Abstract
Context: The roots of Lophira lanceolata Van Tiegh. Ex Keay (Ochnaceae) have numerous medicinal values in the Central African region. Even though the MeOH extract of the roots has shown antimycobacterial activities, the constituents responsible for this inhibitory activity remain unknown.
Objective: Phytochemical investigation of the MeOH root extract of L. lanceolata and determination of the antimycobacterial activities of that extract and constituents against the growth of Mycobacterium tuberculosis.
Materials and methods: Column chromatography was used to provide bioactive phytoconstituents. Those compounds were elucidated using MS and NMR spectroscopic data. Antimycobacterial screening of the extract (4.882–5000 µg/mL in DMSO during 24 h at 37 °C) and isolated compounds (0.244–250 µg/mL in DMSO during 24 h at 37 °C) was performed by microplate alamar blue assay (MABA) against two mycobacterial strains.
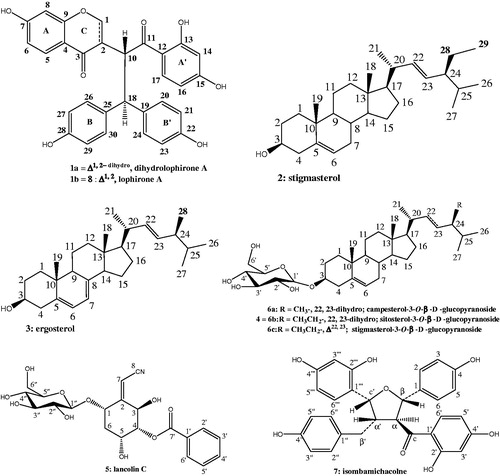
Results: The investigation of L. lanceolata MeOH roots extract provided of mixture of unseparated biflavonoids with a newly described one, dihydrolophirone A (1a) associated to lophirone A (1b). The bioactive compounds that effectively inhibited the growth of M. tuberculosis AC45 were found to be compounds 1 and 2. They exhibited MIC values of 31.25 and 15.75 µg/mL, respectively, and their MIC was found to be 62.5 µg/mL against resistant strain AC83.
Discussion and conclusions: It is clearly evident from the results obtained that the mycobacterial activity of L. lanceolata could be related mainly to its steroid and flavonoid contents. Therefore, this study suggests the potential of the above-mentioned classes of compounds as promising candidate agents for developing new anti-tuberculosis drugs.
Introduction
Tuberculosis (TB) is a chronic contagious and deadly disease that spreads through the air. The number of patients infected with the disease is rising worldwide. In 2015, there were an estimated 10.4 million cases of TB with over 400,000 deaths resulting from TB disease among people living with HIV (World Health Organization [WHO] Citation2016). The proportion of TB cases living with HIV was highest in the African region despite the implementation of the standardized control strategy (Directly Observed Treatment Short course [DOTS]) (WHO Citation1996). Thus, the incidence, prevalence and mortality rates of TB in Africa have continued to be on increase (WHO Citation2016). In addition, the high susceptibility of human immunodeficiency virus-infected persons to this illness, the proliferation of HIV/AIDS, and the emergence of multidrug-resistant strains of Mycobacterium tuberculosis (MDR) are contributing to the worsening impact of this disease (Bloom Citation2002). Currently, the first-line regimen for treating TB is considered old and prescribes rifampicin (RMP) and isoniazid (INH) as component drugs. These antibiotics overtime affect the rise of multi-drug resistant (MDR). This increasing drug resistance incidence has led to an urgent need to develop new antitubercular drugs with low toxicity to overcome these limitations and increase the armamentarium of the existing therapeutic drugs.
The genus Lophira (Ochnaceae) is found throughout tropical regions of Africa (Ghogomu et al. Citation1989a). The genus is widely exploited commercially to build houses and to make furniture (Tih et al. Citation1992). Lophira lanceolata Van Tiegh. Ex Keay is a tall tree reaching up to 60 feet high growing in the woody savannah forests. This plant is used in African folk medicine for the treatment of diseases related mainly to toothache, liver infections, female sterility, cough, fever, heart pains, blood spitting, intercostal pain, stomach pain, dysmenorrhea, respiratory troubles, and to relieve the gripping of dysentery (Persinos et al. Citation1967; Ghogomu et al. Citation1989a). Previous phytochemical analyses of the genus Lophira have resulted in the isolation of biflavonoids and tetraflavonoids (Ghogomu et al. Citation1987, Citation1989a, Citation1989b, Citation1990; Tih et al. Citation1992; Pegnyemb et al. Citation1994; Sani et al. Citation2010, Citation2011; Tih, Ghogomu, et al. Citation2003; Tih, Martin, et al. Citation2003), nitrile glycosides (Murakami et al. Citation1993; Tih et al. Citation1994), benzamides (Persinos et al. Citation1967), a benzoylglucoside (Pegnyemb et al. Citation1998), triterpenoids (Sani et al. Citation2011).
In continuation of our phytochemical studies on Lophira lanceolata, other chemical constituents of the roots of this species have been investigated; newly isolated compounds could support the traditional uses of the plant, based on their medicinal interest. Although there is no information on the antitubercular activity of this plant or its constituents, previous reports on antitubercular activity of steroids and biflavonoids have designated immense prospects in this field (Camacho-Corona et al. Citation2009; Thakur and Gothwal Citation2015). It has been claimed that several plant natural products inhibit several species of mycobacteria (Okunade et al. Citation2004; Pauli et al. Citation2005). The Lophira genus through the richness of its components could be considered as an important source for new antitubercular agents.
We report herein the isolation and identification of a new biflavonoid derivative and other constituents from the methanol extract of Lophira lanceolata with their antitubercular properties.
Materials and methods
General procedures
Melting points were uncorrected and were measured on a Mettler Toledo instrument. IR spectra were recorded on an Alpha FT-IR Spectrometer from Bruker, while 1D and 2D NMR spectra were obtained on a Bruker DRX 500 (500 MHz for 1H and 125 MHz for 13C spectra) spectrometer (Bruker, Rheinstetten, Germany) with chemical shifts reported in δ (ppm) using TMS (δH) as an internal standard. The HR-ESI-MS were obtained on LTQ-FT instrument (Thermo Scientific, Waltham, MA). UPLC–MS were measured by a Shimadzu UPLC–MS system using a L− column 2 ODS (I.D. 2.1 × 100 mm, Chemical Evaluation and Research Institute, Tokyo, Japan), at a flow rate of 0.2 mL/min, a detection wavelength of 350 and 300 nm, and FMW (HCOOH/MeCN/H2O = 1:12:87) as eluent, ESI+ 4.5 kV, ESI− 3.5 kV, 250 °C. Optical rotations were measured on a Perkin-Elmer 341 polarimeter. Silica gel 60 (230–400 mesh E. Merck, Darmstadt, Germany) was employed for column chromatography (CC), the solvent mixing systems for elution were mainly CH2Cl2–MeOH with increasing polarity. Analytical thin layer chromatography (TLC) was carried out on Merck silica gel (Merck, Darmstadt, Germany) 60 PF254 plates (0.25 mm layer thickness).
Plant material
The stem roots of L. lanceolata were collected at Balamba, near Yaoundé in the Center region of Cameroon (4° 26′. 00′′ N, 11° 14′. 00′′ E) in July 2014 and authenticated by Mr. Victor Nana a botanist. A voucher specimen (N° 45596 HCN) was deposited at the National Herbarium in Yaoundé, Cameroon.
Extraction and isolation
Dried and powdered stem roots of L. lanceolata (854 g) was extracted with methanol (6.0 L, three times) at room temperature. The combined methanol extract was evaporated under reduced pressure to give a thick gummy mass (34.6 g) that was suspended in water and successively extracted with n-hexane, dichloromethane and ethyl acetate to afford the corresponding subfractions. The ethyl acetate soluble sub-fraction (13.0 g) was subjected to CC (4 × 120 cm; 150 g of silica gel 230–400 mesh) eluting with a gradient solvent system (CH2Cl2–MeOH) to obtain five fractions I–V. Fraction I (1.98 g) was further purified by CC (1.5 × 80 cm; 20 g of silica gel 230–400 mesh) eluting with CH2Cl2–MeOH (50:1 to 40:1) to afford compounds 2 (8 mg) and 3 (3 mg), respectively. Fraction II (1.86 g) resulting from CH2Cl2–MeOH (35:1 to 20:1) was chromatographed by CC (1.5 × 80 cm; 20 g of silica gel 230–400 mesh) using CH2Cl2–MeOH (40:1 to 15:1) to afford compound 4 (180 mg). Fraction III (2.73 g) resulting from CH2Cl2–MeOH (20:1 to 10:1) was chromatographed by CC (2.5 × 100 cm; 60 g of silica gel 230–400 mesh) to afford three sub-fractions IIIa–IIIc. Sub-fraction IIIa (0.88 g) was further purified by CC (1 × 25 cm; 40 g of silica gel 230–400 mesh) in gradient elution mixture solvent composed of CH2Cl2–MeOH (25:1 to 15:1) to afford compound 5 (10 mg). Sub-fraction IIIc (1.08 g) was further purified by silica gel column (1.5 × 80 cm; 20 g of silica gel 230–400 mesh) using CH2Cl2–MeOH (30:1 to 15:1) to produce a mixture of three components 6 (28 mg). Fraction IV (1.20 g) expected from CH2Cl2–MeOH (10:1 to 5:1) was rechromatographed (2 × 100 cm; 25 g of silica gel 230–400 mesh) using the solvent system CH2Cl2–MeOH (15/1 to 5/1) to give compound 1 (14 mg). Fraction V (4.42 g) afforded from CH2Cl2–MeOH (5:1 to 1:1) was chromatographed by CC over SiO2 eluted with CH2Cl2–MeOH (8:1 to 1:1) and purified by Sephadex LH-20 using pure MeOH as eluent to provide 7 (12 mg).
(2R*)-2,3-Dihydrolophirone A (1a)
Orange amorphous powder; m.p. 265–269 °C; TLC Rf: 0.40 (CH2Cl2–MeOH; 10:1); IR (KBr) cm−1: 3444, 2970, 2854, 1710, 1652, 1603, 1572, 1071, 1054; 1H and 13C NMR spectral data (500 and 125 MHz, DMSO-d6), see ; HR-ESI-MS m/z: 511.1396 [M–H]− (calcd. C30H24O8-H: 511.1394); 535.1363 [M + Na]+ (calcd. C30H24O8Na+: 535.1369).
Table 1. 1H and 13C NMR spectroscopic dataa of compounds 1a and 1b (500 and 125 MHz in DMSO-d6) δ in ppm, with those of reference (compound 8Table Footnotec).
Mycobacterium tuberculosis strains
For the present study, the mycobacteria (M. tuberculosis) used were clinical isolated strains resistant to isoniazid and rifampicin codified AC45 and AC83, respectively (these strains were obtained from Sangmelima district’s Hospital in South Region of Cameroon). The genetic profile of the resistance has been carried out at Laboratory for Tuberculosis Research (Biotechnology Centre, University of Yaoundé I) through Line probe Assay method.
Preparation and growth conditions of M. tuberculosis
The mycobacteria strain has been cultured at 37 °C for two weeks in Middlebrook 7H9 (Himedia, Mumbai, India) supplemented with 0.05% (v/v), 2% glycerol and 10% OADC (oleic acid–albumin–dextrose–catalase of Liofilchem s.r.l, Roseto degli Abruzzi, Italy). The optical density of 0.45–0.55 was measured using spectrophotometer at 550 nm to obtain a suspension of 1.5 × 108 UFC/mL (Collins and Franzblau Citation1997).
Preparation of extract and phytochemicals for antitubercular test
The activity of all phytochemicals (extract and pure compounds) against the aforementioned M. tuberculosis strains was tested using the microplate alamar blue assay (MABA) as described previously by Collins and Franzblau (Citation1997) and Jiménez-Alleranes et al. (Citation2003, Citation2007). In 96-well microplates, all wells received 100 µL of supplemented Middlebrook 7H9 broth, then working metabolites solutions (100 µL) were poured into the first well of each row, from which twofold dilution series were made through the microplate column. The test inoculum (100 µL) was added to all testing wells, as well as to the drug-free control wells. The final concentration of DMSO in wells was 7% v/v.
Drug susceptibility testing of M. tuberculosis
Determination of minimum inhibitory concentration
MIC values were determined using the MABA; rifampicin and isoniazid were employed as references. The mycobacteria strain has been cultured at 37 °C for two weeks in Middlebrook 7H9 (Himedia, Mumbai, India) supplemented with 0.05% (v/v), 2% glycerol and 10% OADC (oleic acid–albumin–dextrose–catalase of Liofilchem s.r.l, Roseto degli Abruzzi, Italy). The 96-well plates received 100 μL of Middlebrook 7H9 broth and serial dilution of compounds was made directly on the plate with drug concentrations of 0.244–250 μg/mL and 5000 to 4.882 μg/mL for extracts. Plates were covered and sealed with parafilm and incubated at 37 °C for 14 days. Then, 40 μL of freshly prepared 1:1 mixture of alamar blue reagent and 7% Tween 80 (Himedia, Mumbai, India) was added to the plate and incubated for 24 h. A blue colour in the well was interpreted as no bacterial growth and pink colour was scored as growth. The MIC was defined as the lowest drug concentration, which prevented colour change from blue to pink. The results of antitubercular activity are depicted in and . Minimum inhibitory concentration (MIC) was determined by the broth micro-dilution method according to the guidelines of the Clinical and Laboratory Standards Institute (Citation2011).
Table 2. MIC and MBC values of the methanol extract and the isolated compounds against Mycobacterium tuberculosis (AC45).
Table 3. MIC and MBC values of the methanol extract and the isolated compounds against Mycobacterium tuberculosis (AC83).
Determination of minimum bactericidal concentration
The minimal bactericidal concentration (MBC) determination, 50 μL of each wells which concentration was ≥ MIC was sub-cultured in 150 μL of Mbk 7H9 medium and incubated at 37 °C for 10 days, then mycobacterial growth was carried out by addition of 40 μL of alamar blue. MBC was defined as the lowest concentration of extract at which no visible growth of the germ was observed. Minimum bactericidal concentration was determined by the broth micro-dilution method according to the guidelines of the CLSI 2011.
Results and discussion
Crude MeOH extract from Lophira lanceolata was evaluated against two strains of M. tuberculosis. The strain AC45 of M. tuberculosis was more susceptible to MeOH root extract than AC83, with MIC values of 312.5 and 1250 µg/mL ( and ), respectively. Suggesting that the strain AC83 is more resistant than AC45. This extract was chromatographed on a column of silica gel eluted with CH2Cl2–MeOH in increasing polarity to afford compounds 1–7. Compounds 2–7 were identified as known; stigmasterol (2) (Habib et al. Citation2007), ergosterol (3) (Feitosa et al. Citation2016), sitosterol-3-O-β-d-glucopyranoside (4) (Ngono Bikobo et al. Citation2015), lanceolin C (5) (Messanga et al. Citation1998), the mixture of campesterol-3-O-β-d-glucopyranoside (6a), sitosterol-3-O-β-D-glucopyranoside (6b), stigmasterol-3-O-β-d-glucopyranoside (6c) (Ngono Bikobo et al. Citation2014), isombamichalcone (7) (Ghogomu et al. Citation1989a, Citation1989b). Those structures were deduced from comparison of their spectral data with those reported in the literature.
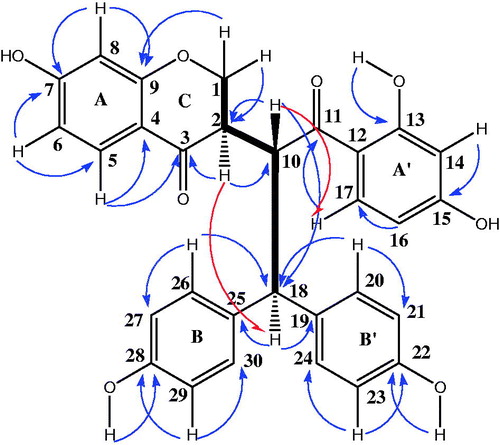
Compound 1 was obtained as an orange amorphous powder and a single peak in the UPLC profile. However, two sets of signals with almost the same intensities in the 1H NMR spectrum suggested that 1 was a mixture of two unseparated components, 1a and 1b, which were present in an approximate 2:1 ratio. This is strengthened by the HR-ESI-MS recorded in the negative and positive ion modes which exhibited different peaks at m/z 511.1396; 509.1238 [M–H]−, and 535.1363; 533.1305 [M + Na]+ corresponding to molecular formulas C30H24O8 and C30H22O8, respectively, suggesting that 1a and 1b had almost the same skeleton, with the presence of two additional hydrogen atoms for the first compound (see Supplemental data). Although 1a and 1b appeared to be homogenous based on TLC monitoring, thorough spectroscopic analyses were carried to determine the structure of these two unseparated compounds. The IR spectrum of 1 showed a broad hydroxyl absorption band at 3444 cm−1, signals at 1710 cm−1 (unconjugated carbonyl group), 1652 cm−1 (conjugated carbonyl groups), 1603 and 1572 cm−1 (aromatic rings) and 1071 cm−1 (C–O–C groups). The 1H NMR and 1H–1H COSY spectra of 1a indicated the presence of two 1,2,4-trisubstituted benzene moieties at δH 6.28 (1H, d, J = 8.4 Hz, H-8), 6.75 (1H, dd, J = 2.3, 8.5 Hz, H-6) and 7.60 (1H, d, J = 8.5 Hz, H-5) (ring A). Other data at δH 6.18 (1H, d, J = 2.3 Hz, H-14), 6.48 (1H, dd, J = 2.3, 8.5 Hz, H-16) and 7.39 (2H, d, J = 8.5 Hz, H-17) suggested ring A′, when two 1,4-disubstitued benzene moieties could be identified at δH 6.52 (2H, d, J = 8.5 Hz, H-27/-29) and 7.29 (2H, d, J = 8.5 Hz, H-26/-30) (ring B); ring B′ appeared through signals at δH 6.73 (2H, d, J = 8.5 Hz, H-21/-23) and 7.28 (2H, d, J = 8.5 Hz, H-20/-24). In addition, the presence of a methine proton and a methylene group for compound 1a was remarkable at δH 2.76 (1H, m, J = 3.3, 6.9, 10.9 Hz, H-2) and 3.39 and 3.79 (2H, m, H-1), respectively. Additionally, an AB system involved in trans-relationship was recognized at δH 4.04 (1H, d, J = 10.6, 12.4 Hz, H-10) and 3.75 (1H, d, J = 6.9, 12.4 Hz, H-18) as depicted in . The 13C NMR of 1a showed the resonances of 30 carbons consisting of 10 quaternary aromatic carbons at δC 114.3 (C-12), 115.8 (C-4), 130.9 (C-19), 133.1 (C-25), 156.0 (C-22), 157.6 (C-28), 159.0 (C-9), 165.0 (C-7), 165.1 (C-13) and 165.6 (C-15), along with two carbonyl carbons at δC 190.6 and 205.4, fourteen sp2 methine carbons (δC 102.6–132.9), two sp3 carbons at δC 71.5 (C-1) (methylene) and at δC 49.4 (C-2) (methine). Moreover, signals of two sp3 methine carbons at δH 49.1 (C-10) and 55.3 (C-18) indicated the presence of partial structure that was in accordance with ethyl group as shown in . The cross-peaks of H-1 with H-2 and H-10 in the 1H–1H COSY spectrum indicated the presence of a partial structure that was consistent with a chromone derivative group. All these signals were in good agreement with those of the Lophirone A skeleton (Ghogomu et al. Citation1987). The complete assignment of all proton and carbon resonances was achieved after careful analysis of COSY, HSQC and HMBC techniques. The chemical structure of 1a was established with the aid of the HMBC spectrum; the chromone moiety (ring AC) was assigned by the long range correlations between H-2 (δH 2.76) and C-1 (δC 71.5), C-3 (δC 190.6) and C-10 (δC 49.1). Additional correlations concerned H-1 (δH 3.39) and C-2 (δC 49.4), suggesting that the ring C of the chromone moiety was hydrogenated, and H-5 (δH 7.60) with C-3 (δC 190.6), C-4 (δC 115.8) and C-9 (δC 159.0) indicating the attachment of ring C to C-10. Further elements from HMBC predict correlations between H-17 and C-11 (δC 205.4), C-15 (δC 165.6), and between H-18 with the ketone carbonyl of C-11 (δC 205.6) corroborating the presence of an ethyl moiety in 1a. This ethyl substructure was linked to C-18 of the molecule based on the HMBC correlations between H-20 (δH 7.28), H-26 (δH 7.29), with C-18 (δH 55.3) as shown in . In addition, further elements from NOESY between H-10 (δH 4.04) and H-17 (δH 7.39) indicated the attachment of ethyl group to benzoyl moiety (ring A′). Moreover, HMBC correlation between an hydroxyl group at δH 12.6 with carbon at δC 165.1 (C-13), other hydroxyl groups at δH 9.48 and δH 9.07 correlating with carbons at δC 157.6 (C-28) and 156.0 (C-22) respectively indicated the attachment of these hydroxyls to ring A′, B and B′ separately. The relative configuration of carbon C-2 was determined from coupling constant of H-2 proton and from NOESY results (). A 10.9 Hz value of this proton suggested the trans-relationships with H-10 (10.6 Hz). The NOESY correlation observed between H-2 (δH 2.76) and H-18 (δH 3.75) indicated the common orientation on the same side of the molecule for these protons. The structure of 1a was therefore assigned as (2R*)-2,3-dihydrolophirone A.
The 1H and 13C NMR spectra of 1b were very similar to those of 1a. In the 1H NMR spectrum, signals due to two 1,2,4-trisubstituted benzene, two 1,4-disubstitued benzene, two methine protons involved in trans-relationships at δH 3.80 (1H, d, J = 12.0 Hz, H-18) and 4.98 (1H, d, J = 12.0 Hz, H-10), and one sp2 methine singlet at δH 8.06 (H-1) were evident, and these signals were in good agreement with those reported in literature (Ghogomu et al. Citation1987). The slight difference in the chemical shifts between H-18 for 1a (δH 3.75) and 1b (δH 3.80) might be explained by the dehydrogenation of chromone moiety for compound 1a. However, 13C NMR spectrum showed significantly different chemical shift values from those of 1a from chromone moiety. The appearance of carbons at δC 155.6 (C-1) and δC 125.4 (C-2) in 1b instead of the methylene and methine carbons (δC 71.5, C-1 and 49.4, C-2) in 1a was evident. The structure of 1b was also determined through the interpretation of the HMBC spectrum, which showed long-range correlations from H-1 (δH 8.06) to C-2 (δC 125.4), C-3 (δC 174.5) and C-9 (δC 159.0) and from H-10 (δH 4.98) to C-2 (δC 125.4) and C-3 (δC 203.0). All these results were very similar to those of reported lophirone A. Furthermore, the NOESY experiment of compound 1b showed cross-peaks from proton signal at δH 4.98 (H-10) to proton signal at δH 7.39 (H-17), indicating the attachment of the benzoyl moiety (nucleus A′) to methine carbon (C-10) of ethyl group as described for compound 1a. Therefore, the structure of 1b was identified as the known lophirone A.
In order to assess the biological effects of the compounds isolated from MeOH extract, antimycobacterial assays were performed. The antimycobacterial effect was assessed by testing compounds against two resistant strains of M. tuberculosis. Moreover, the methanol extract of L. lanceolata showed higher antimycobacterial activity in M. tuberculosis AC45 (MIC 312.5 µg/mL) than M. tuberculosis AC83 (MIC 1250 µg/mL) in the MABA assay, and it indicates that these results can be associated with differences in pathogenesis and virulence of the two strains or different pathogenic phenotypes between AC45 and AC83.
Although plants are an excellent source of diverse molecules, there is no plant based drug for the treatment of TB (Tiwari et al. Citation2013). The present study has brought out the antitubercular activities of compounds 1, 2, 4, 5, 6 and 7 against two clinical isolate strains of M. tuberculosis AC 45 and AC 83 in MABA assay. From these results (listed in and ), compounds 1 and 2 exhibited the most potent antitubercular activity at MIC 31.25 and 15.75 µg/mL, respectively, against M. tuberculosis AC45. There are few studies describing the antimycobacterial activity of biflavonoids especially chalcone derivatives and steroids. In this respect, stigmasterol and the biflavonoids (dihydrolophirone A and lophirone A) can be considered as promising isolated compounds according to Cantrell et al. (Citation2001). Compound 6 possessed antitubercular activity at MIC 250 µg/mL against the two tested strains and was considered inactive ( and ). In addition, compound 2 was more active than analogues (4 and 6) against M. tuberculosis suggesting an impact of polar substituents which showed a decreased inhibitory potency for antimycobacterial effect, implying an important role of the substitution patterns at C-3 (). In assessing structure–activity relationships, the higher activity of compound 1 (MIC 31.25 µg/mL) compared to that of compound 8 (a pure sample of lophirone A, MIC 62.5 µg/mL) might be explained by a higher lipophilicity of the pair (1a and 1b) which destabilizes the cytoplasmic membrane of microorganism, thereby reducing the pH gradient across the membrane and induces the cell death (Zengin and Baysal Citation2014). Nevertheless, the antimycobacterial activity of 1a was not well evaluated because it appeared as mixture knowing that the position of hydroxyl group in phenolic ring is not recognized to strongly influence the degree of antibacterial activity (Shan et al. Citation2005), this is exemplified by the MIC values of compounds 7 and 8 at 62.5 µg/mL. However, according to Castellar et al. (Citation2011), in all researches, no correlation on structure activity of flavonoids against mycobacteria could be drawn. Ergosterol (3) could not be tested due to its insufficient quantity. The antimycobacterial properties of some derivatives such as ergosterol-5,8-endoperoxide isolated from Ajuga remota are documented (Cantrell et al. Citation1999). The activity detected for 2 (MIC 15.75 µg/mL) and 4 (MIC 62.5 µg/mL) is twofold better than those previously reported (Thakur and Gothwal Citation2015). No antimycobacterial activity was reported earlier for the test compounds except for compound 4 which showed similar results against the resistant strain to isoniazid AC 45 (Evina et al. Citation2017; Tiam et al. Citation2017).
It was observed that all the compounds possessed very weak and/or no activity against M. tuberculosis AC83. Regarding the MBC values of both extracts tested ( and ), it seems that they could be similar to their MIC values against the tested organisms. These results also imply that all tested compounds exhibit bactericidal action against the studied strains (Peterson and Shanholtzer Citation1992).
Many plants of our continent are used locally in the treatment of TB, but their anti-tubercular properties have not been investigated due to the lack of appropriated material and scientific facilities. Therefore, it is imperative for African governments in general and in Sub-Sahara Africa in particular to make an effort to fund anti-tubercular drug research.
This study is the first report of antimycobacterial activity of constituents isolated from L. lanceolata against M. tuberculosis.
Conclusions
The data illustrate that MeOH extract of the roots of L. lanceolata has exhibited less pronounced in vitro antimycobacterial activities and a number of active plant derived compounds belonging mainly to two chemical classes, steroid and bioflavonoid derivatives, exhibited good to moderate antimycobacterial activities. These bioactive phytochemicals can be used or utilized as promising candidate substances to develop new tools in antitubercular research. Additionally, there is a strong positive correlation between the antimycobacterial activity results and the ethnomedical/traditional usage on this plant against TB and TB-related diseases.
Supplemental Material
Download MS Word (419.2 KB)Acknowledgements
We thank Mr. V. Nana for the collection and identification of plant material. We thank Mr. Felix Fehr of Department of Chemistry of University of Fribourg and Koert’s team of Philipps-Universität Marburg for spectral analyses.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bloom BR. 2002. Tuberculosis the global view. N Engl J Med. 346:1434–1435.
- Camacho-Corona MR, Favela-Hernández JMJ, González-Santiago O, Garza-González E, Molina-Salinas GM, Said-Fernández S, Delgado G, Luna-Herrera J. 2009. Evaluation of some plant-derived secondary metabolites against sensitive and multidrug-resistant Mycobacterium tuberculosis. J Mex Chem Soc. 53:71–75.
- Cantrell CL, Franzblau SG, Fischer NH. 2001. Antimycobacterial plant terpenoids. Planta Med. 67:685–694.
- Cantrell CL, Rajab MS, Franzblau SG, Froczek FR, Fisher NH. 1999. Antimycobacterial ergosterol-5,8-endoperoxide from Ajuga remota. Planta Med. 65:732–734.
- Castellar A, Coelho TS, Silva PEA, Ramos DF, Lourenço MCS, Lage CLS, Julião LS, Barbosa YG, Leitão SG. 2011. The activity of flavones and oleanolic acid from Lippia lacunosa against susceptible and resistant Mycobacterium tuberculosis strains. Rev Brasil Farmacogn. 21:835–840.
- Clinical and Laboratory Standards Institute. 2011. Susceptibility testing of Mycobacteria, Nocardiae, and other aerobic Actinomycetes. 2nd edition. Approved Standard M24-A2. Wayne (PA): USACLSI.
- Collins L, Franzblau SG. 1997. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother. 41:1004–1009.
- Evina JN, Ngono Bikobo DSAA, Zintchem A, Mbabi Nyemeck II N, Moni Ndedi EDF, Betote Diboue P, Nyegue MA, Atchade AT, Pegnyemb DE, Bochet CG, et al. 2017. In vitro antitubercular activity of extract and constituents from the stem bark of Disthemonanthus benthamianus. Braz J Pharmacogn. 27:739–743.
- Feitosa AO, Dias ACS, Ramos GC, Bitencourt HR, Siqueira JES, Marhino PSB, Ocampos ABF. Marhino AMR 2016. Lethality of cytochalasin B and other compounds isolated from fungus Aspergillus sp (Trichocomaceae) endophyte of Bauhinia guianensis (Fabaceae). Rev Argent Microbiol. 48:259–263.
- Ghogomu RT, Sondengam BL, Martin MT, Bodo B. 1987. Lophirone A, a biflavonoid with unusual skeleton from Lophira lanceolata. Tetrahedron Lett. 28:2967–2968.
- Ghogomu RT, Sondengam BL, Martin MT, Bodo B. 1989a. Lophirones D and E: two new cleaved biflavonoids from Lophira lanceolata. J Nat Prod. 52:284–288.
- Ghogomu RT, Sondengam BL, Martin MT, Bodo B. 1989b. Structure of lophirones B and C, biflavonoids from the bark of Lophira lanceolata. Phytochemistry. 28:1557–1559.
- Ghogomu RT, Sondengam BL, Martin MT, Bodo B. 1990. Structure of chalcone dimers lophirone F, H and G from Lophira lanceolata stem bark. Phytochemistry. 29:2289–2293.
- Habib MR, Nikkon F, Rahman M, Haque EM, Karim RM. 2007. Isolation of stigmasterol and beta-sitosterol from methanolic extract of root bark of Calotropis gigantea (Linn). Pak J Biol Sci: PJBS. 10:4174–4176.
- Jiménez-Alleranes A, Meckes M, Ramirez R, Torres J, Herrera-Luna J. 2003. Activity against multidrug-resistant Mycobacterium tuberculosis in Mexican plants used to treat respiratory diseases. Phytother Res. 17:903–908.
- Jiménez-Alleranes A, Meckes M, Torres J, Herrera-Luna J. 2007. Antimycobacterial triterpenoids from Lantana hispida (Verbenaceae). J Ethnopharmacol. 111:202–205.
- Messanga BB, Tih RG, Sondengam BL, Blond A, Bodo B. 1998. Lanceolin C, a new nitrile glycoside from Lophira alata. Fitoterapia. 69:439–442.
- Murakami A, Ohigashi H, Tanaka S, Hirota M, Irie R, Takeda N, Tatematsu A, Koshimizu K. 1993. Bitter cyanoglucosides from Lophira alata. Phytochemistry. 32:1461–1466.
- Ngono Bikobo DS, Abouem A, Zintchem A, Mbabi Nyemeck IIN, Atchadé AT, Bayiha Ba Njock G, Mosset P, Pegnyemb DE. 2015. Secondary metabolites from Campylospermum oliverianum (Farron), Campylospermum glaucum (Tiegh) and Campylospermum dybowskii (Van Tiegh). Int J Pharmacog Phytochem Res. 7:119–127.
- Ngono Bikobo DS, Mosset P, Abouem A, Zintchem A, Atchadé AT, Balemaken Missi M, Mbabi Nyemeck IIN, Pegnyemb DE. 2014. Campylospermine, an N-hydroxy alkaloid from the leaves of Campylospermum densiflorum (Ochnaceae). Int J Pharmacog Phytochem Res. 6:719–728.
- Okunade L, Elvin-Lewis MP, Lewis WH. 2004. Natural antimycobacterial metabolites: current status. Phytochemistry. 65:1017–1032.
- Pauli GF, Case RJ, Inui T, Wang Y, Cho S, Fisher NH, Franzblau SG. 2005. New perspectives on natural products in TB drug research. Life Sci. 78:485–494.
- Pegnyemb DE, Ghogomu R, Sondengam BL, Martin MT, Bodo B. 1994. Minor biflavonoids of Lophira lanceolata. J Nat Prod. 57:1275–1278.
- Pegnyemb DE, Messanga BB, Ghogomu R, Sondengam BL, Martin MT, Bodo B. 1998. A new benzoylglucoside and a new prenylated isoflavone from Lophira lanceolata. J Nat Prod. 61:801–803.
- Persinos GJ, Quimby MW, Mott AR, Farnsworth NR, Abraham DJ, Fong HHS, Blomster RN. 1967. Studies on Nigerian plants. 3. Biological and phytochemical screening of Lophira lanceolata, and the isolation of benzamide. Planta Med. 15:361–365.
- Peterson LR, Shanholtzer CJ. 1992. Tests for bactericidal effects of antimicrobial agents: technical performance and clinical relevance. Clin Microbiol Rev. 5:420–432.
- Sani AA, Alemika TE, Abdulraheem OR, Sule IM, Ilyas M, Haruna AK, Sikirat AS. 2010. Isolation characterisation of cupressuflavone from the leaves of Lophira lanceolata. J Pharm Bioresour. 7:14–16.
- Sani AA, Rafat OA, Sikirat AS, Alemika TE, Ilyas M. 2011. Structure determination of betulinic acid from the leaves of Lophira lanceolata Van Tiegh. Ex Keay (Ochnaceae). J Appl Pharm Sci. 8:244–245.
- Shan B, Cai YZ, Sun M, Corke H. 2005. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem. 53:7749–7759.
- Thakur JP, Gothwal PP. 2015. Edible plants as a source of antitubercular agents. J Pharmacogn Phytochem. 4:228–234.
- Tiam ER, Ngono Bikobo DS, Abouem A, Zintchem A, Mbabi Nyemeck N II, Moni Ndedi EF, Betote Diboue PH, Nyegue MA, Atchadé AT, Pegnyemb DE, et al. 2017. Secondary metabolites from Triclisia gilletii (De Wild) Staner (Menispermaceae) with antimycobacterial activity against Mycobacterium tuberculosis. Nat Prod Res. doi: 10.1080/14786419.2017.1402324
- Tih AE, Ghogomu RT, Sondengam BL, Martin MT, Bodo B. 1992. Tetraflavonoids of Lophira alata leaves. Phytochemistry. 31:981–984.
- Tih AE, Ghogomu RT, Sondengam BL, Martin MT, Bodo B. 1994. Lanceolins A and B: nitrile glycosides esters from Lophira lanceolata leaves. J Nat Prod. 57:971–974.
- Tih AE, Ghogomu RT, Sondengam BL, Caux CB, Bodo B. 2003a. Minor biflavonoids from Lophira alata leaves. Biochem Syst Ecol. 31:549–551.
- Tih AE, Martin MT, Ghogomu RT, Vuidepot I, Sondengam BL, Bodo B. 2003b. Lophiroflavans B and C, tetraflavonoids of Lophira alata leaves. Phytochemistry. 31:3595–3599.
- Tiwari N, Thakur JP, Saikia D, Gupta MM. 2013. Antitubercular diterpenoids from Vitex trifolia. Phytomedicine. 20:605–610.
- World Health Organization. 1996. Tuberculosis: directly observed treatment short course; [accessed 2006 Nov]. http://www.who.int/tb/dots/dotsplus/faq/en/index.html.
- World Health Organization. 2016. Global tuberculosis report. Global and investments fall far short of those needed to end the global TB epidemic. Geneva, Switzerland: WHO; [accessed 2017 May 3]. http://www.who.int/tb/publications/global_report/gtbr2016_main_text.pdf.
- Zengin H, Baysal AH. 2014. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure–activity relationships evaluated by SEM microscopy. Molecules. 19:17773–17798.