?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Context: Phytochemical and pharmacological data on Ducrosia anethifolia (DC.) Boiss. (Apiaceae), an Iranian medicinal plant, are scarce; however, furocoumarins are characteristic compounds of D. anethifolia.
Objective: Our experiments identify the secondary metabolites of D. anethifolia and assess their antitumor and anti-multidrug resistance activities.
Materials and methods: Pure compounds were isolated from the extract of aerial parts of the plant by chromatographic methods. Bioactivities were tested on multidrug resistant and sensitive mouse T-lymphoma cell lines. The inhibition of the cancer MDR efflux pump ABCB1 was evaluated by flow cytometry (at 2 and 20 µM). A checkerboard microplate method was applied to study the interactions of furocoumarins and doxorubicin. Toxicity was studied using normal murine NIH/3T3 fibroblasts.
Results: Thirteen pure compounds were isolated, nine furocoumarins namely, pabulenol (1), (+)-oxypeucedanin hydrate (2), oxypeucedanin (3), oxypeucedanin methanolate (4), (−)-oxypeucedanin hydrate (5), imperatorin (6), isogospherol (7), heraclenin (8), heraclenol (9), along with vanillic aldehyde (10), harmine (11), 3-hydroxy-α-ionone (12) and 2-C-methyl-erythrytol (13). Oxypeucedanin showed the highest in vitro antiproliferative and cytotoxic activity against parent (IC50 = 25.98 ± 1.27, 40.33 ± 0.63 µM) and multidrug resistant cells (IC50 = 28.89 ± 0.73, 66.68 ± 0.00 µM), respectively, and exhibited slight toxicity on normal murine fibroblasts (IC50 = 57.18 ± 3.91 µM).
Discussion and conclusions: Compounds 2, 3, 5, 7, 10–13 were identified for the first time from the Ducrosia genus. Here, we report a comprehensive in vitro assessment of the antitumor activities of D. anethifolia furocoumarins. Oxypeucedanin is a promising compound for further investigations for its anticancer effects.
Introduction
The genus Ducrosia (Apiaceae) consists of six species: Ducrosia ismaelis Asch., D. flabellifolia Boiss., D. assadii Alava., D. areysiana (Deflers) Pimenov & Kljuykov, D. inaccessa (C.C.Towns.) Pimenov & Kljuykov and D. anethifolia (DC.) Boiss. D. anethifolia is one of the three species growing wild in several areas of Iran, Afghanistan, Pakistan, Syria, Lebanon, Iraq, and some other Arab states and countries along the Persian Gulf (Aynehchi Citation1991; Ghahreman Citation1993; Mozaffarian Citation1996). The whole herb, especially its aerial part, has been used in Iranian folk medicine as an analgesic and in case of anxiety and insomnia (Shalaby et al. Citation2014). The aerial part, including the seed was reported to be carminative and useful for irregularities of menstruation and galactagogue (Amiri and Joharchi Citation2016). The herb is added to a variety of Persian foods for flavouring (Aynehchi Citation1991; Haghi et al. Citation2004).
The phytochemical profile of D. anethifolia has only been partly explored. In the literature, the majority of papers deal with the composition of the essential oil (EO). As major constituents, α-pinene (11.6% (Mostafavi et al. Citation2008), 70.3% (Mottaghipisheh et al. Citation2014), 59.2% (Janssen et al. Citation1984)); n-decanal (1.4-45% (Karami and Bohlooli Citation2017), 45.06% (Vazirzadeh et al. Citation2017), 70% (Hajhashemi et al. Citation2010), 57% (Mahboubi and Feizabadi Citation2009), 25.6–30.3% (Mazloomifar and Valian Citation2015), 18.8% (Sefidkon and Javidtash Citation2002)), dodecanal (28.8% (Shahabipour et al. Citation2013)), cis-chrysanthenyl acetate (72.28%) (Ashraf et al. Citation1979; Habibi et al. Citation2017) have been reported.
Furocoumarins and terpenoids are characteristic components of the Ducrosia genus. From the seeds of D. anethifolia, two new terpenoids, the monoterpene ducrosin A and the sesquiterpene ducrosin B were isolated along with stigmasterol and the furocoumarins heraclenin and heraclenol (Queslati et al. Citation2017). Psoralen, 5-methoxypsoralen, 8-methoxypsoralen, imperatorin, isooxypeucedanin, pabulenol, pangelin, oxypeucedanin methanolate, oxypeucedanin hydrate, 3-O-glucopyranosyl-β-sitosterol and 8-O-debenzoylpaeoniflorin were also isolated from the extract of D. anethifolia (Stavri et al. Citation2003; Shalaby et al. Citation2014). GC analysis of the fatty acids showed high percentages of elaidic acid and oleic acid (Queslati et al. Citation2017), beside 58.8% petroselinic acid in the seed oil of D. anethifolia (Khalid et al. Citation2009). Apart from D. anethifolia, furocoumarins (psoralen, isopsoralen) have been reported only from D. ismaelis from this genus (Morgan et al. Citation2015).
The bioactivities of the extracts of aerial parts of D. anethifolia have been studied in vitro and in vivo. Different extracts of the plant exerted moderate anti-radical scavenging (Mottaghipisheh et al. Citation2014; Shahat et al. Citation2015); and antibacterial effects (Syed et al. Citation1987; Mahboubi and Feizabadi Citation2009). Pangelin isolated from D. anethifolia demonstrated activity against a panel of fast growing mycobacteria (Stavri et al. Citation2003). Essential oil of the seeds and methanol extract showed a weak antibacterial effect against 14 Gram positive and negative bacteria (Javidnia et al. Citation2009; Habibi et al. Citation2017). In an experiment on three human cancer cell lines (K562, LS180 and MCF-7), D. anethifolia EO demonstrated remarkable to moderate cytotoxic activity, while EO of D. flabellifolia showed less pronounced activity (Shahabipour et al. Citation2013). Ducrosin B exerted remarkable cytotoxicity against the human colon HCT-116 and ovary SKOV-3 cancer cell lines in vitro (Queslati et al. Citation2017).
The crude D. anethifolia extract and the isolated furocoumarins exhibited in vivo antidiabetic activities (Shalaby et al. Citation2014). The in vivo anxiolytic (Hajhashemi et al. Citation2010; Shokri et al. Citation2013; Zamyad et al. Citation2016), sedative (Hajhashemi et al. Citation2010), analgesic and anti-inflammatory (Asgari Nematian et al. Citation2017) and also anti-locomotor activities (Zamyad et al. Citation2016) of D. anethifolia EO have been tested. Intra-peritoneal administration of the D. anethifolia EO improved spatial learning and memory in adult male rats (Abbasnejad et al. Citation2017). The intra-peritoneal injection of the hydroalcoholic extract of D. anethifolia effectively reduced the pentylenetetrazole-induced seizure manifestations in male Wistar rats (Nyasty et al. Citation2017). Moreover, D. anethifolia extract reduced the number of germ cells, the level of testosterone and spermatogenesis in male Wistar rats (Rahimi et al. Citation2016).
As presented above, furocoumarins are the most characteristic compounds of the Ducrosia and their activities against cancer cells seem to be promising. Imperatorin showed antiproliferative effect on human hepatoma HepG2 cells (Luo et al. Citation2011); furthermore, this compound and heraclenin induced apoptosis in Jurkat leukemia cells. In Jurkat cells treated for 72 h with heraclenin and imperatorin, most of the DNA fragmentation occurred at the G2/M and G1/S phases of the cell cycle, respectively (Appendino et al. Citation2004). 8-Methoxypsoralen inhibited the growth of neuroblastoma (IC50 = 56.3 µM) and metastatic colon cancer cells (IC50 = 88.5 µM) by triggering both extrinsic and intrinsic apoptotic pathways, independently of photoactivation (Bartnik et al. Citation2017). Isoimperatorin, cnidicin, imperatorin, oxypeucedanin, byakangelicol and oxypeucedanin hydrate exhibited a significant inhibition on cell proliferation in a dose-dependent manner, particularly oxypeucedanin against HCT-15 (colon cancer) cells with ED50 = 3.4 ± 0.3 μg/mL (Kim et al. Citation2007).
Beside direct antiproliferative and cytotoxic activities, furocoumarins affect multidrug resistance (MDR) as well. Among 20 selected furocoumarin derivatives, phellopterin (IC50 = 8.0 ± 4.0 µM) and isopimpinellin (IC50 = 26.0 ± 5.7 µM) exhibited the highest activity against CEM/C1 (lymphoblastic leukaemia) and HL-60/MX2 (MDR) cell lines, respectively (Kubrak et al. Citation2017). Feroniellin A reverted MDR in A549RT-eto lung cancer cells (Kaewpiboon et al. Citation2014). Bergapten (IC50 = 40.29 ± 0.30 nM) and xanthotoxin (IC50 = 1.10 ± 0.91 nM) showed remarkable anticancer activity against EPG85. 257RDB (MDR1 overexpressing human gastric adenocarcinoma cell line) and MCF7MX (BCRP overexpressing human epithelial breast cancer cell line), respectively (Mirzaei et al. Citation2017).
Our work explores the phytochemical composition of D. anethifolia, examines the complex in vitro anticancer activities, including antiproliferative, cytotoxic and anti-MDR effects of its isolated compounds, and analyses the interaction of compounds possessing promising bioactivities with chemotherapeutics.
Materials and methods
General procedures
NMR spectra were recorded in CD3OD and CDCl3 on a Bruker Avance DRX 500 spectrometer at 500 MHz (1H) and 125 MHz (13C). The peaks of the residual solvent (δH 3.31 and 7.26, δC 49.0 and 77.2, respectively) were taken as reference. The data were acquired and processed with MestReNova v6.0.2e-5475 software. Chemical shifts are expressed in parts per million and coupling constants (J) values are reported in Hz. All solvents were used in analytical grade (Molar Chemicals Kft, Halásztelek, Hungary).
Pure compounds were isolated by using open column chromatography (Silica gel 60, 0.063–0.2 mm, Merck, Darmstadt, Germany) (CC), medium pressure liquid chromatography (MPLC, silica gel 60, 0.045–0.063 mm, Merck, Darmstadt, Germany), gel chromatography (Sephadex® LH-20, Pharmacia, Uppsala, Sweden), normal (Silica gel 60, Merck, Darmstadt, Germany) and reverse phase (Silica gel 60 RP-18 F254s, Merck, Darmstadt, Germany) preparative thin layer chromatography (PTLC and RP-PTLC, respectively), centrifugal PTLC (Silica gel 60 GF254, Merck, Darmstadt, Germany) (CPTLC) and reverse phase preparative HPLC (Kinetex® 5 µm C-18 100 Å, 150 × 4.6 mm Phenomenex, Torrance, CA) (RP-HPLC). The HPLC flow was 1.2 mL/min, column oven temperature was 24 °C. Detection was carried out within the range of 190–800 nm. The HPLC system comprised of Waters 600 pump, Waters 2998 PDA detector, Waters in/line degasser AF degasser unit connected with Waters 600 control module using Empower Pro 5.00 software.
Plant material
The aerial parts of Ducrosia anethifolia were collected by JM from south of Iran (Fars, Neyriz, Iran) in April 2016. Identification of the plant was done by Dr. Mohammad Jamal Saharkhiz at Department of Horticultural Science, Faculty of Agriculture, Shiraz University, Iran, and a voucher specimen was deposited in the Herbarium of Department of Pharmacognosy, University of Szeged (voucher no.: 880).
Isolation of compounds
Aerial parts (flower, leaves and stem, 3 kg) were dried in shade at room temperature and powdered, then extracted with methanol (40 L). After filtration, the filtrate was concentrated under reduced pressure to yield the crude extract. The extract (464.1 g) was dissolved with methanol–water 1:1 (1.5 L) and then partitioned successively with n-hexane (4 × 1 L), CHCl3 (4 × 1 L), EtOAc (4 × 1 L) and n-BuOH (4 × 1 L). The solvents were removed from each extract to yield the n-hexane extract, CHCl3 extract, EtOAc extract and n-BuOH extract.
The CHCl3-soluble fraction (20.6 g) was initially subjected to CC with a gradient system consisting of increasing concentration of MeOH in CHCl3 (0–80%); column fractions with similar TLC patterns were combined to get six major fractions D1, D2, D3, D4, D5 and D6. D1 was chromatographed by MPLC, first eluting with n-hexane–CH2Cl2 (50:50; 0:100), then adding MeOH to CH2Cl2 (0–100%), to afford four subfractions (D11, D12, D13 and D14). D11 was separated to 49 subfractions using CPTLC with an isocratic eluting system n-hexane–EtOAc–MeOH (10:3:1), which resulted in the isolation of the pure compound 6 (82.8 mg). The RP-HPLC purification of D11 subfractions with MeOH–H2O (MeOH–H2O 1:1) afforded compound 10 (1.7 mg). D12 was chromatographed by MPLC applying a gradient solvent system with increasing EtOAc in n-hexane (5-100%) to get eight major subfractions (D121–D128). From D123, the pure compound 3 (3.1 mg) was isolated by using CPTLC with EtOAc in n-hexane (5–100%). D124 was successively separated to 81 fractions by CPTLC (same system), then subfractions 49–54 was subjected to RP-HPLC with MeOH–H2O (15–50% H2O in MeOH) yielding compound 9 (2.56 mg).
D13 was separated by MPLC with increasing ratio of EtOAc in n-hexane (5–100%) to get seven fractions (D131–D137). D133 was subjected to MPLC with the same solvent system to gain 19 subfractions. Finally, subfractions 1–2 were purified by using CPTLC with toluene–EtOAc (90:10, 80:20, 70:30, 60:40, 50:50) as eluents to gain compound 2 (100.4 mg). Subfraction 3 from D133 was subjected to CPTLC by eluting with toluene–EtOAc (90:10, 80:20, 70:30, 60:40, 50:50) to yield 32 subfractions. Subfractions 18–19 of D133 were separated by PTLC with toluene–EtOAc (1:1) to get compound 8 (35.3 mg). Besides, subfractions 23–32 were chromatographed by RP-HPLC (MeOH–H2O 1:1) and then by PTLC with toluene–EtOAc (1:1) to yield compounds 12 (1.02 mg) and 1 (1.78 mg). By using CPTLC with increasing concentration of EtOAc in toluene (5–100%) as eluents, subfraction 6 from D133 was chromatographed to get 43 subfractions. Subfractions 24–28 and 36–40 were separated by PTLC with CHCl3–MeOH–n-hexane (5:1:5) to retrieve compounds 4 (2.5 mg) and 7 (21.7 mg), respectively.
D3 was separated to five major fractions (D31–D35) by MPLC with a solvent system containing increasing ratio of MeOH in CHCl3 (0–100%). D33 was chromatographed by CPTLC with raising the concentration of MeOH (0–20%) in the mixture of cyclohexane–EtOAc (1:1) to afford 70 subfractions. Subfractions 21–23 contained the pure compound 11 (1.0 mg). The main fraction D4 was separated by MPLC to seven subfractions (D41–D47) by raising the ratio of MeOH (0–100%) in acetone–toluene (1:1). Subfraction D42 was subsequently chromatographed by PTLC with eluting cyclohexane–EtOAc–MeOH (4.75:4.75:0.5) and compound 5 (2.7 mg) was isolated. Furthermore, D46 was purified with CPTLC by increasing ratio of MeOH (0–100%) in acetone–toluene (1:1); then compound 13 (2.9 mg) was purified by using RP-PTLC [MeOH–H2O (1:1)] from subfractions 35–37 ().
Cell lines
L5178Y mouse T-cell lymphoma cells: parent, PAR cells (ECACC cat. no. 87111908, obtained from FDA, Silver Spring, MD) were transfected with pHa MDR1/A retrovirus. The ABCB1-expressing L5178Y cell line (MDR) was selected by culturing the infected cells in 60 ng/mL colchicine containing medium. L5178Y PAR mouse T-cell lymphoma cells and the L5178Y human ABCB1-transfected subline (MDR) were cultured in McCoy’s 5A medium supplemented with 10% heat-inactivated horse serum, 200 mM l-glutamine, and penicillin–streptomycin mixture in 100 U/L and 10 mg/L concentration, respectively, at 37 °C and in a 5% CO2 atmosphere.
NIH/3T3 mouse embryonic fibroblast cell line (ATCC CRL-1658) was purchased from LGC Promochem (Teddington, UK). The cell line was cultured in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich, St. Louis, MO), containing 4.5 g/L glucose, supplemented with 10% heat-inactivated foetal bovine serum (FBS). The cells were incubated at 37 °C, in a 5% CO2, 95% air atmosphere.
Assay for antiproliferative effect
The effects of increasing concentrations of the analysed compounds on cell proliferation were tested in 96-well flat-bottomed microtiter plates (Poljarević et al. Citation2018). The compounds were diluted in 100 μL of McCoy’s 5A medium. 6 × 103 mouse T-cell lymphoma cells (PAR or MDR) in medium (100 μL) were added to each well, with the exception of the medium control wells. The culture plates were further incubated at 37 °C for 72 h; at the end of the incubation period, 20 μL of MTT solution (thiazolyl blue tetrazolium bromide, Sigma, St. Louis, MO) (from a 5 mg/mL stock) was added to each well. After incubation at 37 °C for 4 h, 100 μL of sodium dodecyl sulphate (SDS, Sigma, St. Louis, MO) solution (10% in 0.01 M HCl) was added to each well and the plates were further incubated at 37 °C overnight. The cell growth was determined by measuring the OD at 540 nm (ref. 630 nm) with a Multiscan EX ELISA reader (Thermo Labsystems, Waltham, MA). IC50 values were calculated via the following equation:
Assay for cytotoxic effect
The effects of increasing concentrations of compounds on cell growth were tested in 96-well flat-bottomed microtiter plates (Poljarević et al. Citation2018). The compounds were diluted in a volume of 100 μL medium. Then, 1 × 104 cells in 100 μL of medium were added to each well, with the exception of the medium control wells. In case of NIH/3T3 cells, the compounds were added after seeding the cells at 37 °C overnight. The culture plates were incubated at 37 °C for 24 h; at the end of the incubation period, 20 μL of MTT solution (from a 5 mg/mL stock) was added to each well. After incubation at 37 °C for 4 h, 100 μL of SDS solution (10% in 0.01 M HCl) was added to each well and the plates were further incubated at 37 °C overnight. Cell growth was determined by measuring the optical density (OD) at 540 nm (ref. 630 nm) with a Multiscan EX ELISA reader. Inhibition of the cell growth was determined according to the formula:
Results are expressed in terms of IC50, defined as the inhibitory dose that reduces by a 50% the growth of the cells exposed to the tested compound.
Assay for multidrug resistance reversing activity
The inhibition of the cancer MDR efflux pump ABCB1 by the tested compounds was evaluated using flow cytometry measuring the retention of rhodamine 123 by ABCB1 (P-glycoprotein) in MDR mouse T-lymphoma cells, as the L5178Y human ABCB1-gene transfected mouse T-lymphoma cell line (MDR) overexpresses P-glycoprotein (Domínguez-Álvarez et al. Citation2016). This method is a fluorescence-based detection system which uses verapamil as reference inhibitor. Briefly, cell number of L5178Y MDR and PAR cell lines was adjusted to 2 × 106 cells/mL, re-suspended in serum-free McCoy’s 5A medium and distributed in 0.5 mL aliquots into Eppendorf centrifuge tubes. The tested compounds were added at different concentrations and the samples were incubated for 10 min at room temperature. Verapamil (Sigma, St. Louis, MO) and tariquidar (Sigma, St. Louis, MO) were applied as positive controls. Next, 10 µL (5.2 µM final concentration) of the fluorochrome and ABCB1 substrate rhodamine 123 (Sigma, St. Louis, MO) were added to the samples and the cells were incubated for 20 min at 37 °C, washed twice and re-suspended in 0.5 mL PBS for analysis. The fluorescence of the cell population was measured with a Partec CyFlow® flow cytometer (Partec, Görlitz, Germany). The percentage of mean fluorescence intensity was calculated for the treated MDR cells as compared with the untreated cells. A fluorescence activity ratio (FAR) was calculated based on the following equation which relates the measured fluorescence values:
The results obtained from a representative flow cytometry experiment in which 20,000 individual cells of the population were evaluated for amount of rhodamine 123 retained with the aid of the Partec CyFlow® flow cytometer, are first presented by the histograms and these data converted to FAR units that define fluorescence intensity, standard deviation, peak channel in the total- and in the gated-populations. Parameters calculated are: forward scatter (FSC, forward scatter count of cells in the samples or cell size ratio); side scatter (SSC, side scatter count of cells in the samples); FL-1 (mean fluorescence intensity of the cells) and FAR, whose values were calculated using the equation given above.
Checkerboard combination assay
A checkerboard microplate method was applied to study the effect of drug interactions between furocoumarins and the chemotherapeutic drug doxorubicin (Takács et al. Citation2015). This assay was carried out using multidrug resistant mouse T-lymphoma cells overexpressing the ABCB1 transporter. Doxorubicin is in the class of anthracycline antitumor agents, and it exerts anticancer activity as a topoisomerase-II (TI-2) inhibitor. The dilutions of doxorubicin (Teva, Debrecen, Hungary, stock solution: 2 mg/mL) were made in a horizontal direction in 100 μL (final concentration: 17.242 μM), and the dilutions of the test compounds vertically in the microtiter plate in 50 μL volume. The cells were re-suspended in McCoy’s 5A culture medium and distributed into each well in 50 μL containing 6 × 103 cells each. The plates were incubated for 72 h at 37 °C in 5% CO2 atmosphere. The cell growth rate was determined after MTT staining. At the end of the incubation period, 20 μL of MTT solution (from a stock solution of 5 mg/mL) was added to each well. After incubation at 37 °C for 4 h, 100 μL of SDS solution (10% in 0.01 M HCl) was added to each well and the plates were further incubated at 37 °C overnight. Optical density was measured at 540/630 nm with Multiscan EX ELISA reader (Thermo Labsystems, Waltham, MA) as described elsewhere (Takács et al. Citation2015). Combination index (CI) values at 50% of the growth inhibition dose (ED50) were determined using CompuSyn software (ComboSyn, Inc., Paramus, NJ) to plot four to five data points to each ratio. CI values were calculated by means of the median-effect equation, where CI <1, CI = 1 and CI >1 represent synergism, additive effect (or no interaction) and antagonism, respectively (Chou and Martin Citation2005; Chou Citation2010).
Results
Isolated compounds
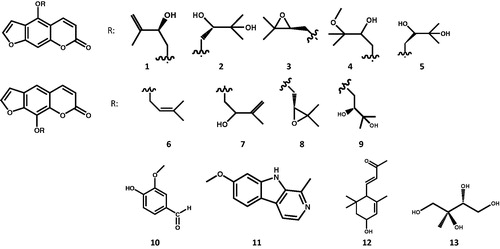
Repeated column chromatography of the bioactive fractions resulted in the isolation of 13 compounds. The compounds were identified by careful interpretation of NMR data and comparison of 1H and 13C chemical shifts with those reported in literature. Nine linear furocoumarin derivatives, namely pabulenol (1) (Sbai et al. Citation2016), (+)-oxypeucedanin hydrate (aviprin) (2) (Sbai et al. Citation2016), oxypeucedanin (3) (Sbai et al. Citation2016), oxypeucedanin methanolate (4) (Fujioka et al. Citation1999), (–)-oxypeucedanin hydrate (prangol) (5) (Rahimifard et al. Citation2018), imperatorin (6) (Lv et al. Citation2013), isogospherol (7) (Macias et al. Citation1990), heraclenin (8) (Poonkodi Citation2016), heraclenol (9) (Harkar et al. Citation1984); along with vanillic aldehyde (10) (Chung et al. Citation2011), harmine (11), 3-hydroxy-α-ionone (12) and 2-C-methyl-erythrytol (13) were identified (1H NMR spectra see in Supporting Information). The diastereomers (+)-oxypeucedanin hydrate and (−)-oxypeucedanin hydrate were distinguished by determining their optical rotations and comparing with literature (Atkinson et al. Citation1974).
The 1H and 13C NMR spectral data of 11, 12 and 13 in CD3OD are reported here for the first time. Compound 11 (harmine): 1H NMR (500 MHz, CD3OD) δ = 8.11 (H-3, d, J = 5.5 Hz), 8.02 (H-5, d, J = 8.7 Hz), 7.86 (H-4, d, J = 5.5 Hz), 7.06 (H-8, d, J = 1.9 Hz), 6.89 (H-6, dd, J = 8.7 Hz, 1.9 Hz), 3.92 (s, 7-OCH3), 2.80 (s, 1-CH3); 13C NMR (125 MHz, CD3OD) δ = 162.9, 144.6, 141.6, 137.0, 136.2, 130.7, 123.7, 116.3, 113.5, 111.4, 95.4, 56.0, 19.1. Compound 12 (3-hydroxy-α-ionone): 1H NMR (500 MHz, CD3OD) δ = 6.67 (H-7, dd, J = 15.8 Hz, 10.3 Hz), 6.13 (H-8, d, J = 15.8 Hz), 5.60 (H-4, br s), 4.22 (H-3, br s), 2.58 (H-6, d, J = 10.3 Hz), 2.27 (H3-10, s), 1.80 (H-2β, dd, J = 13.2 Hz, 5.9 Hz), 1.63 (H3-13, s), 1.38 (H-2α, dd, J = 13.2 Hz, 7.2 Hz), 1.01 (H3-11, s), 0.90 (H3-12, s); 13C NMR (125 MHz, CD3OD) δ = 200.8, 149.8, 135.9, 134.7, 127.3, 65.9, 55.6, 45.0, 35.0, 29.8, 27.1, 24.5, 22.8. Compound 13 (2-C-methyl-erythrytol): 1H NMR (500 MHz, CD3OD) δ = 3.80 (H-4a, dd, J = 10.4 Hz, 2.5 Hz), 3.61 (H-3, m), 3.59 (H-4b, m), 3.52 (H-1a, d, J = 11.1 Hz), 3.44 (H-1b, d, J = 11.1 Hz), 1.11 (2-CH3, s); 13C NMR (125 MHz, CD3OD) δ = 76.2, 75.0, 68.5, 63.8, 19.7.
Antiproliferative and cytotoxic activities on cancer cell lines
Furocoumarins isolated from D. anethifolia were subjected to bioassay for cytotoxic and antiproliferative activity against cancer cell lines. All compounds exerted potent antiproliferative effect on sensitive and resistant mouse T-lymphoma cells (). However, they did not show any selectivity towards the resistant cell line. The most potent compound was oxypeucedanin on both cell lines. Some compounds had no toxic effects ((+)-oxypeucedanin hydrate (2), heraclenol (9), isogospherol (7)); furthermore, pabulenol (1), oxypeucedanin methanolate (4) and imperatorin (6) were more toxic on the sensitive PAR cell line (IC50 between 52 and 57 µM) without any toxicity on MDR cells (). Oxypeucedanin (3) and heraclenin (8) exhibited cytotoxic activity; however, they were more potent on the sensitive PAR cell line (). The cytotoxic activity of furocoumarins was assessed using NIH/3T3 normal murine fibroblast cells. Some compounds showed slight toxic effect on normal fibroblasts, namely (+)-oxypeucedanin hydrate (2), heraclenol (4) and isogospherol (8) with IC50 values of 83.55, 65.78 and 54.82 µM, respectively. Pabulenol (1) possessed similar activity on fibroblast and parental mouse lymphoma cells. In addition, oxypeucedanin (3), oxypeucedin methanolate (5) and heraclenin (9) exhibited mild toxicity on fibroblasts and parental lymphoma cells. Imperatorin (7) had no toxic activity on fibroblasts.
Table 1. Antiproliferative (AA) and cytotoxic activities (CA) of the furocoumarins against PAR, MDR and NIH/3T3 cells presented as IC50 values.
Multidrug resistance reversing activity
Regarding the efflux pump inhibiting activity of the compounds on ABCB1 overexpressing MDR mouse T-lymphoma cells, only oxypeucedanin (3) showed moderate ABCB1 inhibiting effect (FAR: 2.22); however, this inhibition was lower than in case of the positive controls tariquidar (FAR: 100) and verapamil (FAR: 8.2) (, figures see in Supporting Information).
Table 2. Efflux pump inhibiting activities of furocoumarins.
Table 3. Checkerboard combination assay of selected compounds with doxorubicin.
Combination assay results on MDR cells
The two most promising compounds in the previous assays were investigated in combination with the standard chemotherapeutic drug doxorubicin. The compounds oxypeucedanin (3) and heraclenin (8) showed slight synergistic effect with doxorubicin, for this reason, they might be potential adjuvants in combined chemotherapy applying standard anticancer drugs with compounds that can act synergistically ().
Discussion
Chromatographic separation of the extract of D. anethifolia herbs resulted in the isolation of 13 compounds, among them were nine furocoumarins. Compounds 2, 3, 5, 7, 10–13 were identified for the first time from Ducrosia genus.
The tested furocoumarins exerted antiproliferative effects on sensitive and resistant mouse T-lymphoma cells with no selectivity towards the resistant cell line. This is the first comprehensive analysis of this plant and its furocoumarins on these cells. Oxypeucedanin (3) had the most remarkable activity on both cell lines. The most effective furocoumarins, oxypeucedanin (3) and heraclenin (9) exhibited marginal toxicity on normal fibroblast cells and sensitive parental mouse lymphoma cells; furthermore, they were less toxic on multidrug resistant lymphoma cells. From the tested compounds, only oxypeucedanin showed moderate MDR reversing activity. In the checkerboard assay, oxypeucedanin and heraclenin showed slight synergistic effect with doxorubicin. These compounds might improve the cytotoxic effect of the standard chemotherapeutic drug doxorubicin.
Supplemental Material - Supporting_information.docx
Download MS Word (1.8 MB)Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Abbasnejad M, Mostafavi A, Kooshki R, Hamzenejad P, Esmaeili-Mahani S. 2017. Effect of Ducrosia anethifolia (DC.) Boiss essential oil on spatial learning and memory in rats. J Gorgan Univ Med Sci. 18:9–15.
- Amiri MS, Joharchi MR. 2016. Ethnobotanical knowledge of Apiaceae family in Iran: a review. Avicenna J Phytomed. 6:621–635.
- Appendino G, Bianchi F, Bader A, Campagnuolo C, Fattorusso E, Taglialatela-Scafati O, Blanco-Molina M, Macho A, Fiebich BL, Bremner P. 2004. Coumarins from Opopanax chironium. New dihydrofuranocoumarins and differential induction of apoptosis by imperatorin and heraclenin. J Nat Prod. 67:532–536.
- Asgari Nematian M, Yaghmaei P, Mohammadi S. 2017. Assessment of the antinociceptive, antiinflammatory and acute toxicity effects of Ducrosia anethifolia essential oil in mice. Sci J Kurdistan Univ Med Sci. 22:74–84.
- Ashraf M, Karim A, Bushra B. 1979. Studies on the essential oils of the Pakistani species of the family Umblliferae. Pak J Sci Ind Res. 22:252–254.
- Atkinson E, Boyd DR, Grundon MF. 1974. Coumarins of Skimmia japonica. Phytochemistry. 13:853–855.
- Aynehchi Y. 1991. Materia medica and Iranian medicinal plants. Tehran: Tehran University Publications.
- Bartnik M, Sławińska-Brych A, Żurek A, Kandefer-Szerszeń M, Zdzisińska B. 2017. 8-methoxypsoralen reduces AKT phosphorylation, induces intrinsic and extrinsic apoptotic pathways, and suppresses cell growth of SK-N-AS neuroblastoma and SW620 metastatic colon cancer cells. J Ethnopharmacol. 207:19–29.
- Chou T, Martin N. 2005. A computer program for quantitation of synergism and antagonism in drug combinations, and the determination of IC50 and ED50 and LD50 values. CompuSyn for drug combinations: PC software and user’s guide. Paramus: ComboSynInc.
- Chou TC. 2010. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 70:440–446.
- Chung CP, Hsia SM, Lee MY, Chen HJ, Cheng F, Chan LC, Kuo YH, Lin YL, Chiang W. 2011. Gastroprotective activities of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) on the growth of the stomach cancer AGS cell line and indomethacin-induced gastric ulcers. J Agric Food Chem. 59:6025–6033.
- Domínguez-Álvarez E, Gajdács M, Spengler G, Palop JA, Marć MA, Kieć-Kononowicz K, Amaral L, Molnár J, Jacob C, Handzlik J, et al. 2016. Identification of selenocompounds with promising properties to reverse cancer multidrug resistance. Bioorg Med Chem Lett. 26:2821–2824.
- Fujioka T, Furumi K, Fujii H, Okabe H, Mihashi K, Nakano Y, Matsunaga H, Katano M, Mori M. 1999. Antiproliferative constituents from Umbelliferae plants. V. A new furanocoumarin and falcarindiol furanocoumarin ethers from the root of Angelica japonica. Chem Pharm Bull. 47:96–100.
- Ghahreman A. 1993. Flora of Iran/Flore de l’Iran, vol. 12. Tehran: Research Institute of Forests and Rangelands.
- Habibi H, Ghahtan N, Kohanmoo MA, Eskandari F. 2017. Research in molecular medicine chemical composition and antibacterial effect of medicinal plants against some food-borne pathogens. Res Mol Med. 5:14–21.
- Haghi G, Safaei A, Safari J. 2004. Extraction and determination of the main components of the essential oil of Ducrosia anethifolia by GC and GC/MS. Iran J Pharm Res. 3:90–99.
- Hajhashemi V, Rabbani M, Ghanadi A, Davari E. 2010. Evaluation of antianxiety and sedative effects of essential oil of Ducrosia anethifolia in mice. Clinics (Sao Paulo, Brazil). 65:1037–1042.
- Harkar S, Razdan TK, Waight ES. 1984. Steroids, chromone and coumarins from Angelica officinalis. Phytochemistry. 23:419–426.
- Janssen AM, Scheffer JJ, Baerheim Svendsen A, Aynehchi Y. 1984. The essential oil of Ducrosia anethifolia (DC.) Boiss. Chemical composition and antimicrobial activity. Pharm Weekblad Sci Ed. 6:157–160.
- Javidnia K, Miri R, Assadollahi M, Gholami M, Ghaderi M. 2009. Screening of selected plants growing in Iran for antimicrobial activity. Iran J Sci Technol Trans A. 33:329–333.
- Kaewpiboon C, Surapinit S, Malilas W, Moon J, Phuwapraisirisan P, Tip-Pyang S, Johnston RN, Koh SS, Assavalapsakul W, Chung YH. 2014. Feroniellin A-induced autophagy causes apoptosis in multidrug-resistant human A549 lung cancer cells. Int J Oncol. 44:1233–1242.
- Karami A, Bohlooli A. 2017. Essential oil chemical diversity of Ducrosia anethifolia (DC.) Boiss. accessions from Iran. J Essent Oil Bear Pl. 20:1342–1348.
- Khalid B, Hamid S, Liaqat L, Khan JI. 2009. Seed oils of Pakistani wild species of Umbelliferae family: Ducrosia anethifolia, Bunium persicum, Bunium cylindricum and Ammi majus; as potential industrial raw material. Pak J Sci Ind Res. 52:260–263.
- Kim Y-K, Kim YS, Ryu SY. 2007. Antiproliferative effect of furanocoumarins from the root of Angelica dahurica on cultured human tumor cell lines. Phytother Res. 21:288–290.
- Kubrak T, Bogucka-kocka A, Komsta Ł, Za D, Bogucki J, Galkowski D, Kaczmarczyk R, Feldo M, Cioch M, Kocki J. 2017. Modulation of multidrug resistance gene expression by coumarin derivatives in human Leukemic cells. Oxid Med Cell Longev. 2017:1–13.
- Luo KW, Sun JG, Chan JYW, Yang L, Wu SH, Fung KP, Liu FY. 2011. Anticancer effects of imperatorin isolated from Angelica dahurica: induction of apoptosis in HepG2 cells through both death-receptor- and mitochondria-mediated pathways. Chemotherapy. 57:449–459.
- Lv X, Liu D, Hou J, Dong P, Zhan L, Wang L, Deng S, Wang C, Yao J, Shu X, et al. 2013. Biotransformation of imperatorin by Penicillium janthinellum. Anti-osteoporosis activities of its metabolites. Food Chem. 138:2260–2266.
- Macias FA, Massanet GM, Rodriguez-Luis F, Salvá J. 1990. 13C NMR of coumarins. Magn Reson Chem. 28:219–222.
- Mahboubi M, Feizabadi MM. 2009. Antimicrobial activity of Ducrosia anethifolia essential oil and main component, decanal against methicillin-resistant and methicillin-susceptible Staphylococcus aureus. J Essent Oil Bear Pl. 12:574–579.
- Mazloomifar A, Valian M. 2015. GC–MS analysis of the leaves essential oil of Ducrosia anethifolia (DC.) Boiss. obtained with three extractions. J Essent Oil Bear Pl. 18:904–907.
- Mirzaei SA, Gholamian Dehkordi N, Ghamghami M, Amiri AH, Dalir Abdolahinia E, Elahian F. 2017. ABC-transporter blockage mediated by xanthotoxin and bergapten is the major pathway for chemosensitization of multidrug-resistant cancer cells. Toxicol Appl Pharmacol. 337:22–29.
- Morgan AMA, Kim JH, Lee HW, Lee SH, Lim CH, Jang HD, Kim YH. 2015. Phytochemical constituents from the aerial part of Ducrosia ismaelis Asch. Nat Prod Sci. 21:6–13.
- Mostafavi A, Afzali D, Mirtadzadini SM. 2008. Chemical composition of the essential oil of Ducrosia anethifolia (DC.) Boiss. from Kerman province in Iran. J Essent Oil Res. 20:509–512.
- Mottaghipisheh J, Maghsoudlou MT, Valizadeh J, Arjomandi R. 2014. Antioxidant activity and chemical composition of the essential oil of Ducrosia anethifolia (DC.) Boiss. from Neyriz. J Med Plants By-Prod. 3:215–218.
- Mozaffarian V. 1996. A dictionary of Iranian plant names. Tehran: Farhang Moaser.
- Nyasty F, Oryan S, Sofiabadi M, Eslimi Esfahani D. 2017. Effect of intraperitoneal injection of hydroalcoholic extract of Ducrosia anethifolia on pentylenetetrazol-induced anticonvulsion in male Wistar rats. Horizon Med Sci. 23:49–53.
- Poljarević JM, Tamás Gál G, May NV, Spengler G, Dömötör O, Savić AR, Grgurić-Šipka S, Enyedy ÉA. 2018. Comparative solution equilibrium and structural studies of half-sandwich ruthenium(II)(η6-toluene) complexes of picolinate derivatives. J Inorg Biochem. 181:74–85.
- Poonkodi K. 2016. Phytoconstituents from Richardia scabra L. and its biological activities. Asian J Pharm Clin Res. 9:1–4.
- Queslati MH, Bouajila J, Belkacem MA, Harrath AH, Alwasel SH, Ben Jannet H. 2017. Cytotoxicity of new secondary metabolites, fatty acids and tocols composition of seeds of Ducrosia anethifolia (DC.) Boiss. Nat Prod Res. 6419:1–7.
- Rahimi N, Samani Jahromi E, Zolghadri Jahromi S. 2016. The effect of the hydro-alcoholic extract of Ducrosia anethifolia on testosterone hormone and the histological changes of the testicle in male adult rats. Armaghane-danesh. 21:682–693.
- Rahimifard M, Manayi A, Baeeri M, Gholami M, Saeidnia S, Abdollahi M. 2018. Investigation of β-sitosterol and prangol extracted from Achillea tenoifolia along with whole root extract on isolated rat pancreatic islets. Iran J Pharm Res. 17:317–325.
- Sbai H, Saad I, Ghezal N, Greca M, Della, Haouala R. 2016. Bioactive compounds isolated from Petroselinum crispum L. leaves using bioguided fractionation. Ind Crops Prod. 89:207–214.
- Sefidkon F, Javidtash I. 2002. Essential oil composition of Ducrosia anethifolia (DC.) Boiss. from Iran. J Essent Oil Res. 14:278–279.
- Shahabipour S, Firuzi O, Asadollahi M, Faghihmirzaei E, Javidnia K. 2013. Essential oil composition and cytotoxic activity of Ducrosia anethifolia and Ducrosia flabellifolia from Iran. J Essent Oil Res. 25:160–163.
- Shahat AA, Ibrahim AY, Alsaid MS. 2015. Antioxidant capacity and polyphenolic content of seven Saudi Arabian medicinal herbs traditionally used in Saudi Arabia. Indian J Tradit Know. 14:28–35.
- Shalaby NMM, Abd-Alla HI, Aly HF, Albalawy MA, Shaker KH, Bouajila J. 2014. Preliminary in vitro and in vivo evaluation of antidiabetic activity of Ducrosia anethifolia Boiss. and its linear furanocoumarins. Biomed Res Int. 2014:1–13.
- Shokri H, Hekmatpou D, Ebrahimi Fakhar HR, Nyazi A, Azadi M, Taghizadeh M. 2013. Effect of Ducrosia anethifolia (Barilax) on anxiety after acute myocardial infarction. Arak Med Univ J. 16:28–34.
- Stavri M, Mathew KT, Bucar F, Gibbons S. 2003. Pangelin, an antimycobacterial coumarin from Ducrosia anethifolia. Planta Med. 69:956–959.
- Syed M, Iqbal MJ, Chaudhary FM, Bhatty MK. 1987. Antimicrobial activity of essential oils of Umbelliferae family. Part VI. Stewartiella baluchistanica, Penstemon canescens and Ducrosia anethifollia. Pak J Sci Ind Res. 30:595–598.
- Takács D, Csonka Á, Horváth Á, Windt T, Gajdács M, Riedl Z, Hajós G, Amaral L, Molnár J, Spengler G. 2015. Reversal of ABCB1-related multidrug resistance of colonic adenocarcinoma cells by phenothiazines. Anticancer Res. 35:3245–3251.
- Vazirzadeh A, Dehghan F, Kazemeini R. 2017. Changes in growth, blood immune parameters and expression of immune related genes in rainbow trout (Oncorhynchus mykiss) in response to diet supplemented with Ducrosia anethifolia essential oil. Fish Shellfish Immunol. 69:164–172.
- Zamyad M, Abasnejad M, Esmaeili-Mahani S, Mostafavi A. 2016. Alpha-pinene as the main component of Ducrosia anethifolia (Boiss) essential oil is responsible for its effect on locomotor activity in rats. Avicenna J Neuro Psych Physio. 3:e38787.