Abstract
Context
Palmatine, a biologically active isoquinoline alkaloid, possesses multiple pharmaceutical activities against mucosal infection and inflammation.
Objective
There are no reports about the influence of palmatine on uterine mucosal epithelial cells.
Materials and methods
We used proteomics to analyse differentially expressed proteins (DEPs) in goat endometrial epithelial cells (EECs) stimulated by lipopolysaccharide (LPS, 5 μg/mL, the dosage can induce inflammatory response, according to our previous study) for 12 h and then treated with palmatine (80 μg/mL) for 8 h; the dosage was selected based on MTT assay. The EECs without any treatment were used as controls. Every group was treated in triplicate.
Results
A total of 428 DEPs in LPS-stimulated group and 486 DEPs in the palmatine-treated group were identified. Functional annotation analysis showed that palmatine mainly regulated the protein expression of structural molecules involved in the response to stimuli. Pathway analysis showed that cell adhesion molecule (CaM) pathways were most significant enriched due to palmatine treatment. Junction adhesion molecule 1 (JAM1), nectin 1 (NECT1) and cadherin 5 (CDH5), which play important roles in the transepithelial migration (TEpM) of leukocytes, were significantly downregulated by palmatine. Meanwhile, other proteins essential to the maintenance of cell adhesion and those that facilitate leukocyte migration were upregulated after palmatine treatment.
Discussion and conclusions: The results suggested that palmatine regulates the expression of CaMs to affect TEpM during uterine mucosal inflammation and provides novel insight to understanding and developing palmatine pharmacology. Palmatine is a promising drug for treatment of mucosal inflammation.
Introduction
Palmatine, an isoquinoline alkaloid distributed in many botanical families, has been reported to possess multiple pharmaceutical and biological activities, involving its antibacterial (Chae et al. Citation1999), antiviral (Jia et al. Citation2010), gastroprotective (Wang et al. Citation2017) and anticolitis activities (Mai et al. Citation2019), and has been used in gastritis and peptic ulcer treatment (Zhou et al. Citation2017). Palmatine has also been shown to increase the effects of available antibiotics and decrease the emergence of multi-drug resistant (MDR) bacteria (Aghayan et al. Citation2017) and possesses promising therapeutic potential against cardiac hypertrophy (Yuan et al. Citation2017). Clinically, palmatine has been mainly used for the treatment of gastrointestinal tract infection, respiratory tract infection, urinary system infection, surgery-related infection, pelvic inflammation, chronic endometritis and gynaecological inflammation (Chao et al. Citation2009; Fang and Li Citation2016).
The mucosal epithelium is a tissue common to both the gastrointestinal tract and female reproductive tract. Known to form an efficient physical barrier against infection, epithelial cells have evolved innate immune functions as well as the ability to modulate the recruitment and activity of immune cells of both the innate and adaptive immune systems (Schaefer et al. Citation2004). Luminal epithelial cells are inextricably related to inflammation, whether in enteritis, gastritis or gynaecological inflammation. Regarding female mammals, uterine epithelial cells constitute the mucus layer and act as the first line of defence against pathogens that invade the uterine lumen (Davies et al. Citation2008). However, limited reports have been published on the effects of palmatine on uterine epithelial cells. But palmatine can reduce lipopolysaccharide (LPS)-induced inflammatory responses in endometrial epithelial cells (EECs) by inhibiting the TRIF-dependent NF-κB pathway according to our previous research (Yan et al. Citation2017). Hence, further knowledge about the most comprehensive mechanism of palmatine against inflammation would contribute to improving therapeutic options for disease.
Proteomic analysis based on mass spectrometry and bioinformatics is a powerful and high-throughput technique to identify global differentially expressed proteins (DEPs) (Yang et al. Citation2018). The desire to fully understanding the pharmacological effects of palmatine and its derivatives and elucidate their mechanisms using genomics, proteomics and other advanced approaches has attracted scientists (Mi et al. Citation2015). However, differences in the global expression of proteins in mucosal epithelial cells treated with palmatine compared to control cells have not been reported.
In this study, we used isobaric tags for relative and absolute quantitation (iTRAQ) coupled with 2D nano LC–MS/MS to quantify and analyse DEPs in cells stimulated by LPS and treated with palmatine compared with control cells. The results improve our pharmacological understanding of palmatine and shed light on its mechanism and, importantly, pathways involved in the regulation of cellular inflammation.
Materials and methods
Reagents
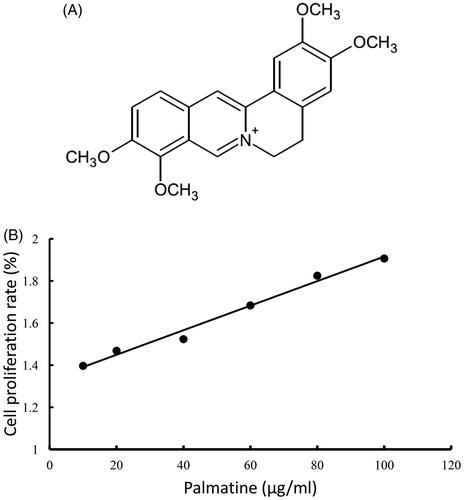
Palmatine (purity >98%, ) was purchased from Shanghai Source Leaf Biological Technology Corporation (Shanghai, China). LPS (O111:B4, L4391, Sigma) was purchased from Sigma Chemical Co. (St. Louis, MO). Dulbecco's modified Eagle's medium/F12 (DMEM/F12) and foetal bovine serum (FBS) were supplied by HyClone Corporation (HyClone-Pierce, Logan, UT).
Cell culture and treatment
Goat EECs, which are immortalized cells, were cultured in DMEM/F12 containing 10% FBS, 100 µg/mL penicillin and 100 µg/mL streptomycin at 37 °C in a humidified atmosphere containing 5% CO2. After preliminary culture for 24 h, two groups of EECs (2 × 105 cells/mL) were exposed to LPS (5 μg/mL) for 12 h, after which one group was treated with 80 μg/mL palmatine for an additional 8 h. The dosage of palmatine was selected based on MTT assay (). Another group of cells that was not administered any treatment was used as a negative control. Every group was treated in triplicate.
Protein isolation, digestion and labelling with iTRAQ reagent
After treatment, the cells were washed twice with PBS (pH 7.2), digested with trypsin and extracted with lysis buffer (7 M urea (Sangon, Shanghai, China, UB0148), 2 M thiourea (Sangon, Shanghai, China, TB1943), 4% CHAPS (Sigma, St. Louis, MO, 19899), 40 mM Tris–HCl (BBI, TB0194), pH 8.5, containing complete protease inhibitor. The cells were lysed by sonication at 200 W for 15 min and then centrifuged at 4 °C and 30,000×g for 15 min. The supernatant was mixed well with a 5× volume of chilled acetone (Sangon, Shanghai, China, A6854) containing 10% (v/v) trichloroacetic acid (Sangon, Shanghai, China, TB0968), incubated at −20 °C overnight, and centrifuged at 4 °C and 30,000×g, after which the precipitate was retained and washed with chilled acetone three times. The pellet was air-dried and dissolved in lysis buffer (7 M urea, (Sangon, Shanghai, China, SB0485), 2 M thiourea, 4% NP40 (Fluka, Buchs, Switzerland, 74385), 20 mM Tris–HCl, pH 8.0–8.5). The suspension was sonicated, centrifuged at 4 °C and 30,000×g for 15 min, and transferred to another tube. Disulphide bonds were reduced with 10 mM dithiothreitol, and cysteines were blocked with 55 mM iodoacetamide (Sigma, St. Louis, MO, I6125). Then, the supernatant was washed with acetone and centrifuged under the former conditions. The pellet was removed, air-dried, and dissolved in 500 μL of 0.5 M tetraethylammonium bromide (Fluka, Buchs, Switzerland, T0761) before being sonicated one more time. Finally, the samples were centrifuged, and the supernatants were transferred to a new tube, following which proteins were quantified using the Bradford method (Sangon, Shanghai, China, C503041). The protein-containing supernatants were stored at –80 °C for further analysis. The total protein (100 μg) was extracted from each pooled sample solution and digested with Trypsin Gold (Promega, Madison, WI, PRV5280) at a protein:trypsin ratio of 30:1 at 37 °C for 16 h. After trypsin digestion, peptides were dried by vacuum centrifugation. The peptides were reconstituted in 0.5 M tetraethylammonium bromide and processed with 8-plex iTRAQ reagent (Applied Biosystems Sciex, Foster City, CA, 4390812) according to the manufacturer's protocol. Briefly, one unit of iTRAQ reagent was thawed and reconstituted in 24 μL of isopropanol. Samples were individually labelled with different isobaric tags, with control samples labelled with 113 and 114 tags; LPS-stimulated samples labelled with 115, 116 and 117 tags; and palmatine-treated samples labelled with 118, 119 and 121 tags. Then, the labelled samples were incubated at room temperature for 2 h. The labelled peptide mixtures were pooled and dried by vacuum centrifugation.
LC–MS/MS analysis
The iTRAQ-labelled peptide mixtures were fractionated by strong cation exchange chromatography on an LC-20AB HPLC pump system (Shimadzu, Kyoto, Japan) using a 4.6 × 250 mm Ultremex SCX column containing 5 µm particles (Phenomenex, Torrance, CA). The peptides were eluted at a flow rate of 1 mL/min with a gradient of buffer A [25 mM NaH2PO4 in 25% acetonitrile (Fisher, San Jose, CA, A998-4), pH 2.7] for 10 min, 5–60% buffer B (25 mM NaH2PO4, 1 M KCl in 25% acetonitrile, pH 2.7) for 27 min and 60–100% buffer B for 1 min. The system was then maintained at 100% buffer B for 1 min before equilibration with buffer A for 10 min prior to the next injection. With absorbance monitoring at 214 nm, a total of 20 fractions were collected, desalted with a Strata-X C18 column (Phenomenex, Torrance, CA) and vacuum dried. Each fraction was dissolved in buffer [2% acetonitrile, 0.1% formic acid (Dikma, Lake Forest, CA, 50144)] and centrifuged at 20,000×g for 10 min. Then, 10 µL of supernatant was loaded on an LC-20AD nano HPLC (Shimadzu, Kyoto, Japan) by the auto sampler onto a 2 cm C18 trap column (inner diameter of 75 µm). The peptides were subjected to nanoelectrospray ionization followed by tandem mass spectrometry (MS/MS) in a Q Exactive mass spectrometer (Thermo Fisher Scientific, San Jose, CA) coupled online to the HPLC. Intact peptides were detected in the Orbitrap at a resolution of 70,000. Peptides were selected for MS/MS using high-energy collision dissociation operating mode with a normalized collision energy setting of 27.0; ion fragments were detected in the Orbitrap at a resolution of 17,500. A data-dependent procedure that alternated between one MS scan followed by 15 MS/MS scans was applied for the 15 most abundant precursor ions above a threshold ion count of 20,000 in the MS survey scan with subsequent dynamic exclusion duration of 15 s. The electrospray voltage applied was 1.6 kV. Automatic gain control (AGC) was used to optimize the spectra generated by the Orbitrap. The AGC target for full MS was 3E6 and 1E5 for MS2. For MS scans, the m/z scan range was 350–2000 Da. For MS2 scans, the m/z scan range was 100–1800.
Data analysis
For identification and quantitation, we adopted iQuant software (Wen et al. Citation2014), which was independently developed by BGI (Shenzhen, China) and integrates the Mascot Percolator algorithm, which automatically rescores the database search results by using a machine learning algorithm to improve the identification rate of the results, to quantitate the iTRAQ data. First, the results were filtered at the spectrum/peptide level by 1% FDR (psm-level FDR ≤ 0.01) to obtain the spectrogram and list of significance identified peptides. Then, on the basis of the parsimony principle, protein assembly was conducted with the peptides, and a variety of proteins were produced. To control the protein false-positive rate, in this process, the data were filtered again at the protein level with a 1% FDR (protein-level FDR ≤0.01). iQuant software processing primarily involves the following steps: protein filtration, determination and reporting of the group tag correction purity, determination of missing values, calculation of quantitative protein values, statistical analysis, and presentation of the final results. Ratios with a p value < 0.05 and a threshold of ±1.2-fold change were considered significant.
Bioinformatic analysis
Gene ontology (GO) protein annotation was performed against the nonredundant protein database (NR, NCBI) using the Blast2GO program. Most types of annotations were obtained from the Gene Ontology Consortium website (http://www.geneontology.org). Then, the DEPs were mapped to different biological pathways according to functional categories in the Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathway database (http://www.genome.ad.jp/kegg/pathway.html). GO terms and KEGG pathways significantly enriched in the DEPs were identified based on the presence of two DEPs and the results of a hypergeometric test. DEPs identified by GO and KEGG analyses were used to obtain further biological information by cluster analysis. Cluster analysis was carried out with Cluster 3.0, and the results were visualized and browsed using the TreeView programme. A Venn diagram was drawn with the online tool Venny 2.0 (http://bioinfogp.cnb.csic.es/tools/venny/index.html). The p value used to reflect whether a result was statistically significant in statistical analyses was calculated with a hypergeometric distribution probability formula. For the analyses in this study, p< 0.05 indicated statistical significance.
Quantitative real-time PCR analysis
After treatment with LPS or palmatine, cells were harvested, and total RNA was extracted using the TRIzol method. Then, 1 µg of total RNA was reverse transcribed into cDNA with a one-step RT-PCR kit (Takara, Dalian, China) following the manufacturer’s directions. Real-time fluorescent was performed using the following cycling conditions: 40 cycles of preincubation at 95 °C for 30 s, denaturation at 95 °C for 15 s and annealing at 60 °C for 20 s. Each real-time PCR sample was analysed for the target gene and a housekeeping gene (GAPDH). Real-time PCR to quantify the relative expression of three target genes was performed using quantitative PCR Super Mix and 2× SYBR Green in a final reaction volume of 25 µL according to the manufacturer’s protocol. The relative expression of target genes was quantified by calculating the ratio between the concentration of the target gene and that of the housekeeping gene with the 2–△△Ct method. Each PCR result of experiments performed in triplicate underwent statistical analysis. The three genes selected for RT-PCR assays were nectin 1 (NECT1), junction adhesion molecule 1 (JAM1) and cadherin 5 (CDH5). The specific primers were as follows: forward primer 5′-AGCCCTGTTCTCGTCTTGTG-3′ and reverse primer 5′-GTTCCTCTTGGAGCCCCTTC-3′ for NECT1; forward primer 5′-AAAGGCGAAAGTCGGCAGTA-3′ and reverse primer 5′-GGACAGCTTGGCAGGGTTAT-3′ for JAM1; forward primer 5′-GGTCGCCGTGTACAATCTGA-3′ and reverse primer 5′-GGTGAACTCAGGGGCATTGT-3′ for CDH5; forward primer 5′-AAGTTCCACGGCACAGTCAA-3′ and reverse primer 5′-ACCACATACTCAGCACCAGC-3′ for GAPDH.
Results
Number and distribution of DEPs
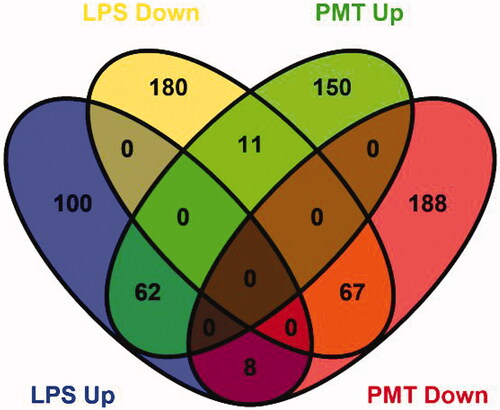
A total of 6985 proteins were identified by iTRAQ-coupled 2D LC–MS/MS analysis of the cells. Among them, proteins that displayed significantly altered expression levels compared with those in the control group with a threshold of ±1.2-fold change and p < 0.05 was considered DEPs. A total of 428 DEPs in the LPS-stimulated group and 486 DEPs in the palmatine-treated group were identified. Among the DEPs in the LPS-stimulated group, 170 proteins were upregulated, and 258 proteins were downregulated (Table S1 and Table S2). In addition, 223 proteins were upregulated, and 263 proteins were downregulated in the palmatine-treated group. Venn diagram analysis further showed that the LPS-stimulated group and palmatine-treated group shared 62 upregulated proteins and 67 downregulated proteins. Additionally, the expression levels of 19 proteins changed in opposite ways in the two groups ().
Figure 2. Venn diagram showing the distribution of DEPs from the LPS-stimulated group and palmatine-treated group. The blue oval represents upregulated proteins in the LPS-stimulated group (LPS Up), the yellow oval represents downregulated proteins in the LPS-stimulated group (LPS Down), the green oval represents upregulated proteins in the palmatine-treated group (PMT Up), and the red oval represents downregulated proteins in the palmatine-treated group (PMT Down). Overlap between ovals indicates the number of proteins common to different groups.
Cluster analysis of DEPs
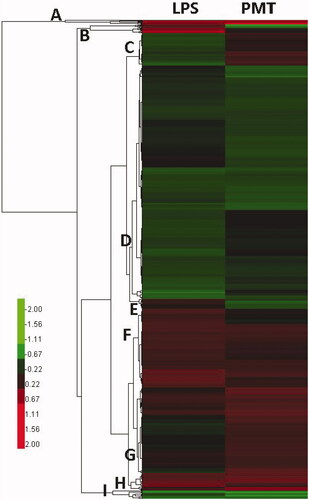
All DEPs were clustered through hierarchical clustering. Protein expression levels in the two groups are indicated by a colour gradient in . Both groups of DEPs consist of specific proteins that may indicate the different effects of LPS stimulation and palmatine treatment. In general, the DEPs in subfamilies A, D, F, H and I were clustered in a similar pattern and associated with LPS-induced inflammation, whereas the DEPs in subfamilies B, C, E and G were clustered in an inverted pattern and may reflect the effect of palmatine treatment on LPS-induced inflammation.
Functional classification of the DEPs
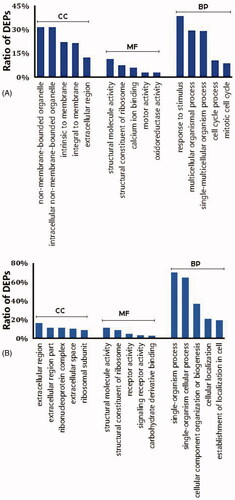
The DEPs were functionally annotated using the Blast2GO program. Three main types of annotations were obtained from the Gene Ontology Consortium website: cellular components, metabolic functions and biological processes. Gene ontology enrichment analysis showed that DEPs between the LPS-stimulated cells and control cells are mainly extracellular, possess structural molecular activity and are involved in single-organism process pathways (). Enrichment analysis of DEPs between palmatine-treated cells and control cells indicated that the DEPs are primarily involved in processes related to the response to stimuli and that these proteins are structural molecules located in nonmembrane-bound organelles ().
Figure 4. Classification of the top 5 DEPs based on their functional annotation using gene ontology enrichment analysis. (A) LPS-stimulated group and (B) palmatine-treated group. BP: biological process; CC: cellular component; MF: molecular function. Vertical coordinates indicate different functional annotations, and the abscissas indicate the percentage of proteins enriched in the corresponding annotation among all DEPs.
KEGG pathway analysis
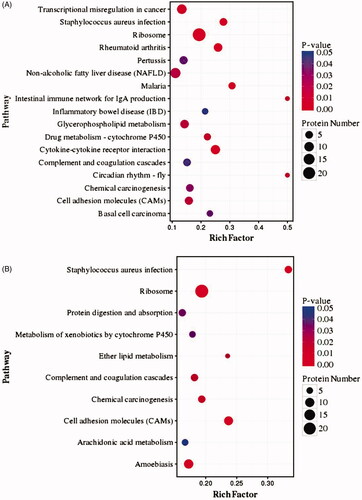
shows the results of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of the DEPs in both groups. Based on the rich factor (p< 0.05), the DEPs in LPS-stimulated cells compared with control cells are mainly enriched in pathways related to the ribosome (24 DEPs), cytokine–cytokine receptor interactions (nine DEPs), cell adhesion molecules (CaMs) (six DEPs) and Staphylococcus aureus infection (five DEPs), in addition to other pathways. In contrast, the DEPs in palmatine-treated cells compared with control cells are mainly enriched in pathways related to the ribosome (24 DEPs), CaMs (nine DEPs), S. aureus infection (six DEPs) and ether lipid metabolism (four DEPs).
Clustering of cellular adhesion molecules
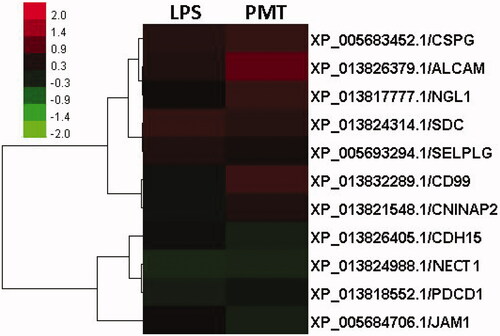
Clusters of differentially expressed CaMs are shown in , which reveals that activated leukocyte cell adhesion molecule (ALCAM), P-selectin glycoprotein ligand (SELPLG), syndecan (SDC) and chondroitin sulphate proteoglycan (CSPG) were upregulated, whereas programmed cell death 1 (PDCD1) and NECT1 were downregulated in the LPS-stimulated group compared to the control group. After treatment with palmatine, CD99, Netrin-G1 ligand (NGL1), ALCAM, CSPG, contactin-associated protein-like 2 (CNTNAP2) and SDC were upregulated, whereas JAM1, NECT1 and CDH5 were downregulated. Among these proteins, SDC, CSPG and ALCAM were upregulated in both groups, whereas NECT1 was downregulated.
Quantitative real-time PCR to assess gene expression
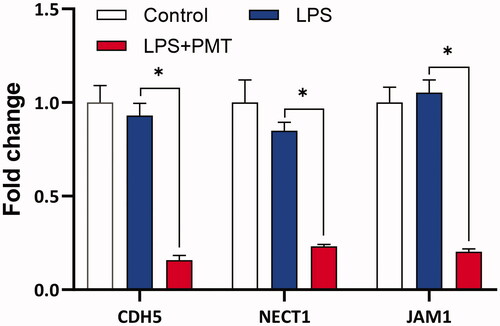
The mRNA expression levels of three adhesion molecules were independently determined by RT-qPCR. shows the fold change in gene expression relative to that of GAPDH. The gene expression levels of CHD5, NECT1 and JAM1 were not significantly altered when the mucosal epithelial cells were stimulated with only LPS but significantly decreased (p < 0.05) after treatment with palmatine.
Discussion
In this study, comparative proteomics showed that palmatine may play a role in the regulation of transepithelial migration (TEpM) through altering the expression of some CaMs. This may be a new mechanism by which palmatine attenuates inflammation.
Palmatine () is an important traditional medicine compound that is widely used to treat jaundice, hypertension, dysentery, inflammation and liver-related diseases (Zhou et al. Citation2016). This compound has been recorded as an inflammation-resolving drug in the Chinese Pharmacopoeia and suggested as a clinical medicine to relieve bacillary dysentery and gynaecological inflammation. In particular, palmatine is generally used to treat gynaecological inflammation in China (Chinese Pharmacopoeia Committee Citation2015). However, there have been few reports about the mechanism by which palmatine resolves reproductive inflammation. For a comprehensive understanding of palmatine activity, proteomic research was carried out with the aim of identifying alterations in the protein expression profile and mechanism of uterine mucosal epithelial cells, which are known to mount a significant inflammatory response upon stimulation with bacteria or bacterial LPS, an important virulence factor of Gram-negative bacteria (Salilew-Wondim et al. Citation2016). The results of iTRAQ analysis showed that palmatine altered cellular proteome expression patterns () and the DEP distribution (). Moreover, in addition to alterations in the cellular proteome, functions () and biological pathways () were changed due to changes in protein expression by palmatine; this was especially true for the CaM pathway, which was identified as the pathway most significantly enriched in the DEPs (). Thus, for the first time, these results provide novel insight into the potential pharmacology of palmatine, supporting the idea of the therapeutic use of this drug.
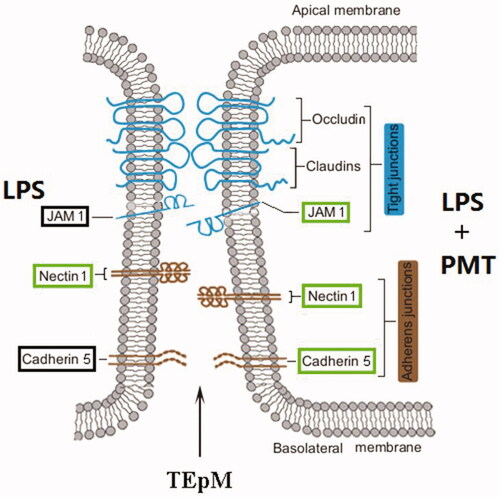
Palmatine may play a role in the regulation of intercellular junctions. Mucosal epithelial cells often form functional barriers that separate organs or tissues from the external environment. Formation of these barriers by epithelial cells requires neighbouring cells to interact via intercellular junctions, including tight junctions (TJs) and adherens junctions (AJs) (Balda and Matter Citation2016). These junctions consist of some important components. Claudins, occludins and junctional adhesion molecules (JAMs) are the major CaMs in TJs, whereas cadherins and nectins are the major CaMs in AJs (Ooshio et al. Citation2010). Many studies have reported that pathogenic bacteria can disrupt the function and structure of cell–cell junctions and epithelial polarity (Tapia et al. Citation2017). In this study, LPS downregulated NECT1, and palmatine downregulated the crucial molecules JAM1, NECT1 and CHD5 (). This finding demonstrates that palmatine can inhibit the formation of two junctional complexes, particularly AJs, in LPS-stimulated cells.
Compared with AJs, TJs consist of more components, and our results showed that palmatine significantly inhibited all core components of AJs but inhibited only one component of TJs, JAM1 (). Both TJs and AJs are major junctional apparatuses of epithelial cells (Campbell et al. Citation2017). These two adhesion complexes are structurally and functionally intertwined and exhibit a high level of interdependency (Balda and Matter Citation2016). TJs are often accompanied by AJs, and the assembly of TJs is dependent upon their formation and maturation between epithelia (Ooshio et al. Citation2010; Maiers et al. Citation2013). Thus, TJ assembly may have been negatively affected by palmatine, whereas AJ formation was downregulated by palmatine in LPS-stimulated cells.
Most major adhesion molecules have been shown to contribute to cell connection and communication. For example, TJs mediate signal transduction to regulate cytoskeletal dynamics, cell proliferation, migration and survival (Zihni et al. Citation2014) and maintain apical-basal polarity by restricting the mixing of apical and lateral plasma membrane components (Van Itallie and Anderson Citation2014), while AJs contribute to the epithelium in metazoans (Miller et al. Citation2013) and control some dynamic behaviours in epithelial cells, such as their arrangement, movement and shape (Takeichi Citation2014). Therefore, with the breakdown of these two adhesion complexes, palmatine may also change the function and structure of cell–cell junctions and epithelial polarity in LPS-stimulated uterine mucosal epithelial cells.
Moreover, TJs often control leukocyte transmigration, such as the transmigration of PMNs from the apical to basal aspect of the endothelium in transendothelial migration (TEndoM) and their migration from the basal to apical aspect of the epithelium in TEpM (Nourshargh and Alon Citation2014). While leukocyte migration into sites of inflammation or damaged tissue is a fundamental host defence reaction, excessive or aberrant leukocyte trafficking can be damaging to the host (Medzhitov Citation2010; Parkos Citation2016). Fortunately, TEndoM can mediate the systemic dissemination of a local inflammatory response, and TEpM is a protective physiological event that facilitates inflammation resolution through leukocyte clearance (Nourshargh et al. Citation2016). Our work indicated that palmatine could downregulate the assembly of AJs and TJs in LPS-stimulated epithelial cells, and this effect lead to a decrease in the barrier function of TJs when leukocyte migration occurs. Thus, we tentatively propose that palmatine facilitates the TEpM of leukocytes to relieve inflammation and prevent excessive or aberrant leukocyte trafficking in the local endometrium.
Additionally, some other cellular adhesion molecules beyond those in AJs and TJs were upregulated in LPS-stimulated cells after their treatment with palmatine, including CD99, NGL1, SDC, CSPG, CNTNAP2 and ALCAM (), and the cell adhesion pathway was also activated (). These molecules often play a role in cell adhesion, migration and proliferation, which is especially true for CD99 and ALCAM, which are involved in leukocyte migration and adhesion (Pasello et al. Citation2018), cell–cell interactions among the epithelia, and TEndoM (Hebron et al. Citation2018), respectively. These results demonstrated that palmatine may increase cell adhesion and promote leukocyte migration during the inflammatory response in cells.
Finally, to verify the results of proteomic analysis, the mRNA expression of three adhesion molecules, NECT1, CDH5 and JAM1, was investigated by real-time PCR, as there are no available commercial antibodies to validate expression of these proteins. The expression profiles of these gene showed the same tendency revealed by proteomic analysis and were not influenced by LPS but rather significantly decreased by palmatine (). Although protein expression was not closely correlated with changes in the corresponding mRNA levels (Fournier et al. Citation2010; Lan et al. Citation2012), here, palmatine downregulated CDH5, NECT1 and JAM1 at both the gene and protein levels.
Conclusions
This study is the first to report that palmatine may regulate cell adhesion and leukocyte migration. As shown in , palmatine downregulated expression of the adhesion junction proteins NECT1 and CDH5 and even the TJ protein JAM1 in uterine mucosal epithelial cells stimulated with LPS. This could facilitate the TEpM of leukocytes residing in the endometrium, such as neutrophils and macrophages. Therefore, the breakdown of junctional apparatuses between epithelial cells by palmatine may be an important mechanism to alleviate uterine mucosal inflammation. These results enrich current knowledge of the mechanism by which palmatine resolves inflammation and provide important clues for the development of novel drugs against mucosal inflammation.
Disclosure statement
There are no conflicts of interest regarding the publication of this article.
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/13880209.2021.1917927).
Additional information
Funding
References
- Aghayan S, Kalalian M, Fazli M, Darban-Sarokhalil D, Khoramrooz S, Jabalameli F, Yaslianifard S, Mirzaii M. 2017. The effects of berberine and palmatine on efflux pumps inhibition with different gene patterns in Pseudomonas aeruginosa isolated from burn infections. Avicenna J Med Biotechnol. 9(1):2–7.
- Balda M, Matter K. 2016. Tight junctions as regulators of tissue remodelling. Curr Opin Cell Biol. 42:94–101.
- Campbell HK, Maiers JL, DeMali KA. 2017. Interplay between tight junctions & adherens junctions. Exp Cell Res. 358(1):39–44.
- Chae S, Jeong I, Choi D, Oh J, Ahn Y. 1999. Growth-inhibiting effects of Coptis japonica root-derived isoquinoline alkaloids on human intestinal bacteria. J Agric Food Chem. 47(3):934–938.
- Chao J, Lu T-C, Liao J-W, Huang T-H, Lee M-S, Cheng H-Y, Ho L-K, Kuo C-L, Peng W-H. 2009. Analgesic and anti-inflammatory activities of ethanol root extract of Mahonia oiwakensis in mice. J Ethnopharmacol. 125(2):297–303.
- Chinese Pharmacopoeia Committee. 2015. Pharmacopoeia of the People's Republic of China. Part II. Beijing: China Medical Science Press; p. 592.
- Davies D, Meade KG, Herath S, Eckersall PD, Gonzalez D, White JO, Conlan RS, O'Farrelly C, Sheldon IM. 2008. Toll-like receptor and antimicrobial peptide expression in the bovine endometrium. Reprod Biol Endocrinol. 6:53.
- Fang Q, Li H. 2016. Effect of palmatine sequential combination of exercise therapy on serum inflammatory markers in pelvic inflammation and its clinical effect. Chin J Biochem Pharm. 6:164–166.
- Fournier ML, Paulson A, Pavelka N, Mosley AL, Gaudenz K, Bradford WD, Glynn E, Li H, Sardiu ME, Fleharty B, et al. 2010. Delayed correlation of mRNA and protein expression in rapamycin-treated cells and a role for Ggc1 in cellular sensitivity to rapamycin. Mol Cell Proteomics. 9(2):271–284.
- Hebron KE, Li EY, Arnold Egloff SA, von Lersner AK, Taylor C, Houkes J, Flaherty DK, Eskaros A, Stricker TP, Zijlstra A, et al. 2018. Alternative splicing of ALCAM enables tunable regulation of cell–cell adhesion through differential proteolysis. Sci Rep. 8(1):3208.
- Jia F, Zou G, Fan J, Yuan Z. 2010. Identification of palmatine as an inhibitor of West Nile virus. Arch Virol. 155(8):1325–1329.
- Lan P, Li W, Schmidt W. 2012. Complementary proteome and transcriptome profiling in phosphate-deficient Arabidopsis roots reveals multiple levels of gene regulation. Mol Cell Proteomics. 11(11):1156–1166.
- Mai C, Wu M, Wang C, Su Z, Cheng Y, Zhang X. 2019. Palmatine attenuated dextran sulfate sodium (DSS)-induced colitis via promoting mitophagy-mediated NLRP3 inflammasome inactivation. Mol Immunol. 105:76–85.
- Maiers JL, Peng X, Fanning AS, Demali KA. 2013. ZO-1 recruitment to alpha-catenin - a novel mechanism for coupling the assembly of tight junctions to adherens junctions. J Cell Sci. 126:3904–3915.
- Medzhitov R. 2010. Inflammation 2010: new adventures of an old flame. Cell. 140(6):771–776.
- Mi R, Tu B, Bai X, Chen J, Ouyang Y, Hu Y. 2015. Binding properties of palmatine to DNA: spectroscopic and molecular modeling investigations. Luminescence. 30(8):1344–1351.
- Miller P, Clarke D, Weis W, Lowe C, Nelson W. 2013. The evolutionary origin of epithelial cell–cell adhesion mechanisms. Curr Top Membr. 72:267–311.
- Nourshargh S, Alon R. 2014. Leukocyte migration into inflamed tissues. Immunity. 41(5):694–707.
- Nourshargh S, Renshaw S, Imhof B. 2016. Reverse migration of neutrophils: where, when, how, and why? Trends Immunol. 37(5):273–286.
- Ooshio T, Kobayashi R, Ikeda W, Miyata M, Fukumoto Y, Matsuzawa N, Ogita H, Takai Y. 2010. Involvement of the interaction of afadin with ZO-1 in the formation of tight junctions in Madin-Darby canine kidney cells. J Biol Chem. 285(7):5003–5012.
- Parkos C. 2016. Neutrophil–epithelial interactions: a double-edged sword. Am J Pathol. 186:404–1416.
- Pasello M, Manara M, Scotlandi K. 2018. CD99 at the crossroads of physiology and pathology. J Cell Commun Signal. 12(1):55–68.
- Salilew-Wondim D, Ibrahim S, Gebremedhn S, Tesfaye D, Heppelmann M, Bollwein H, Pfarrer C, Tholen E, Neuhoff C, Schellander K, et al. 2016. Clinical and subclinical endometritis induced alterations in bovine endometrial transcriptome and miRNome profile. BMC Genomics. 17:218.
- Schaefer T, Desouza K, Fahey J, Beagley K, Wira C. 2004. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology. 112(3):428–436.
- Takeichi M. 2014. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol. 15(6):397–410.
- Tapia R, Kralicek S, Hecht G. 2017. Modulation of epithelial cell polarity by bacterial pathogens. Ann N Y Acad Sci. 1405(1):16–24.
- Van Itallie CM, Anderson JM. 2014. Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol. 36:157–165.
- Wang L, Wang X, Zhang S-L, Zhu X-M, Liu Y-Q, Song Z-J, Du W-J, Ji J, Cui C-L, He X, et al. 2017. Gastroprotective effect of palmatine against acetic acid-induced gastric ulcers in rats. J Nat Med. 71(1):257–264.
- Wen B, Zhou R, Feng Q, Wang Q, Wang J, Liu S. 2014. IQuant: an automated pipeline for quantitative proteomics based upon isobaric tags. Proteomics 14:2280–2285.
- Yan B, Wang D, Dong S, Cheng Z, Na L, Sang M, Yang H, Yang Z, Zhang S, Yan Z, et al. 2017. Palmatine inhibits TRIF-dependent NF-κB pathway against inflammation induced by LPS in goat endometrial epithelial cells. Int Immunopharmacol. 45:194–200.
- Yang N, Liu Y, He P, Ke R, Zhao Y, Feng Y, Jing R, Ma S, Liu C, Geng Y, et al. 2018. iTRAQ-based differential proteomic analysis reveals the pathways associated with tigecycline resistance in Acinetobacter baumannii. Cell Physiol Biochem. 51(3):1327–1339.
- Yuan Y, Peng W, Liu Y, Xu Z. 2017. Palmatine attenuates isoproterenol-induced pathological hypertrophy via selectively inhibiting HDAC2 in rats. Int J Immunopathol Pharmacol. 30(4):406–412.
- Zhou J-T, Li C-L, Tan L-H, Xu Y-F, Liu Y-H, Mo Z-Z, Dou Y-X, Su R, Su Z-R, Huang P, et al. 2017. Inhibition of Helicobacter pylori and its associated urease by palmatine: investigation on the potential mechanism. PLoS One. 12(1):e0168944.
- Zhou X, Lin X, Yan X, Jiang L, Li W, Jin L, Wu L. 2016. Chondroprotective effects of palmatine on osteoarthritis in vivo and in vitro: a possible mechanism of inhibiting the Wnt/β-catenin and hedgehog signaling pathways. Int Immunopharmacol. 34:129–138.
- Zihni C, Balda M, Matter K. 2014. Signalling at tight junctions during epithelial differentiation and microbial pathogenesis. J Cell Sci. 127(Pt 16):3401–3413.