Abstract
Context: Withania (Solanaceae) species are known to be a rich source of withanolides, which have shown several biological properties.
Objective: To identify the compounds responsible for Withania adpressa Coss. antioxidant activity and further test them for their NF-κB inhibition and antiproliferative activity in multiple myeloma cells.
Materials and methods: Compounds were obtained from the EtOAc extract of W. adpressa leaves. Structure elucidation was carried out mainly by 1D- and 2D-NMR, and mass spectrometry. Isolated compounds were tested in a dose-response for their in vitro NF-κB inhibition and antiproliferative activity in multiple myeloma cells after 5 and 72 h treatment, respectively.
Results: The fractionation resulted in the isolation of a new glycowithanolide named wadpressine (5) together with withanolide F, withaferin A, coagulin L, and nicotiflorin. The latter showed a moderate ability to scavenge free radicals in DPPH (IC50 = 35.3 µM) and NO (IC50 = 41.3 µM) assays. Withanolide F and withaferin A exhibited low µM antiproliferative activity against both multiple myeloma cancer stem cells and RPMI 8226 cells. Furthermore, they inhibited NF-κB activity with IC50 values of 1.2 and 0.047 µM, respectively. The other compounds showed a moderate inhibition of cell proliferation in RPMI 8226 cells, but were inactive against cancer stem cells and did not inhibit NF-κB activity.
Discussion and conclusions: One new glycowithanolide and four known compounds were isolated. Biological evaluation data gave further insight on the antitumor potential of withanolides for refractory cancers.
Introduction
The genus Withania (Solanaceae) consists of eight species (The Plant List Citation2013) occurring predominantly in North Africa and from the Mediterranean basin to India (Hepper Citation1991). This genus is known for elaborating withanolides, which are steroidal lactones characterized by a C28 basic skeleton. Since the isolation of withaferin A (Lavie et al. Citation1965), more than 750 withanolides with various functional groups have been isolated, largely but not exclusively, from about 25 genera of Solanaceae (Hang et al. Citation2012). In recent years, withanolides have attracted a significant attention from numerous researchers owing to their structural features and their multiple bioactivities such as cytotoxic (Cordero et al. Citation2009; Hang et al. Citation2012), anti-inflammatory (Jayaprakasam and Nair Citation2003), immunomodulatory (Mesaik et al. Citation2006), anticholinesterase (Choudhary et al. Citation2005), and antioxidant (Bhattacharya et al. Citation2001) properties.
The flora of Morocco includes three Withania species: W. frutescens Pauquy, W. somnifera (L.) Dunal, and W. adpressa Coss. The latter is a medicinal plant endemic to Moroccan Sahara, locally known as “aglim” or “hjuju”, and used to treat food intoxication (Bellakhdar Citation1997). Our previous phytochemical study on this species led to the isolation of a new withanolide named (22R)-14α,15α,17β,20β-tetrahydroxy-1-oxowitha-2,5,24-trien-26,22-olide together with withanolides F and J (Abdeljebbar et al. Citation2007). To date, there is only one pharmacological study on W. adpressa, which mainly refers to the potent cytotoxicity of its withanolides against human cancer cells (Abdeljebbar et al. Citation2009).
The crude extracts of W. somnifera and W. frutescens were reported to have in vitro and in vivo antioxidant activities (Bhattacharya et al. Citation2001; El Bouzidi et al. Citation2011). The compounds responsible for the antioxidant activity included glycowithanolides (Bhattacharya et al. Citation1997). Interest has increased in naturally occurring antioxidants since they may be used to protect humans from oxidative stress damage and to lower the incidence of various cancers. Furthermore, Withania spp. extracts and several withanolides isolated from them have shown potential for their antitumor activity (Mathur et al. Citation2006).
To investigate the potential of W. adpressa leaves for cancer chemoprevention and therapy, the EtOAc extract was selected for bioactivity-guided fractionation based on its antioxidant activity. Four known compounds (1–4, ) and a new glycowithanolide, named wadpressine (5), were isolated. To further investigate their potential to control carcinogenesis, pure compounds were tested for their ability to inhibit NF-κB, a transcription factor involved in various aspects of the carcinogenesis process such as inflammation, cell survival, proliferation, migration, and angiogenesis (Aggarwal Citation2004). Finally, these compounds were tested against two multiple myeloma (MM) cell lines. MM is a hematological cancer in which the over activation of the NF-κB pathway is particularly crucial (Demchenko and Kuehl Citation2010).
Materials and methods
General experimental procedures
UHPLC was performed on an Ultimate 3000 UPLC System (ThermoFisher Scientific, Waltham, MA) with an Acquity BEH C18 column (50 × 2.1 mm i.d.; 1.7 μm, Waters, Milford, MA) using an optimized gradient (MeCN and H2O both containing 0.1% formic acid) of 5–98% MeCN in 4 min and followed by a washing step with 98% MeCN for 2 min. HRMS spectra were obtained on a Q Exactive Plus Hybrid quadripole-orbitrap mass spectrometer (ThermoFisher Scientific) using electrospray in positive and negative modes. The spray voltage was set at 4.0 and 2.5 kV, and the sheath gas flow rate (N2) at 47.5 and 50 units, respectively, the capillary temperature at 255 °C; the S lens RF level at 50 and the probe heater temperature at 412.5 °C. 1H- and 13C-NMR spectra were recorded on a Varian Unity Inova 500 MHz NMR (Palo Alto, CA) instrument. Chemical shifts are reported in parts per million (δ) using the residual CD3OD signal (δH 3.31; δC 49.0) or the CDCl3 signal (δH 7.26; δC 77.2) as internal standards for 1 H- and 13 C-NMR, respectively, and coupling constants (J) are reported in Hz. Complete assignment was performed based on 2 D experiments (COSY, ROESY, NOESY, edited-HSQC and HMBC).
Silica gel 60 (230–400 mesh, Fluka Analytical, Darmstadt, Germany), Sephadex LH-20 (Pharmacia Biotech, Uppsala, Sweden) and Lichroprep RP-18 (40–63 μm, Merck, Darmstadt, Germany) were used for column chromatography. Fractions were monitored by thin layer chromatography (TLC) on precoated silica gel 60F254 (0.20 mm film thickness, Merck). Spots were visualized by exposure to UV light and/or by spraying with Godin or sulphuric vanillin reagents followed by heating at 105 °C.
Plant material
The leaves of W. adpressa were collected at the flowering stage in Taznaght (South of Morocco) in April 2015 and authenticated by Dr Aziz Abbad, Department of Biology, Cadi Ayyad University. A voucher specimen (N° Mar 4223) was deposited at the Botany Department Herbarium, Faculty of Sciences-Semlalia, Cadi Ayyad University, Marrakech, Morocco.
Extraction
Air-dried powdered leaves of W. adpressa (2 kg) were defatted with hexane and then extracted with methanol using a Soxhlet apparatus for 72 h for each solvent. After removing the solvent under reduced pressure, the methanol extract (168 g) was suspended into distilled water, filtered and then partitioned successively with dichloromethane, ethyl acetate, and n-butanol to obtain 28, 15, and 48 g of crude extracts, respectively.
Biological assays
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay
The DPPH radical scavenging activity was carried out as described previously (Şahin et al. Citation2004) with slight modifications. Samples (1–200 µg/mL) were added to a 60-μM methanol solution of DPPH. The absorbance of the solution was measured at 517 nm after a 30-min incubation period at room temperature in the dark.
NF-κB inhibition assay
NF-κB inhibitory activity was assessed using a HEK293/NF-κB-luc cell line (Panomics, Fremont, CA) as previously described (Ndongo et al. Citation2017). Briefly, cells were cultured at 37 °C and 5% CO2 atmosphere in high glucose Dulbecco's modified Eagle's medium (ThermoFisher Scientific) with 100 IU/mL penicillin, 100 μg/mL streptomycin, 100 μg/mL hygromycin B (ThermoFisher Scientific) and 10% foetal bovine serum (Biowest, Nuaillé, France). The day before the assay, cells were treated for 1 h with 2.5 μM of Cell Tracker Green CMFDA (ThermoFisher Scientific), a fluorescent dye used to quantify cell viability, in FBS-free medium. Then, 104 cells/well were seeded in 96-well plates and incubated overnight. After that, cells were treated with either the vehicle control (0.5% DMSO in culture medium) or the samples and stimulated for 5 h with 20 ng/mL of TNF-α (Sigma-Aldrich, Saint Louis, MO). Finally, cells were lysed with reporter lysis buffer (Promega, Madison, WI) and fluorescence signals were read on a Cytation 3 multimode plate reader (Biotek, Winooski, VT). Luciferase assay reagent (Promega) was then added to each well using the auto-injector and the luminescence signals were read. The fluorescence signal was used to normalize the luminescence signal for each well to limit the influence of cell toxicity in luminescence signal intensity. Relative NF-κB activity was quantified by comparing the normalized luminescence signal of vehicle treated nonstimulated cells with the one of vehicle treated stimulated cells. All pure compounds were first screened at 20 μM. Compounds able to inhibit more than 50% of TNF-α induced NF-κB activity at this concentration were considered as active and were selected for calculation of their IC50. Nonlinear regression (with sigmoidal dose response) was used to calculate the IC50 values using GraphPad Prism. Each compound was tested in duplicate and three independent experiments were performed using parthenolide as positive control.
Antiproliferative activity
Human multiple myeloma cancer stem cells (MM-CSCs) derived from the bone marrow of a MM patient, were obtained from Celprogen (Torrance, CA). MM-CSCs were used between passages 4 and 12 for experiments. The tumour plasma cells RPMI 8226 were obtained from LGC standards (Middlesex, United Kingdom). RPMI 8226 cells were used between passages 10 and 25 for experiments. The two cell lines were cultured at 37 °C and 5% CO2 atmosphere in RPMI 1640 culture medium supplemented with 10% foetal bovine serum (Biowest), 100 IU/mL penicillin and 250 μg/mL streptomycin. MTT and XTT assays were used to evaluate the antiproliferative activity of the pure compounds in MM-CSCs and RPMI 8226 cells, respectively (Issa and Cuendet Citation2017). MM-CSCs were seeded in 96 well plates at a density of 5,000 cells per well and allowed to adhere for 24 h, whereas RPMI 8226 cells were plated at a density of 15,000 cells per well and treated immediately. MM-CSCs and RPMI 8226 cells were treated with increasing concentrations (0–20 µM) of compounds for 72 h. Twenty microlitres of MTT solution (5 mg/mL) or 50 μL XTT solution (1 mg/mL) were added in each well and incubated for 2 h (MTT assay) or 4 h (XTT assay). The media and MTT solution were aspirated and the cells containing formazan were solubilized in 100 µL DMSO. Absorbance was measured at 590 nm (MTT assay) or 450 nm (XTT assay). The percentage of cell viability was calculated as the absorbance of each well was divided by that of the vehicle control wells (0.5% DMSO in culture medium) and multiplied by 100. IC50 values were calculated using GraphPad Prism. Each compound was tested in triplicate and three independent experiments were performed using bortezomib as positive control.
Results and discussion
Compound isolation and characterization
Three extracts of increasing polarity (CH2Cl2, EtOAc, and n-BuOH) were prepared from the leaves of W. adpressa and tested for their antioxidant properties. The EtOAc extract exhibited the highest radical scavenging activity (IC50 = 4.8 µg/mL). Based on this result, this extract (15 g) was fractionated by low pressure chromatography on a silica gel column (100 × 2.5 cm i.d.) and eluted with a gradient made of CH2Cl2–EtOAc–MeOH (95:5:1 to 0:10:90). Fractions were combined based on their TLC profile to afford 13 fractions (F1–F13). Fractions F3, F6, F7, and F8 showed the highest DPPH free radical scavenging activity with IC50 values ranging between 10.5 and 25.4 µg/mL. Fraction F3 (783 mg) was further separated on a RP-18 column with a H2O–MeOH mixture of increasing polarity (75:25 to 25:75). The active subfractions F3-2 (230 mg) and F3-3 (133 mg) were subjected to a final purification on a Sephadex LH-20 column with MeOH as eluent to yield compounds 1 (50 mg) and 2 (25 mg), respectively. Fraction F6 (1.4 g) was further fractionated on a silica gel column with a CH2Cl2-MeOH mixture of increasing polarity (9:1 to 2:8) to give six subfractions. Subfraction F6-6 (600 mg) was submitted to a final purification on a Sephadex LH-20 eluted with MeOH to yield compound 3 (150 mg). Fraction F7 was subjected to column chromatography on a RP-18 column and eluted with a gradient of H2O-MeOH (75:25 to 25:75) and afforded six subfractions. The active subfraction F7-5 was submitted to purification on a Sephadex LH-20 column with MeOH to give compound 4 (25 mg). Fraction F8 (520 mg) was separated on Sephadex LH-20 with MeOH and afforded five subfractions. Subfraction F8-4 was submitted to column chromatography on a RP-18 column and eluted with H2O–MeOH (75:25 to 25:75). Final purification was performed by preparative TLC using CH2Cl2–EtOAC–MeOH (8:2:0.1) to afford compound 5 (34 mg).
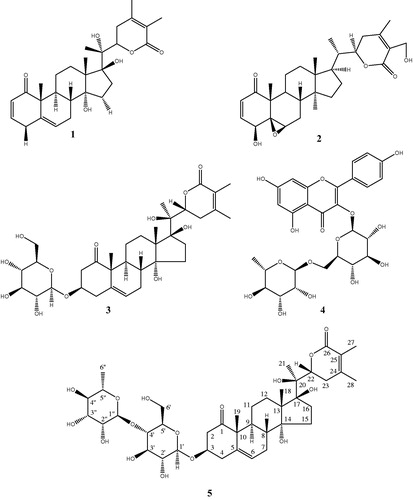
Compounds 1–4 were identified as withanolide F (Abdeljebbar et al. Citation2007), withaferin A (Lavie et al. Citation1965; Erazo et al. Citation2008), coagulin L (Atta-ur-Rahman et al. Citation1998), and nicotiflorin (De Sousa et al. Citation2014), respectively. Their structure elucidation was performed by high resolution mass spectrometry, 1D- and 2D-NMR experiments and comparison with spectroscopic data reported in the literature. These compounds are described for the first time in this species except for withanolide F (1), which was previously isolated from the leaves of this species (Abdeljebbar et al. Citation2007). Withaferin A (2) was previously isolated from W. somnifera, W. coagulans, W. aristata, and W. frutescens (Gonzalez et al. Citation1982; Neogi et al. Citation1988; Llanos et al. Citation2010) and coagulin L (3) from W. coagulans (Atta-ur-Rahman et al. Citation1998). It is noteworthy that this is the first report on the isolation of nicotiflorin (4) from a Withania species.
Compound 5 was isolated as a white amorphous powder. HRESIMS showed a pseudo molecular ion peak at m/z 797.3853 [M + H]+ (calcd. 797.3864), indicating a molecular formula of C40H60O16. IR spectrum revealed the presence of a hydroxyl (3,420 cm−1), an α,β-unsaturated δ-lactone moiety (1715 cm−1) and a carboxyl (1690 cm−1). 1 H- and 13 C-NMR data are given in . The structure of compound 5 was partly deduced from that of coagulin L (3). The comparison of their 13 C-NMR chemical shifts (Table S1) showed that 5 contains an additional sugar unit in position 4′ compared to 3 (8 ppm deviation). The planar structure of compound 3 was determined by the analysis of 1 H, 13 C, COSY, HSQC and HMBC spectra and found to be identical to the one of coagulin L (Atta-ur-Rahman et al. Citation1998). The comparison of 13C-NMR chemical shifts of 2,3-dihydro-3β-hydroxywithanolide F (the aglycone of coagulin L) (Zhang and Timmermann Citation2016) or of the aglycone part of tetra-acetylated coagulin L (Atta-ur-Rahman et al. Citation1998), with those of compound 3, let us to conclude the identity of the latter was coagulin L, even though chemical shift comparison was biased by the use of different NMR solvents (CDCl3 and CD3OD) (). The absence of reported stereoisomers of coagulin L provides further support for our conclusion. The sugar unit in 5 is bound to the aglycone in position 3, as shown by the H-1′/C-3 correlation. A series of COSY correlations, starting from H-1′, revealed the chemical shifts of H-2′ to H-5′, all of these being in axial position, as deduced from the high value of their coupling constants. The HSQC spectrum indicated the chemical shift of H-6′a and H-6′b as those of a methylene group in a primary alcohol functional group whose connection with C-5′ was proven by the HMBC spectrum. The anomeric configuration of this glucose unit is β, as indicated by the high H-1′/H-2′ coupling constant (7.8 Hz).
Table 1. 1H-(500 MHz) and 13C-(125 MHz) NMR data of compound 5 (CDCl3).
The anomeric position 1″ of the sugar unit present in 5 was characterized by 1 H and 13 C chemical shifts clearly identified in the HSQC spectrum. The H-1″ signal, hidden under the strong HOD signal of the solvent, correlated with the one of H-2″ in the TOCSY spectrum. The COSY spectrum sequentially correlated with the signal of H-2″ to those of H-3″ to H-6″. The latter appeared as a shielded doublet of a methyl group. The small value of the H-1″/H-2″ and H-2″/H-3″ coupling constants and the high value of the H-3″/H-4″ and H-4″/H-5″ coupling constants are compatible with a rhamnose unit in α configuration. The H-1″/C-4′ HMBC correlation confirmed the binding of this sugar to position 4′ of the glucose unit. Therefore, the structure of compound 5 was determined as (14R,17S,20R,22R)-3β-O-(α-l-rhamnopyranosyl-(1→4)-β-d-glucopyranosyl)-14,17,20-trihydroxy-1-oxo-witha-5,24-dienolide and named wadpressine. The common characteristic of coagulin L (3) and wadpressine (5) is the presence of a 5-en-1-one structure that had only been reported in withanolides from Withania coagulans (Atta-ur-Rahman et al. Citation1998) and Ajuga parviflora Benth. (Lamiaceae) (Khan et al. Citation1999).
NF-κB inhibitory activity
More recently, it has become clear that an over activation of the NF-κB pathway plays a critical role in cancer development and progression (Aggarwal and Sung Citation2011). Hence, each compound was also tested for its NF-κB inhibition. Only withanolide F (1) and withaferin A (2), an already described NF-κB inhibitor (Heyninck et al. Citation2014), inhibited TNF-α-induced NF-κB activity with IC50 values of 1.2 and 0.05 μM, respectively ().
Table 2. NF-ĸB inhibition mediated by the isolated compounds (1-5)Table Footnotea.
Antiproliferative activity
MM remains an incurable malignancy, with a median survival of only 5 years, despite all the treatment advances (Fonseca et al. Citation2017). The presence of CSCs in MM is considered to contribute to disease relapse through their drug-resistant nature. Targeting MM-CSCs is therefore a promising strategy for MM treatment. The effect of pure compounds on cell growth in MM-CSCs and RPMI 8226 cells was investigated using MTT and XTT assays (). Withaferin A (2) exhibited the strongest activity with IC50 values of 0.33 and 0.17 µM in MM-CSCs and RPMI 8226 cells, respectively. Similar results were reported for withaferin A in a previous study (Issa and Cuendet Citation2017).
Table 3. Antiproliferative activity of the isolated compounds (1–5) in MM-CSCs and RPMI 8226 cellsTable Footnotea.
Withanolide F (1) also showed activity against both cell lines (). Previous results have shown that withanolide F strongly inhibited the growth of several cancer cell lines (Jayaprakasam et al. Citation2003; Abdeljebbar et al. Citation2009). Compounds 3–5 were only active against RPMI 8226 cells. Interestingly, the two compounds able to inhibit NF-κB activity were also the ones active on MM-CSCs, highlighting the potential interest of NF-κB inhibitors for the treatment of refractory cancers.
Conclusions
One new glycowithanolide (5) and four known compounds (1–4) were identified from the leaves of W. adpressa. Compounds (2–4) were isolated from this plant for the first time. In addition, results from the biological evaluation of the compounds isolated from W. adpressa leaves highlight the interest of this plant for its antitumor properties.
Supplementary Material
Download MS Word (18.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abdeljebbar LH, Humam M, Christen P, Jeannerat D, Vitorge B, Amzazi S, Benjouad A, Hostettmann K, Bekkouche K. 2007. Withanolides from Withania adpressa. Helv Chim Acta. 90:346–352.
- Abdeljebbar LH, Benjouad A, Morjani H, Merghoub N, El Haddar S, Humam M, Christen P, Hostettmann K, Bekkouche K, Amzazi S. 2009. Antiproliferative effects of withanolides from Withania adpressa. Therapie. 64:121–127.
- Aggarwal BB. 2004. Nuclear factor-kappaB: the enemy within. Cancer Cell. 6:203–208.
- Aggarwal BB, Sung B. 2011. NF-κB in cancer: a matter of life and death. Cancer Discov. 1:469–471.
- Atta-ur-Rahman A-R, Yousaf M, Gul W, Qureshi S, Choudhary MI, Voelter W, Hoff A, Jens F, Naz A. 1998. Five new withanolides from Withania coagulans. Heterocycles. 48:1801–1811.
- Bellakhdar J. 1997. La Pharmacopée marocaine traditionnelle, médicine arabe ancienne et savoirs. Paris: Ibis Press.
- Bhattacharya SK, Satyan KS, Ghosal S. 1997. Antioxidant activity of glycowithanolides from Withania somnifera. Indian J Exp Biol. 35:236–239.
- Bhattacharya A, Ghosal S, Bhattacharya SK. 2001. Anti-oxidant effect of Withania somnifera glycowithanolides in chronic footshock stress-induced perturbations of oxidative free radical scavenging enzymes and lipid peroxidation in rat frontal cortex and striatum. J Ethnopharmacol. 74:1–6.
- Choudhary MI, Nawaz SA, Zaheer-ul-Haq LMA, Ghayur MN, Jalil S, Riaz N, Yousuf S, Malik A, Gilani AH, Atta-ur-Rahman A-R. 2005. Withanolides, a new class of natural cholinesterase inhibitors with calcium antagonistic properties. Biochem Biophys Res Commun. 334:276–287.
- Cordero CP, Morantes SJ, Páez A, Rincón J, Aristizábal FA. 2009. Cytotoxicity of withanolides isolated from Acnistus arborescens. Fitoterapia. 80:364–368.
- De Sousa EA, da Silva AA, Cavalheiro AJ, Lago JHG, Chaves MH. 2014. A new flavonoid derivative from leaves of Oxandra sessiliflora. J Braz Chem Soc. 25:704–708.
- Demchenko YN, Kuehl WM. 2010. A critical role for the NFκB pathway in multiple myeloma. Oncotarget. 1:59–68.
- El Bouzidi L, Larhsini M, Markouk M, Abbad A, Hassani L, Bekkouche K. 2011. Antioxidant and antimicrobial activities of Withania frutescens. Nat Prod Commun. 6:1447–1450.
- Erazo S, Rocco G, Zaldivar M, Delporte C, Backhouse N, Castro C, Belmonte E, Monache FD, Garcia R. 2008. Active metabolites from Dunalia spinosa resinous exudates. Z Naturforsch C J Biosci. 63:492–496.
- Fonseca R, Abouzaid S, Bonafede M, Cai Q, Parikh K, Cosler L, Richardson P. 2017. Trends in overall survival and costs of multiple myeloma, 2000-2014. Leukemia. 31:1915–1921.
- Gonzalez AG, Darias V, Martin H, Suarez MC. 1982. Cytostatic activity of natural withanolides from Spanish Withania. Fitoterapia. 53:85–88.
- Hang H, Samadi AK, Cohen MS, Timmermann BN. 2012. Antiproliferative withanolides from the Solanaceae: a structure–activity study. Pure Appl Chem. 84:1353–1367.
- Hepper FN. 1991. Old World Withania (Solanaceae): A taxonomic review and key to the species. In: Hawkes JG, Lester RN, Nee M, Estrada N, editors. Solanaceae III taxonomy, chemistry, evolution. Richmond: The Royal Botanic Gardens Kew, p. 211–227.
- Heyninck K, Lahtela-Kakkonen M, Van der Veken P, Haegeman G, Vanden Berghe W. 2014. Withaferin A inhibits NF-kappaB activation by targeting cysteine 179 in IKKβ. Biochem Pharmacol. 91:501–509.
- Issa ME, Cuendet M. 2017. Withaferin A induces cell death and differentiation in multiple myeloma cancer stem cells. Med Chem Commun. 8:112–121.
- Jayaprakasam B, Nair MG. 2003. Cyclooxygenase-2 enzyme inhibitory withanolides from Withania somnifera leaves. Tetrahedron. 59:841–849.
- Jayaprakasam B, Zhang Y, Seeram NP, Nair MG. 2003. Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves. Life Sci. 74:125–132.
- Khan PM, Nawaz HR, Ahmad S, Malik A. 1999. Ajugins C and D, new withanolides from Ajuga parviflora. Helv Chim Acta. 82:1423–1426.
- Lavie D, Glotter E, Shvo Y. 1965. Constituents of Withania somnifera-III-the side chain of withaferin A. J Org Chem. 30:1774–1778.
- Llanos GG, Araujo LM, Jiménez IA, Moujir LM, Vázquez JT, Bazzocchi IL. 2010. Withanolides from Withania aristata and their cytotoxic activity. Steroids. 75:974–981.
- Mathur R, Gupta SK, Singh N, Mathur S, Kochupillai V, Velpandian T. 2006. Evaluation of the effect of Withania somnifera root extracts on cell cycle and angiogenesis. J Ethnopharmacol. 105:336–341.
- Mesaik MA, Zaheer-ul-Haq, Murad S, Ismail Z, Abdullah NR, Gill HK, Atta-ur-Rahman, Yousaf M, Siddiqui RA, Ahmad A, et al. 2006. Biological and molecular docking studies on coagulin-H: Human IL-2 novel natural inhibitor. Mol Immunol. 43:1855–1863.
- Ndongo JT, Ngo Mbing J, Feussi Tala M, Monteillier A, Pegnyemb DE, Cuendet M, Laatsch H. 2017. Indoline alkaloids from Tabernaemontana contorta with cancer chemopreventive activity. Phytochemistry. 144:189–196.
- Neogi P, Kawai M, Butsugan Y, Mori Y, Suzuki M. 1988. Withacoagin, a new withanolide from Withania coagulans roots. Bull Chem Soc Jpn. 61:4479–4481.
- Şahin F, Güllüce M, Daferera D, Sökmen A, Sökmen M, Polissiou M, Agar G, Özer H. 2004. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control. 15:549–557.
- The Plant List. 2013. Version 1.1. Published on the Internet. [accessed 2018 Mar 27]. http://www.theplantlist.org/.
- Zhang H, Timmermann BN. 2016. Withanolide structural revisions by 13C NMR spectroscopic analysis inclusive of the γ-gauche effect. J Nat Prod. 79:732–742.