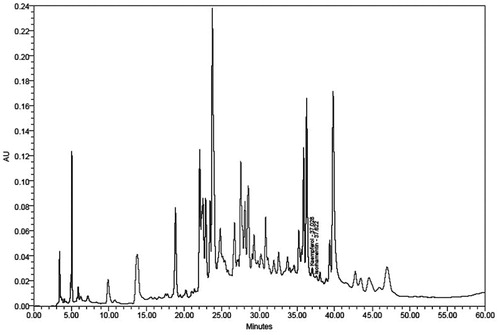
HPLC analysis of SBTE was performed using Waters HPLC system (Waters Corporation, Milford, MA) equipped with Waters 515 HPLC pump, Waters 717 plus autosampler and Waters 2487 PDA detector. Separation was performed in a symmetry C18 250 mm ×4.7 mm ID; 5 μm column by maintaining a flow rate of 1 mL/min for the mobile phase (1% acetic acid (A):methanol (B) as linear gradient run consisting of (A) 90–85% in 0–2 min, 85–70% in 3–20 min, 70–60% in 21–45 min, 60–90% in 46–60 min). Each run was followed by a 10 min equilibrium period. Two flavonoids, kaempferol and isorhamnetin, were quantified in the similar conditions. Peaks were assigned by spiking the samples with standard compounds and comparison with the retention times and spectral matching.
Antigen and adjuvant
Inactivated rabies (Rb) virus (CVS-BHK strain) antigen and the adjuvant, algel (aluminium hydroxide gel) were provided by Indian Immunological Ltd. (Hyderabad, India).
Experimental animals
Healthy, male/female Swiss albino mice, weighing 20–25 g, were collected from the Experimental Animal Facility at DIPAS. The animals were maintained in the Institute's animal house under controlled environment at 25 ± 1 °C and 12 h light–dark cycle. All the experiments were performed according to the regulations specified by the Institute's Animal Ethical Committee and conform to the National guidelines on the care and use of laboratory animals, India.
Animal immunization regimen
Mice were challenged intraperitonially (i.p.) with previously optimized SBTE dose of 100 mg/kg body weight or intramuscularly (i.m.) with algel (5% v/v) formulated with or without Rb antigen (5% v/v). Isorhamnetin was administered (i.p.) with or without Rb antigen (5% v/v). Mice immunized with PBS, SBTE or Rb antigen alone comprised the control groups. Pre-immune sera samples of all the mice were collected on day 0. Priming was done on day 1, followed by a booster dose on day 14. Subsequently, sera sample from each mouse was collected on days 7, 14, 21, 28 and 35. Samples collected on days 7 and 14 and days 21 and 28 were pooled and stored at −80 °C, until analysed for determination of rabies virus neutralizing antibody (RVNA) titers.
In vitro hemolytic assay
Heparinized human blood samples were obtained from healthy individuals after getting informed consent. Blood (5 mL) was washed three times with sterile saline solution (0.9% w/v NaCl, endotoxin free) by centrifugation at 1500 rpm for 5 min. Cell suspension was prepared by diluting the pellet to 0.5% in saline solution. Equal volumes of 0.5 mL of the cell suspension and different concentrations of SBTE (1000, 500, 250, 125, 62.5, 31.2, 15.6 and 7.8 µg/mL) were mixed in saline solution. The mixture was incubated at 37 °C for 30 min, and centrifuged at 2000 rpm for 10 min. Haemoglobin in the supernatants was measured spectrophotometrically at 412 nm (Biotek Instruments, Winooski, VT). Saline and distilled water were used as negative and positive controls, respectively.
Estimation of RVNA titers
Rapid fluorescent focus inhibition test (RFFIT) was used to measure RVNA titers as per WHO recommended procedure (Smith et al. Citation1996) with some modifications. BHK 21 (ATCC CCL 10) and BHK21 adapted CVS 13 strain of rabies virus, were used in this study. An in-house reference serum was used having titer of 30 IU/mL and was calibrated against 2nd International Reference Standard (National Institute of Biological standards, UK). In brief, doubling dilutions of sera samples and reference sera (heat inactivated at 56 °C for 30 min in a water bath) in duplicate were prepared in 96 well plates using Iscove's Modified Dulbecco's Medium (IMDM) (Sigma, St. Louis, MO). To each of the well, 100 µL of the sera dilution and 100 µL of CVS (100 FFD50) were added and the plate was incubated at 37 °C for 1 h. BHK 21 cells were trypsinized and resuspended in 10 mL of IMDM with 10% FCS (Sigma, St. Louis, MO). Cell control and virus controls were also included. To each well of the 96-well plate, 100 µL of cell suspension was added and the plate was incubated at 37 °C in a CO2 incubator (Sanyo, Osaka, Japan) for 24 h. After the incubation, cells were fixed in cold acetone for 30 min and stained by direct fluorescence antibody technique (FAT) using commercially available Rb N conjugate (Light Diagnostics, Sunnyvale, CA). The 96-well plates were then observed under an inverted fluorescence microscope (Nikon Eclipse, Tokyo, Japan). The end point dilution was taken as the highest dilution of serum showing 50% inhibition of fluorescence foci. The antibody titers were converted to IU/mL in comparison with reference serum. Protective neutralization antibody titer limit was considered >0.5 IU/mL.
Cytotoxic T lymphocyte (CTL) and plasma cell responses
Heparinized blood was collected on 5th day post booster from immunized mice. Briefly, RBCs were lysed using FACS lysing solution, washed twice with PBS followed by the surface staining of the cells with FITC labelled CD8 (BD Biosciences, Franklin Lakes, NJ) or PE labelled CD138 (BD Biosciences, Franklin Lakes, NJ) antibody for 30 and 45 min, respectively, in dark at room temperature. For intracellular staining, CD8-FITC stained cells were washed with PBS, fixed in Cytofix, permeabilized using Cytoperm and labelled with PE conjugated Granzyme B antibody. Stained cell population was counted in FACS Calibur Flowcytometer using Cell Quest Pro software. All the reagents used in the assay were purchased from BD Biosciences (Franklin Lakes, NJ).
Memory T cell responses
Heparinized blood samples were collected from immunized mice by retro-orbital puncture, four months after the booster. Briefly, whole blood cells were stained with FITC labelled CD62L and PE labelled CD44 antibodies for 45 min in dark at room temperature. After RBC lysis cells were washed twice with PBS, re-suspended in PBS and acquired using Flowcytometer (BD Biosciences, Franklin Lakes, NJ) by gating 10,000 events using Cell Quest Pro software.
CD11c+ cell recruitment and ex vivo estimation of IL-1β production by peritoneal cells
Intra-peritoneal injection of thioglycollate (4%) (Sigma, St. Louis, MO) was given after administration of booster on day 14. Mice were euthanized after 72 h. Peritoneal cells were aseptically isolated as described elsewhere (Jayashankar et al. Citation2012). Peritoneal cells (1 × 106 cells/mL) with or without LPS (0.1 µg/mL) were cultured in RPMI supplemented with 10% FBS in 5% CO2 incubator at 37 °C for 48 h. Cell culture supernatants were harvested after incubation and concentrations of cytokines were measured using commercially available ELISA kits (eBiosciences, San Diego, CA). For flowcytometric analysis, cells were stained with FITC labelled CD11c antibody for 45 min in dark, immediately after isolation from peritoneal lavage. Cells were acquired in Flowcytometer (BD Biosciences, Franklin Lakes, NJ).
Statistical analyses
SPSS 16.0 software was used for calculating statistical significance by applying non-parametric Wilcoxon’s signed ranks test and independent t-tests for comparing the control groups with the experimental groups (SPSS Inc., Chicago, IL). p < 0.05 was considered to be significant.
Results
HPLC analysis
The presence of kaempferol and isorhamentin in SBTE was confirmed with a retention time of 37.02 and 37.62, respectively. The content of kaempferol and isorhamnetin was found to be 0.29 and 0.14% w/w, respectively ().
In vitro hemolytic assay
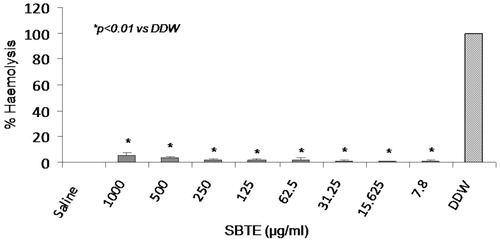
This assay was performed to rule out the hemolytic effect of SBTE at different concentrations in human blood with distilled water as positive control and 0.5% saline as negative control. No significant increase in the % hemolysis was observed in cells treated with any of the SBTE dose in comparison to the saline treated cells ().
Figure 2. Hemolytic activity of SBTE. Hemolytic activity of different concentrations of SBTE (1000–7.8 mg/L) was measured in blood samples collected from healthy volunteers (n = 4). The graph represents the mean values of % hemolysis ± SD. Saline was used as negative control which shows minimum hemolysis and double distilled water (DDW) was used as positive control showing maximum hemolysis. One-way ANOVA Dunett’s T3 test was applied to calculate significance between the hemolytic activity of different concentrations of SBTE and positive control. p value <0.05 was considered as significant.
RVNA titers
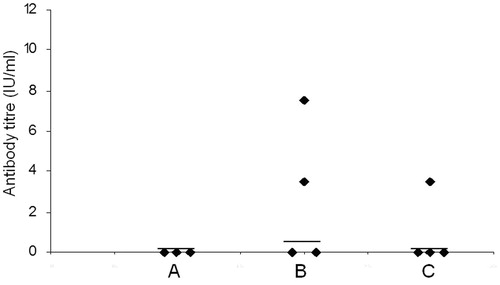
Difference in the humoral immune response generated by different groups of mice immunized with SBTE or algel with inactivated Rb virus antigen was analysed. SBTE was used for determination of its adjuvant activity and compared with commercially available algel. Mice immunized with PBS were considered as controls. Protective RVNA titers were not detected in pre-immune sera samples in any of the groups (). After priming, increase in the antibody titers was observed in mice immunized with SBTE + Rb and algel + Rb, however, it was not found statistically significant as compared to the PBS group () whereas after days 21, 28 and 35 there was a significant increase in RVNA titers (>0.5 IU/mL) in both SBTE–Rb and algel + Rb immunized mice as compared to control group of mice (). A combination of SBTE, algel and Rb antigen not only showed significant increase in RVNA titers after priming and booster administration but also increased the number of responders () as compared to that in the case of SBTE or algel immunized mice (). Isorhamnetin with rabies antigen also showed some increase in RVNA titers after priming ().
Figure 3. Rabies virus neutralizing antibody titers in mice sera. Sera samples were collected on (A) day 0 (pre-immunization); (B) days 7 and 14 (pooled); (C) days 21 and 28 (pooled); (D) day 35. PBS: mice immunized with PBS (n = 10); SBTE + Rb: mice immunized with SBTE + Rb antigen (n = 20); Al + Rb: mice immunized with algel + rabies antigen (n = 20). Experiments were done thrice. Booster was administered on day 14. Horizontal lines indicate the median values. Non-parametric Wilcoxon’s signed ranks test was applied for comparing different groups. p < 0.05 was considered to be significant; ns = not significant. Protective neutralization antibody titer limit was considered >0.5 IU/mL. Mice immunized with Rb antigen alone (n = 4) showed no protective RVNA titers (data not shown).
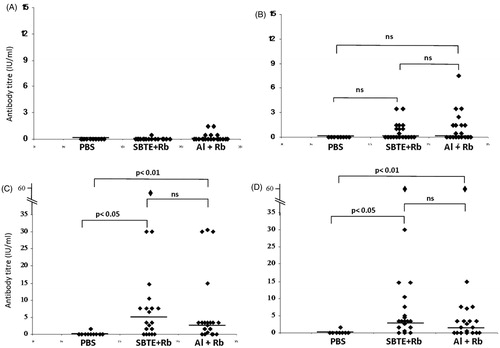
Figure 4. RVNA titers in mice sera from SBTE + Al + Rb immunized mice. Sera samples from immunized mice (n = 10) collected on (A) day 0 (pre-immunization); (B) days 7 and 14 (pooled); (C) days 21 and 28 (pooled); (D) day 35. Horizontal lines indicate the median values. Non-parametric Wilcoxon’s signed ranks test was applied for comparing antibody titers of pre-immunized group with post-immunized group. p < 0.05 was considered to be significant.
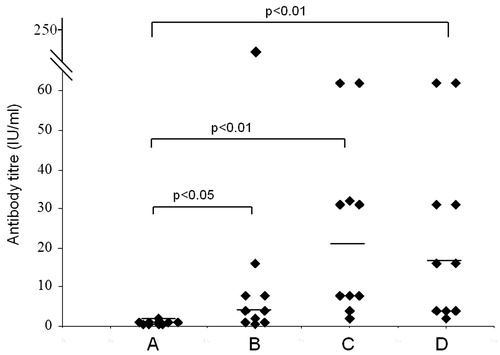
Figure 5. RVNA titers in mice sera from isorhamnetin + Rb immunized mice. Sera samples from immunized mice (n = 4) collected on (A) day 0 (pre-immunization); (B) days 7 and 14 (pooled); (C) days 21 and 28 (pooled). Horizontal lines indicate the median values. Mice immunized with Rb antigen alone (n = 4) showed no protective RVNA titers (data not shown).
CTL response with SBTE and rabies antigen
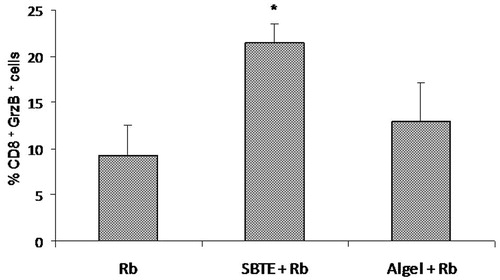
Cytotoxic T lymphocyte (CD8+ Granzyme B+) cell population in peripheral blood of immunized mice was evaluated by flowcytometry. The percentage of CD8+ Granzyme B+ cell population was significantly higher (p < 0.05) in SBTE + Rb immunized mice as compared to the Rb antigen immunized group. Algel + Rb immunized mice also showed increase in the CTL population which was not statistically significant ().
Figure 6. CTL response by SBTE. Bar graph showing the mean ± SEM (n = 3) of % CD8+ Gr B+ cell population in gated peripheral blood lymphocytes of different groups immunized with Rb antigen, SBTE + Rb antigen and algel + Rb antigen. Independent-samples t-test was applied for calculating significance between the immunized groups. *p value <0.05 vs. Rb.
Memory T cell and plasma cell responses and recruitment of CD11c+ cells in peritoneum after immunization with SBTE and Rb antigen
Resting memory T cells (CD44+ CD62L+) and CD138+ plasma cells from peripheral blood of immunized mice were evaluated in gated lymphocyte population by flowcytometry. CD44+ CD62L+ cell population was 27.9% in SBTE + Rb immunized group which was higher than the 20.3% memory cell population in Rb antigen immunized group (). The CD138+ cell population in SBTE + Rb immunized groups was 15.9% which was higher compared to 11.3% CD138+ cells in Rb immunized group (). CD11c+ cells from peritoneal lavage of immunized mice were cell surface stained with FITC conjugated antibody. SBTE + Rb immunized mice showed 25.8% CD11c+ cells as compared to 9.4% cell population in Rb antigen immunized group ().
Table 1. Percent memory T cells, plasma cells and DCs in SBTE + Rb immunized mice.
IL-1β production by peritoneal cells in response to SBTE
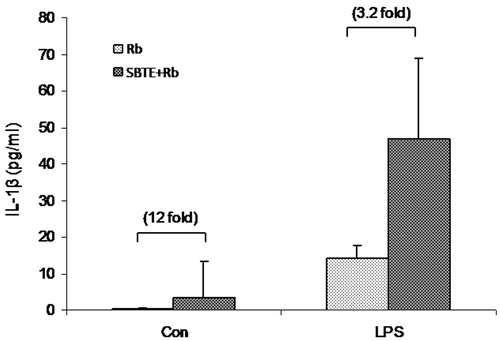
Peritoneal cells from immunized mice were cultured for 48 h to assess LPS stimulated IL-1β production in cell supernatants. Although, statistically not significant, spontaneous IL-1β production in SBTE + Rb immunized mice increased to 12-fold in SBTE + Rb immunized mice as compared to Rb immunized mice. Also a 3.2-fold increase was observed in the LPS stimulated IL-1β production by cells from SBTE + Rb immunized mice as compared to Rb immunized mice ().
Figure 7. IL-1β production by peritoneal lavage cells in response to SBTE. Peritoneal cells were isolated from different groups of immunized mice (n = 3) as indicated: Rb: rabies antigen immunized; SBTE + Rb: SBTE + Rb antigen immunized and were cultured with or without LPS. Cells supernatants after 48 h were assayed for IL-1β. Values in bracket indicate the fold increase IL-1β production in comparison to the Rb antigen immunized group.
Discussion
Adjuvants are the immune enhancing additives and defined as any substance that, when added into a vaccine formulation, act generally to accelerate, prolong and enhance the quality of specific immune response. Vaccine adjuvants target the innate immune system to enhance humoral and cellular responses to the antigens administered. In the last decade, a better understanding of the innate immunity pathways lead to the characterization of the mechanism of action of the vaccine adjuvants derived from microbial structures (Seubert et al. Citation2011).
Plant-originated adjuvants are gaining much attention, known to promote different branches of the immune system and have the potential to be used in design of new vaccines so as to induce a desired immune response. Saponin adjuvants are known to stimulate secretion of a broad range of cytokines, suggesting that they may act by triggering innate immunity. Saponin (Quil A) extracted from Quillaja saponaria and its reverse phase HPLC-released saponin QS-21A have been used in a series of commercial veterinary vaccines as well as human vaccines formulation (Sun et al. Citation2009). In some recent reports (Song and Hu Citation2009; Liu et al. Citation2012; Su et al. Citation2014), the adjuvant activity of Chinese herbal polysaccharides and saponins in inactivated veterinary rabies vaccine is also demonstrated.
Commercially available inactivated rabies virus vaccines either do not contain adjuvant or are adjuvanted with alum, which promotes a Th2 response in mice (Lindblad Citation2004; Abeer et al. Citation2011). Here, we report that an extract from SBT leaves has potential adjuvant activity with inactivated rabies virus antigen. SBTE is safe for administration as it showed least hemolytic activity in human RBCs and is comparatively more efficacious than algel.
Antibodies play a central role in prophylaxis against many infectious agents. Animal models of protection against rabies have demonstrated the essential role of neutralizing antibodies (Hooper et al. Citation1998). RVNA results in viral clearance from the central nervous system of experimentally infected mice (Moore and Hanlon Citation2010). In the present study, we have demonstrated that the SBTE significantly enhanced the RVNA titers in Swiss albino mice. The antibody titer was higher in SBTE–Rb antigen immunized mice compared to algel–Rb antigen immunized mice. A combination of SBTE, algel and Rb antigen when administered in mice showed enhanced RVNA as well as number of responders immediately after priming prior to booster administration. Therefore, SBTE and algel together can be combined to achieve an early vaccine response.
SBT is known to contain many flavonoids such as catechin, rutin, quercetin, isorhamnetin and kaempferol that contribute to its various pharmacological properties (Zu et al. Citation2006; Upadhyay et al. 2010). Since isorhamnetin was also identified in SBTE, we tested this flavonoid with rabies antigen for RVNA titers in mice. Isorhamentin showed enhanced protective RVNA titers, although less than that of SBTE induced RVNA titers suggesting the contribution from other flavonoids for SBTE bioactivity.
Antibodies are produced by terminally differentiated plasma cells. Within haematopoietic system, CD138 expression is restricted to plasma cells (O’Connell et al. Citation2004). In our study, SBTE + Rb administration showed higher CD138+ cell population compared to CD138+ cells in Rb immunized mice (). This data corroborates with the increased RVNA titers.
In rabies virus vaccination, it is widely accepted that neutralizing Abs are essential for protection but experimental infections in mice suggest that cell-mediated immune responses are required for efficient viral clearance (Johnson et al. Citation2010). The contribution of T cells to antiviral immunity in humans has been well established for many viral pathogens. CD8+ T cells follow a program of proliferation and differentiation into CTL armed with effector functions that facilitate pathogen clearance or containment. The CTLs utilize granzymes and perforins to kill virus-infected cells as a major line of defence (Rock et al. Citation2005). After viral clearance, a pool of virus-specific memory CD8+ T cells survive long term in the host (Byers et al. Citation2003). A subset of CD8+ T cells, called the resting memory T cell population are designated as CD62LhiCD44hi (Singh Citation2007). Our study also showed that CD8+ Granzyme B+ CTL () and the resting memory T cell populations () increased in SBTE-rabies antigen immunized group compared to that in rabies antigen immunized group.
Recent insights into the mechanism of action of adjuvants have revealed the recruitment of monocytes and DCs at the site of injection and role of inflammasome pathway in production of proinflammatory cytokine, IL-1β (Kool et al. Citation2008; Lambrecht et al. Citation2009). Myeloid dendritic cells and macrophages are known to express CD11c cell surface marker (Murray and Wynn Citation2011). Proinflammatory cytokines enhance immune response to vaccine, leading to significantly higher neutralizing Ab titers (Horowitz et al. Citation2010). Here also, we investigated the recruitment of CD11c expressing DCs in the peritoneal lavage of immunized mice as well as ex vivo LPS induced IL-1β production by these cells. SBTE–Rb immunized mice showed relatively higher population of CD11c+ cells () and increased IL-1β production () clearly indicating the increased recruitment of APCs at the site of injection. Further, increment in IL-1β production by the peritoneal cells suggests the activation of innate immune pathways.
Our study strongly shows the potential of SBTE as a novel adjuvant for rabies vaccines that can be extrapolated for use in larger animals like dogs which are the main vectors of transmission of rabies to humans. Based on our results, the bioactive components, isorhamnetin and other flavonoids from SBTE, can further be evaluated with rabies vaccine. This adjuvant may play a significant role in mass vaccination campaigns of dogs and in the control of rabies as presently the dogs are getting a single dose of vaccine which most often does not induce protection against rabies.
Acknowledgements
The authors thank Director, Defence Institute of High Altitude Research (Leh, India), DRDO, for providing the plant material. Sincere thanks to Dr. Kshipra Misra, DIPAS, for analysing the HPLC profile of SBTE. Authors sincerely thank Indian Immunologicals Limited, Hyderabad, India, for providing inactivated Rb virus antigen, algel and immunization protocol for conducting this study.
Disclosure statement
The authors have no conflict of interest.
Additional information
Funding
References
- Abeer AH, Afaf B, Messalam AH. 2011. Immunostimulant effect of different fractions of Nigella sativa L. seeds against rabies vaccine. Nat Sci. 9:90–96.
- Arakawa T. 2011. Adjuvants: no longer a 'dirty little secret', but essential key players in vaccines of the future. Expert Rev Vac. 10:1–5.
- Byers AM, Kemball CC, Moser JM, Lukacher AE. 2003. Cutting edge: rapid in vivo CTL activity by polyoma virus-specific effector and memory CD8+ T cells. J Immunol. 171:17–21.
- Gupta A, Kumar R, Pal K, Banerjee PK, Sawhney RC. 2006. Influence of Sea buckthorn (Hippophae rhamnoides L.) flavone on dermal wound healing in rats. Mol Cell Biochem. 290:193–198.
- Hooper DC, Morimoto K, Bette M, Weihe E, Koprowski H, Dietzschold B. 1998. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J Virol. 72:3711–3719.
- Horowitz A, Behrens RH, Okell L, Fooks AR, Riley EM. 2010. NK cells as effectors of acquired immune responses: effector CD4+ T cell-dependent activation of NK cells following vaccination. J Immunol. 185:2808–2018.
- Jain M, Ganju L, Katiyal A, Padwad Y, Mishra KP, Chanda S, Karan D, Yogendra KMS, Sawhney RC. 2008. Effect of Hippophae rhamnoides leaf extract against dengue virus. Phytomedicine. 15:793–799.
- Jayashankar B, Mishra KP, Kumar MSY, Udayasankar K, Misra K, Ganju L, Singh SB. 2012. A supercritical CO2 extract from seabuckthorn leaves inhibits pro-inflammatory mediators via inhibition of mitogen activated protein kinase p38 and transcription factor nuclear factor-κB. Int Immunopharmacol. 13:461–467.
- Johnson N, Cunningham AF, Fooks AR. 2010. The immune response to rabies virus infection and vaccination. Vaccine. 28:3896–3901.
- Kool M, Soullié T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. 2008. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 205:869–882.
- Lambrecht BN, Kool M, Willart MAM, Hammad H. 2009. Mechanism of action of clinically approved adjuvants. Curr Opin Immunol. 21:23–29.
- Lim SY, Matt M, Richard AK, Ghosh SK. 2006. Phytol-based novel adjuvants in vaccine formulation: 1. Assessment of safety and efficacy during stimulation of humoral and cell-mediated immune responses. J Immune Based Ther Vac. 4:6.
- Lindblad EB. 2004. Aluminium compounds for use in vaccines. Immunol Cell Biol. 82:497–505.
- Liu Y, Zhang S, Zhang F, Hu R. 2012. Adjuvant activity of Chinese herbal polysaccharides in inactivated veterinary rabies vaccines. Int J Biol Macromol. 50:598–602.
- Mishra KP, Chanda S, Karan D, Ganju L, Sawhney RC. 2008. Effect of Seabuckthorn (Hippophae rhamnoides) flavone on immune system: an in-vitro approach. Phytother Res. 22:1490–1495.
- Mishra KP, Mishra R, Yadav AP, Jayashankar B, Chanda S, Ganju L. 2011. A comparative analysis of immunomodulatory potential of Seabuckthorn leaf extract in young and old mice. Biomed Aging Pathol. 1:61–64.
- Moore SM, Hanlon CA. 2010. Rabies-specific antibodies: measuring surrogates of protection against a fatal disease. PLoS Negl Trop Dis. 4:e595.
- Murray PJ, Wynn TA. 2011. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 11:723–727.
- O’Connell FP, Pinkus JL, Pinkus GS. 2004. CD138 (Syndecan-1), a plasma cell marker: immunohistochemical profile in hematopoietic and nonhematopoietic neoplasms. Am J Clin Pathol. 121:254–263.
- Okubo HY, Saade F, Petrovsky N. 2012. Advax™, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine. 30:5373–5381.
- Petrovsky N, Aguilar JC. 2004. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 82:488–496.
- Philippa M, McKee AS, Munks MW. 2009. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 9:287–293.
- Rock MT, Yoder SM, Wright PF, Talbot TR, Edwards KM, Crowe Jr JE. 2005. Differential regulation of granzyme and perforin in effector and memory T cells following smallpox immunization. J Immunol. 174:3757–3764.
- Seubert A, Calabro S, Santini L, Galli B, Genovese A, Valentini S, Aprea S, Colaprioc A, D’Oro U, Giuliani MM, et al. 2011. Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. PNAS. 108:11169–11174.
- Singh M. 2007. Vaccine adjuvants and delivery systems (2007 ed.). Hoboken (NJ): John Wiley & Sons, Inc. p. 33–52.
- Smith JS, Yager PA, Baer GM. 1996. A rapid fluorescent focus inhibition (RFFIT) for determining rabies virus-neutralizing antibody. In: Laboratory techniques in rabies. 4th ed. Geneva: World Health Organization.
- Song X, Hu S. 2009. Adjuvant activities of saponins from traditional Chinese medicinal herbs. Vaccine. 27:4883–4890.
- Su X, Pei Z, Hum S. 2014. Ginsenoside Re as an adjuvant to enhance the immune response to the inactivated rabies virus vaccine in mice. Int Immunopharmacol. 20:283–289.
- Sun HX, Xiea Y, Yec YP. 2009. Advances in saponin-based adjuvants. Vaccine. 27:1787–1796.
- Suryakumar G, Gupta A. 2011. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.). J Ethnopharmacol. 138:268–277.
- Upadhyay NK, Yogendra Kumar MS, Gupta A. 2010. Antioxidant, cytoprotective and antibacterial effects of Sea buckthorn (Hippophae rhamnoides L.) leaves. Food Chem Toxicol. 48:3443–3448.
- Xie Y, Pan H, Sun H, Li D. 2008. A promising balanced Th1 and Th2 directing immunological adjuvant, saponins from the root of Platycodon grandiflorum. Vaccine. 26:3937–3945.
- Zu Y, Li C, Fu Y, Zhao C. 2006. Simultaneous determination of catechin, rutin, quercetin kaempferol and isorhamnetin in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. J Pharm Biomed Anal. 41:714–719.