Abstract
Context
23-Hydroxybetulinic acid (23-HBA), a major active constituent of Pulsatilla chinensis (Bunge) Regel (Ranunculaceae), exhibits potential antitumor activity. Its metabolism, however, has not yet been studied.
Objective
This study focuses on the metabolism of 23-HBA in vitro by human liver microsomes.
Materials and methods
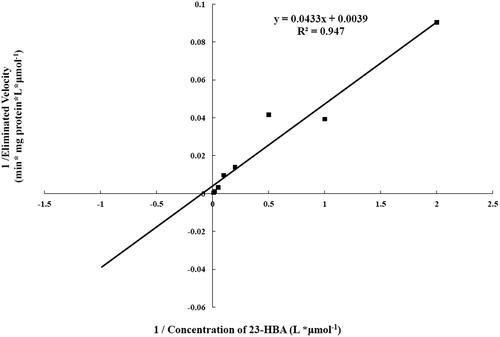
The metabolic kinetics of 23-HBA (0.5–100 µM) and the effects of selective CYP450 (CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4) inhibitors on metabolism of 23-HBA were evaluated in human liver microsomes incubation system and then determined by LC-MS method. The Michaelis–Menten parameters Km and Vmax were initially estimated by analysing Lineweaver–Burk plot. The clearance (CLint) was also calculated.
Results
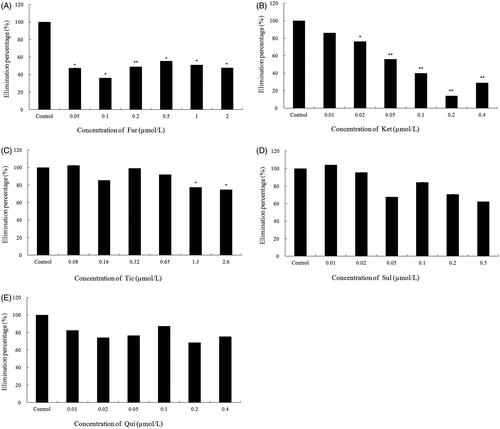
The Vmax, Km, and CLint of 23-HBA were 256.41 ± 11.20 pmol/min/mg, 11.10 ± 1.07 μM, and 23.10 ± 1.32 μL/min/mg, respectively. The metabolism of 23-HBA was significantly inhibited by furafylline (0.05 μM, p < 0.01) and ketoconazole (0.02 μM, p < 0.05). Ticlopidine (1.3 μM, p < 0.05) could inhibit the metabolism of 23-HBA, while the other inhibitors (sulfaphenazole and quinidine) showed nonsignificant inhibition on the metabolism of 23-HBA.
Discussion and conclusions
This is the first investigation of the metabolism of 23-HBA in human liver microsomes. The in vitro study indicates that CYP1A2 and CYP3A4 are mainly involved in the metabolism of 23-HBA. Special attention should be given to the pharmacokinetic and clinical outcomes when 23-HBA was co-administrated with other compounds mainly undergoing CYP1A2/CYP3A4-mediated metabolism. Further studies are needed to evaluate the significance of this interaction and strengthen the understanding of traditional Chinese medicine.
Introduction
Pulsatilla chinensis (Bunge) Regel (Ranunculaceae) is a botanical with a long history of medical use in China, the roots are widely used in the traditional Chinese medicine for adjunctive treatment of malaria, vaginal trichomoniasis, intestinal amoebiasis, bacterial infections, and malignant tumour (Cheng et al. Citation2008).
23-Hydroxybetulinic acid (23-HBA, ), an isolated pentacyclic triterpene, is the major active constituent of Pulsatilla chinensis (Ye et al. Citation1996). 23-HBA has been found to have cytotoxicity against a variety of tumour cell lines (Ji et al. Citation2002; Zhou et al. Citation2007; Zhang et al. Citation2015), such as the human non-small-cell lung cancer cell line NCI-H460, human gastric carcinoma cell line SGC7901 and human hepatocellular carcinoma cell line HepG2 and possesses synergistic effects on the cytotoxicity of doxorubicin in vitro and in vivo (Zheng et al. Citation2010). Herein, 23-HBA has a high potential to be developed as a novel safe chemosensitizer. However, despite the pharmacological importance of 23-HBA, there are no reports about its metabolism pathways, and especially whether it is metabolized by CYP450 enzyme and which subtypes are involved.
This study investigates the metabolic kinetics of 23-HBA and confirm which subtypes of CYP450 are responsible for the metabolism of 23-HBA in human liver microsomes.
Materials and methods
Chemicals and reagents
23-HBA (99% purity) was a kind gift from Professor WC Ye (Jinan University, Guangzhou, China). Furafylline (Fur), sulfaphenazole (Sul), ticlopidine (Tic), quinidine (Qui), ketoconazole (Ket), and NADPH were purchased from Sigma-Aldrich (St. Louis, MO, USA). In vitro CYP H-class 10-Donor pooled human liver microsomes (Lot No. X008061) were obtained from Research Institute for Liver Disease Co., Ltd. (Shanghai, China).
Incubation system
The incubation mixture, with a total volume of 200 μL, contained 100 mM potassium phosphate buffer (pH 7.4), NADPH generating system (1 mM NADP+, 10 mM glucose 6-phosphate, 1 unit/mL glucose-6-phosphate dehydrogenase and 5 mM MgCl2), human liver microsomes (0.2 mg/mL), CYP isoform-specific inhibitors and 23-HBA (1 μM). Incubation time, substrate concentration and liver microsomal concentration were all optimized in the study. The reaction was initiated by adding the NADPH-generating system after a 5 min pre-incubation at 37 °C. After incubation for 15 min in a shaking water bath, the reaction was terminated by the addition of ethyl acetate (800 μL, containing 5 μM oleanolic acid as internal standard). The mixture was kept on ice until it was centrifuged at 20,000 g for 10 min at 4 °C. Aliquots of supernatants were transferred for LC-MS analysis. Control incubations without NADPH or without substrate or without microsomes were included to ensure that metabolism of 23-HBA were microsomes and NADPH dependent. All incubations were performed in triplicate.
LC-MS method
LC-MS was carried out on a Shimadazu (Japan) LCMSD quadrupole mass spectrometer equipped with a series 2010 HPLC system. A Zorbax Extend-C18 column (50 mm × 2.1 mm, 5 µm, Agilent, USA) was used to separate 23-HBA. The mobile phase, consisted of acetonitrile (A) and water containing 0.05% (v/v) triethylamine (B) (A:B = 70:30, v:v), was run at a flow rate of 0.2 mL/min. Negative ion electrospray ionization (ESI) was used to form deprotonated molecules at m/z 471.20 of 23-hydroxybetulinic acid and m/z 455.35 of the internal standard oleanolic acid. Selected ion monitoring (SIM) was used. The optimum ESI conditions for 23-hydroxybetulinic acid and oleanolic acid included a probe voltage of 4500 V, a detector gain of 1600 V, a nitrogen nebulizer pressure of 35 psi, and nitrogen drying gas temperature of 250 °C at 4.5 L/min.
Kinetic study
To evaluate kinetic parameters, 23-HBA (0.5, 1, 2, 5, 10, 20, 50, 100 μM) was incubated with pooled human liver microsomes (0.2 mg/mL) for 15 min. Preliminary experiments were performed to make sure that the disappearance of 23-HBA was in the linear range of both reaction time and the concentration of microsomes. The apparent Vmax and Km values were calculated from non-linear regression analysis of experimental data according to the Michaelis–Menten equation using GraphPad Prism software (version 5.0, GraphPad Software, Inc., San Diego, CA). Accordingly, CLint of 23-HBA was calculated from the value of Vmax/Km.
Chemical inhibition study
Chemical inhibition studies were carried out by adding different human CYP inhibitors to the incubation mixture of 23-HBA (1 µM) before the addition of the NADPH-generating system. The inhibitors utilized were as follows: Fur (CYP1A2, 0.05, 0.1, 0.2, 0.5, 1, 2 μM), Sul (CYP2C9, 0.01, 0.02, 0.05, 0.1, 0.2, 0.5 μM), Tic (CYP2C19, 0.08, 0.16, 0.32, 0.65, 1.3, 2.6 μM), Qui (CYP2D6, 0.01, 0.02, 0.05, 0.1, 0.2, 0.4 μM) and Ket (CYP3A4, 0.01, 0.02, 0.05, 0.1, 0.2, 0.4 μM). The concentrations of inhibitors used in the study were verified to inhibit the specific activities of corresponding CYP isoforms in human liver microsomes (Weaver et al. Citation2003; Lim et al. Citation2013).
Results
The enzyme kinetics of 23-HBA
Preliminary studies indicated that the elimination of 23-HBA was linear up to 15-min incubation time when the concentration of liver microsomes was 0.2 mg/mL at 37 °C. Thus, the kinetic study of 23-HBA in liver microsomes was evaluated using a protein concentration of 0.2 mg/mL and an incubation time of 15 min. Under the experimental conditions used, the metabolism of 23-HBA in human liver microsomes obeyed typical Michaelis–Menten kinetics (shown in ). The kinetic parameters (apparent Vmax and Km) were calculated to be 256.41 ± 11.20 pmol/min/mg and 11.10 ± 1.07 μM. Accordingly, apparent CLint was 23.10 ± 1.32 μL/min/mg.
The effects of inhibitors on the metabolism of 23-HBA
The effect of various chemical inhibitors on the metabolism of 23-HBA was investigated in pooled human liver microsomes. Among the selective inhibitors of five CYP isoforms, furafylline (the selective inhibitor of CYP1A2) could significantly inhibit the disappearance of 23-HBA at all the tested concentration (. Also, ketoconazole (the selective inhibitor of CYP3A4) could significantly inhibit the metabolism of 23-HBA at low concentration (0.02 μM, p < 0.05) (. Ticlopidine (the selective inhibitor of CYP2C19) produced a slight inhibitory effect on the metabolism of 23-HBA at high concentration (>1.3 μM, p < 0.05) (. However, sulfaphenazole (the selective inhibitor of CYP2C9) () and quinidine (the selective inhibitor of CYP2D6) () showed non-significant inhibition on the metabolism of 23-HBA through the tested concentration. It showed that CYP1A2 and CYP3A4 were mainly involved in the metabolism of 23-HBA. CYP2C19 contributed to the metabolism of 23-HBA to some extent, while CYP2C9 and CYP2D6 might have no effect on the metabolism of 23-HBA.
Figure 3. Effects of various CYP450s selective inhibitors on metabolism of 23-HBA (1 µM) in human liver microsomes: (A) furafylline (Fur); (B) ketoconazole (Ket); (C) ticlopidine (Tic); (D) sulfaphenazole (Sul); (E) quinidine (Qui). Significance indicated by: *p < 0.05, **p < 0.01 versus control. Data are presented as mean ± SD of three independent experiments.
Discussion
Herbal medicines are widely used in the treatment of different diseases because of the efficacy and low toxicity. Pulsatilla chinensis has been used as adjuvant in chemotherapy in traditional Chinese medicine for a long time. 23-HBA is the major active constituent of Pulsatilla chinensis and has a high potential to be developed as a novel safe chemosensitizer. Studies on the metabolism of a new drug have an important role for determining the safety and therapeutic potential of the drug (Lin and Lu Citation1997; Fang et al. Citation2011). Moreover, drug metabolism is the major route of drug clearance and CYP450 is the main factor most frequently responsible for inter-individual differences in pharmacokinetics owing to polymorphic or inducible expression. Therefore, it is necessary to identify the drug-metabolizing enzymes involved in metabolism of 23-HBA.
Cytochrome P450s is the most important drug-metabolizing enzyme family, among which CYP3A, CYP2C and CYP2D are responsible for metabolism of about 50%, 25%, and 20% of drugs (Rendic and Carlo Citation1997; Guengerich Citation2006). To understand about the metabolism of 23-HBA in vitro by human liver microsomes, we firstly investigated the characteristics of enzyme kinetic of 23-HBA. The Michaelis–Menten parameters Km and Vmax were initially estimated by analysing Lineweaver–Burk plot. The clearance (CLint) was also calculated.
Chemical inhibition study showed that CYP1A2 and CYP3A4 were mainly involved in the metabolism of 23-HBA. CYP2C19 contributed to the metabolism of 23-HBA to some extent, while CYP2C9 and CYP2D6 might have no effect on the metabolism of 23-HBA. 23-HBA possesses synergistic effects on the cytotoxicity of doxorubicin in vitro and in vivo (Zheng et al. Citation2010). As we known, doxorubicin is a typical substrate of CYP3A4, the synergism may be associated with the inhibition of the metabolism of doxorubicin by 23-HBA.
Therefore, special attention should be paid on pharmacokinetic and clinical outcomes when 23-HBA was co-administrated with other compounds mainly undergoing CYP1A2/CYP3A4-mediated metabolism.
Conclusions
The present study is the first to investigate the metabolism of 23-HBA in human liver microsomes. It was found that CYP450s appeared to be involved in the metabolism of 23-HBA. CYP1A2 and CYP3A4 were the major drug-metabolizing CYP enzymes involved in the metabolism of 23-HBA. The results are helpful for a deeper understanding of metabolic and pharmacokinetic behaviour of 23-HBA. In the later, further study can be made to explore the effect of 23-HBA on the activity of CYP450s in vitro and in vivo, so as to provide some useful information for safe and effective use of 23-HBA in clinical practice and strengthen the understanding of traditional Chinese medicine.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Cheng L, Zhang M, Zhang P, Song Z, Ma Z, Qu H. 2008. Silver complexation and tandem mass spectrometry for differentiation of triterpenoid saponins from the roots of Pulsatilla chinensis (Bunge) Regel. Rapid Commun Mass Spectrom. 22(23):3783–3790.
- Fang ZZ, Zhang YY, Wang XL, Cao YF, Huo H, Yang L. 2011. Bioactivation of herbal constituents: simple alerts in the complex system. Expert Opin Drug Metab Toxicol. 7(8):989–1007.
- Guengerich FP. 2006. Cytochrome P450s and other enzymes in drug metabolism and toxicity. AAPS J. 8(1):E101–11.
- Ji ZN, Ye WC, Liu GG, Hsiao WL. 2002. 23-Hydroxybetulinic acid-mediated apoptosis is accompanied by decreases in bcl-2 expression and telomerase activity in HL-60 Cells. Life Sci. 72(1):1–9.
- Lim KB, Ozbal CC, Kassel DB. 2013. High-throughput mass spectrometric cytochrome p450 inhibition screening. Methods Mol Biol. 987:25–50.
- Lin JH, Lu AY. 1997. Role of pharmacokinetics and metabolism in drug discovery and development. Pharmacol Rev. 49(4):403–449.
- Rendic S, Carlo F. 1997. Human cytochrome P450 enzymes: a status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metab Rev. 29(1-2):413–580.
- Weaver R, Graham KS, Beattie IG, Riley RJ. 2003. Cytochrome P450 inhibition using recombinant proteins and mass spectrometry/multiple reaction monitoring technology in a cassette incubation. Drug Metab Dispos. 31(7):955–966.
- Ye WC, Ji NN, Zhao SX, Liu JH, Ye T, McKervey MA, Stevenson P. 1996. Triterpenoids from Pulsatilla chinensis. Phytochemistry. 42:799–802.
- Zhang DM, Xu HG, Wang L, Li YJ, Sun PH, Wu XM, Wang GJ, Chen WM, Ye WC. 2015. Betulinic acid and its derivatives as potential antitumor agents. Med Res Rev. 35(6):1127–1155.
- Zheng Y, Zhou F, Wu X, Wen X, Li Y, Yan B, Zhang J, Hao G, Ye W, Wang G. 2010. 23-Hydroxybetulinic acid from Pulsatilla chinensis (Bunge) Regel synergizes the antitumor activities of doxorubicin in vitro and in vivo. J Ethnopharmacol. 128(3):615–622.
- Zhou JP, Li D, Wu XM, Ye WC, Zhang LY. 2007. Synthesis and antitumor activity of derivatives of 23-hydroxybetulinic acid. Chin Chem Lett. 18(10):1195–1198.